Abstract
A severe deficiency in von Willebrand factor–cleaving protease (ADAMTS13) activity (< 5% that in normal plasma) has been observed in most patients with a diagnosis of thrombotic thrombocytopenic purpura (TTP) but not in those with a diagnosis of hemolytic uremic syndrome. However, ADAMTS13 deficiency has been claimed not to be specific for TTP, since it was observed in various thrombocytopenic and other conditions. We studied 68 patients with thrombocytopenia due to severe sepsis or septic shock (n = 17), heparin-induced thrombocytopenia (n = 16), idiopathic thrombocytopenic purpura (n = 10), or other hematologic (n = 15) or miscellaneous conditions (n = 10). Twelve of the 68 patients had subnormal levels of ADAMTS13 activity (≤ 30%), but none had less than 10%. Thus, the study showed that ADAMTS13 activity is decreased in a substantial proportion of patients with thrombocytopenia of various causes. A severe deficiency of ADAMTS13 (< 5%), identified in more than 120 patients during 1996 to 2001 in our laboratory, is specific for a thrombotic microangiopathy commonly labeled TTP.
Introduction
In 1997, 4 patients with chronic relapsing thrombotic thrombocytopenic purpura (TTP) were shown to completely lack plasma activity of von Willebrand factor (VWF)–cleaving protease,1 an enzyme identified in 19962,3 and shown to cleave the peptide bond tyrosine 842–methionine 843 in VWF.2 Two large retrospective studies of patients with thrombotic microangiopathy showed severely deficient VWF-cleaving protease activity in most patients with acute TTP,4,5 due either to a constitutional deficiency4 or to abolishment of the activity by autoantibodies.4,5 Patients given a diagnosis of the clinically similar hemolytic uremic syndrome (HUS) had normal VWF-cleaving protease activity.4 This finding seemed surprising because distinguishing between TTP and HUS is often not possible because of the frequently overlapping clinical and laboratory features of the 2 disorders.6 Still, a prospective study by Veyradier et al7 essentially confirmed these findings: all 25 patients with idiopathic TTP totally lacked VWF-cleaving protease and all 17 patients with idiopathic HUS had normal levels. Among the 69 patients with secondary thrombotic microangiopathy, the distinction was somewhat less clear-cut; 7 of 41 patients with a diagnosis of secondary TTP had normal protease levels, whereas 6 of the 28 patients with a diagnosis of secondary HUS had partial or complete protease deficiency.7
The reported N-terminal amino acid sequence8,9 and complementary DNA10,11 of VWF-cleaving protease characterize it as a new member of the ADAMTS family of metalloproteinases (ADAMTS13). Genetic linkage studies in 4 pedigrees with congenital TTP,12 sometimes referred to as Upshaw-Schulman syndrome,13,14 led to identification of 12 different mutations in the gene for ADAMTS13 that accounted for 14 of the 15 disease alleles studied.12 Clinically affected patients with constitutional TTP were homozygous or doubly heterozygous for ADAMTS13 mutations and had protease activity levels less than 10% of normal values.12
Nevertheless, the specificity of ADAMTS13 deficiency for the thrombotic microangiopathy commonly labeled TTP has been strongly challenged.15-17 Moore et al15 found deficient protease activity in only 9 of 20 patients with TTP but also in 6 of 20 with idiopathic thrombocytopenic purpura (ITP), 6 of 10 with disseminated intravascular coagulation (DIC), 5 of 10 with systemic lupus erythematosus, 1 of 5 with leukemia, and even 2 of 20 healthy controls. Furthermore, partial deficiency of ADAMTS13 (36% ± 24%) was reported in 14 patients with DIC.16 Mannucci et al17 found decreased ADAMTS13 activity in newborns; during the second and third trimesters of pregnancy; in patients with liver cirrhosis, uremia, or acute inflammatory disorders; and during the postoperative period. They concluded that a low ADAMTS13 level was not a specific beacon of TTP.
In this study, we investigated ADAMTS13 activity in patients with thrombocytopenia due to different causes in order to clarify the specificity of decreased ADAMTS13 activity for TTP.
Study design
Patients
We recruited 68 patients with thrombocytopenia (platelet count, <140 × 109/L), including 17 patients from a previous study18 with severe sepsis or septic shock with or without DIC; 16 patients with heparin-induced thrombocytopenia type 2 (HIT) studied between 1995 and 2001 (all with a high clinical probability of HIT and the diagnosis confirmed by a high titer of antiheparin–platelet factor 4 antibodies; L. Alberio, et al, manuscript submitted); and 35 patients, prospectively enrolled from July 2001 to December 2001, with thrombocytopenic states with the following causes: ITP (n = 10), idiopathic osteomyelofibrosis (n = 3), myelodysplastic syndrome (MDS; n = 4), acute leukemia (n = 6), severe aplastic anemia (n = 2), and miscellaneous (n = 10) (Table 1). In none of the 68 patients with thrombocytopenia had TTP or HUS been considered as an alternative diagnostic possibility. Platelet count, hemoglobin level, and leukocyte count were determined for each patient. VWF-cleaving protease activity was measured in citrated plasma samples stored at −20°C until assay. The study was approved by the responsible ethical committee (Kantonale Ethische Kommission, Bern, Switzerland).
ADAMTS13 activity
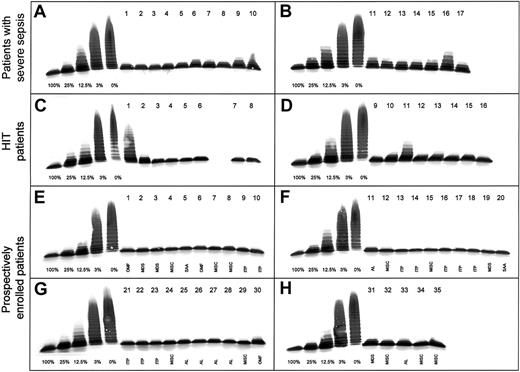
We determined the activity of ADAMTS13 by using a previously described immunoblotting test.1,2 Briefly, plasma samples diluted (1:20) in 0.01 M Tris and 0.15 M sodium chloride (pH 7.4) containing 1 mM Pefabloc SC (Boehringer Mannheim, Germany) were incubated with 10 mM barium chloride at 37°C for 5 minutes and then added to purified protease-free VWF substrate. The reaction mixture was dialyzed on the surface of a hydrophilic filter membrane for 16 to 20 hours at 37°C against a buffer containing 1.5 M urea and 5 mM Tris (pH 8.0). The reaction was stopped by adding EDTA, and the extent of VWF degradation was analyzed by multimer analysis on 1.4% sodium dodecyl sulfate–agarose gels and immunoblotting using peroxidase-conjugated rabbit antihuman VWF antibodies.1 2Dilutions of a pool of normal human plasma (NHP) from 42 healthy male donors were used for calibration of the protease assay in plasma samples from patients. This technique allows accurate determination in the range below 25% of normal activity, permitting discrimination of activity levels of 3% of normal plasma from those below 1% (Figure1). All plasma samples from patients were tested at least twice, and samples with protease activity of 30% or less were reassayed several times on different days. Each patient's pattern of VWF degradation was compared with the standard curve (Figure 1).
Activity of VWF-cleaving protease (ADAMTS13) in 68 patients with thrombocytopenia.
Multimer analysis of VWF substrate digested by diluted (1:20) plasma samples from patients. On each gel (A-H), a calibration curve using dilutions of pooled plasma from healthy donors (1:20 dilution corresponding to 100%) is included. Seventeen patients with severe sepsis (gels A,B), 16 with HIT (gels C,D) and 35 with thrombocytopenia due to various causes (gels E-H) were analyzed. Plasma samples were applied on top of the gel. OMF indicates osteomyelofibrosis; AL, acute leukemia; SAA, severe aplastic anemia; and MISC, miscellaneous.
Activity of VWF-cleaving protease (ADAMTS13) in 68 patients with thrombocytopenia.
Multimer analysis of VWF substrate digested by diluted (1:20) plasma samples from patients. On each gel (A-H), a calibration curve using dilutions of pooled plasma from healthy donors (1:20 dilution corresponding to 100%) is included. Seventeen patients with severe sepsis (gels A,B), 16 with HIT (gels C,D) and 35 with thrombocytopenia due to various causes (gels E-H) were analyzed. Plasma samples were applied on top of the gel. OMF indicates osteomyelofibrosis; AL, acute leukemia; SAA, severe aplastic anemia; and MISC, miscellaneous.
Results and discussion
Among the 68 patients with thrombocytopenia, 12 (18%) had ADAMTS13 activity of 30% or less, the lowest value being 10% (Figure1, gel C, no. 1). Patients with thrombocytopenia associated with severe sepsis or septic shock, often accompanied by DIC (Figure 1, gels A and B), had a median ADAMTS13 activity level of 40% (range, 15%-80%) and 6 of 17 had levels between 15% and 30%. This is in agreement with findings by Loof et al,16 who reported ADAMTS13 activity of 36% ± 24% in 14 patients with DIC.
The 16 patients with confirmed HIT (Figure 1, gels C and D) had a median ADAMTS13 activity level of 70% (range, 10%-100%). Among the 35 prospectively enrolled patients with thrombocytopenia due to various hematologic or other conditions (Figure 1, gels E-H), one patient with MDS had a 25% level of ADAMTS13 activity (Figure 1, gel H, no. 31), whereas all others had levels of 40% or higher (Table 1). All plasma samples with an activity level of 30% or less were reanalyzed at least once on different days; essentially concordant results were obtained (Table 1), thus confirming reproducibility of the immunoblotting assay.
The ADAMTS13 values in this series of patients with thrombocytopenia contrast sharply with those obtained in our earlier multicenter study4 of patients with TTP or HUS: 20 of 24 patients with acute nonfamilial TTP had ADAMTS13 activity below 5% of normal levels, due to a circulating inhibitor detected in 20 of the 24 patients. All 6 patients with constitutional TTP had ADAMTS13 activity levels below 5% as well, and no inhibitor was detected in their plasma.
By December 2001, we had identified more than 120 patients with ADAMTS13 activity levels below 5% in our laboratory. According to the (sometimes incomplete) clinical information available to us, all had clinical and laboratory findings compatible with TTP except 2 apparently asymptomatic siblings of patients with hereditary TTP14 and one child with a diagnosis of Escherichia coli–associated HUS who had a transient acquired severe deficiency of ADAMTS13 activity.19
Therefore, because of the results of the current study in thrombocytopenic patients—all with ADAMTS13 levels of at least 10% that in NHP—as well as the previous analyses of 120 healthy subjects4 and 74 hospitalized or healthy controls5—all with ADAMTS13 levels of at least 45% of NHP—we conclude that a severe ADAMTS13 deficiency (< 5% of the activity in NHP) is a specific finding for a form of thrombotic microangiopathy most often diagnosed as TTP. Our data contradict the findings of Moore et al,15 who reported severe ADAMTS13 deficiency not only in some patients with TTP but also in several patients with thrombocytopenia due to other conditions. Nevertheless, it is also evident from our study that slightly decreased (25%-50%) or moderately decreased ADAMTS13 activity (10%-25%) is rather common in thrombocytopenic patients with severe sepsis or HIT, a finding that is compatible with the observation of mild ADAMTS13 deficiency in various inflammatory disease states17 and in patients with metastasizing neoplasia20 or neoplasia-associated thrombotic microangiopathy.21
Even though very low ADAMTS13 activity (< 5% of the activity in NHP) is a specific feature of the clinical condition labeled TTP, the sensitivity of this laboratory finding for the diagnosis of TTP remains questionable. Although, in retrospective studies, Tsai and Lian5 observed severe ADAMTS13 deficiency in 37 of 37 patients and Furlan et al4 in 26 of 30 patients with a diagnosis of acute TTP, the prospective study by Veyradier et al7 found a severe deficiency in only 47 of 66 patients presenting with idiopathic or secondary TTP (sensitivity, 71%). Therefore, besides severe acquired or hereditary ADAMTS13 deficiency, other pathogenetically relevant factors may cause a thrombotic microangiopathy clinically indistinguishable from TTP.
Further prospective studies are needed to delineate whether specific clinical or laboratory features (including response to therapy) in patients with thrombotic microangiopathy with severe ADAMTS13 deficiency are different from those in patients without such a deficiency. If such differences are observed, a new classification scheme for thrombotic microangiopathies might be justified, with severe acquired ADAMTS13 deficiency and severe constitutional ADAMTS13 deficiency categorized as 2 distinct entities.14
Finally, it is important to distinguish ADAMTS13 activity levels of 10% or higher from those under 5%. Patients with chronic recurring TTP caused by severe hereditary ADAMTS13 deficiency are kept in remission by regular plasma infusions (every 2 to 3 weeks) that raise ADAMTS13 activity to just above 10% to 15%, and 5% of activity may be sufficient to degrade the unusually large VWF multimers and prevent intravascular platelet clumping.14 22
We thank Drs W. A. Wuillemin, S. Zeerleder, and C. Caliezi for providing plasma samples from patients with severe sepsis or septic shock.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2002-02-0344.
Supported in part by a grant from the Fondazione per la ricerca sulla trasfusione e sui trapianti, Lugano, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bernhard Lämmle, Central Hematology Laboratory, University Hospital, Inselspital, Freiburgstrasse, CH-3010, Bern, Switzerland; e-mail: bernhard.laemmle@insel.ch.