Abstract
Heterozygous mutations in neutrophil elastase have been detected in many sporadic cases of congenital neutropenia. However, a convincing pathogenetic mechanism has not been established, and it is unclear whether the effects of the mutant enzyme occur within the cell of production or are paracrine in nature. The healthy father of a patient was demonstrated to be mosaic for his daughter's Cys42Arg elastase mutation. Using semiquantitative polymerase chain reaction, approximately half of his T cells were shown to carry the mutation in contrast to less than 10% of neutrophils. Individual hematopoietic colonies grown from peripheral blood were heterozygous for the mutation or were homozygous wild type. These results demonstrate that precursors containing the mutation are selectively lost during myelopoiesis or fail to develop into neutrophils. This is the first in vivo confirmation of the pathogenic nature of elastase mutations in humans. The normal neutrophil count in the father suggests that the mutant elastase does not have paracrine effects.
Introduction
Severe congenital neutropenia (SCN) is manifest by persistent severe neutropenia, recurrent bacterial infection, and maturation arrest in the bone marrow at the promyelocyte–myelocyte stage.1 Until recently, the pathogenesis of SCN was unknown, but genetic studies have now suggested a causal role for heterozygous mutations in the ELA2 gene encoding neutrophil elastase in approximately three fourths of sporadic cases.2,3 However, a convincing pathogenetic mechanism has not yet been established; neutrophil elastase knock-out mice are not neutropenic,4,5 and preliminary knock-in experiments have failed to produce an SCN phenotype.6 Recently, doubts have been raised as to whether the mutations described are actually sufficient to cause the phenotype of SCN.7
We present here evidence of mosaicism for an ELA2 mutation in the hematologically healthy father of a child with SCN, strongly supporting the pathogenic nature of this mutation in myelopoiesis.
Study design
Case history
The patient had typical SCN diagnosed in infancy. Current white blood cell (WBC) count at 4 years of age is 3.52 × 109/L (neutrophils, 0.04 × 109/L; monocytes, 0.63 × 109/L). Cytogenetic analysis results have always been normal. She had no numerical or clinical response to granulocyte colony stimulating factor (G-CSF), even to doses greater than 100 μg/kg per day, or to the combination of prednisolone and G-CSF. Her parents were unrelated and of northern European origin and had normal full blood counts and white cell differentials (father: WBC, 7.03 × 109/L; neutrophils, 4.18 × 109/L; monocytes, 0.61 × 109/L). Neither had a history of recurrent infections.
Mutational analysis
The ELA2 gene was amplified by polymerase chain reaction (PCR) and sequenced as described previously.3 To confirm the presence of the mutation, a fragment covering nucleotides 1768 to 1959 (GenBank accession number Y00477) was amplified using a 3′ mismatch primer that introduced a SacII restriction endonuclease (RE) digestion site in mutant alleles only. Semiquantitative PCR was performed with a γ–32P–end-labeled forward primer and 25 cycles of amplification on DNA from cells purified from peripheral blood by density gradient centrifugation and isolation with the MiniMACS magnetic bead system (Miltenyi Biotec, Bisley, United Kingdom).SacII-digested products were electrophoresed through 6% denaturing polyacrylamide gels, and the bands were quantified by densitometry.
Peripheral blood colony assays
Fresh mononuclear cells (50 × 103/mL) were cultured in semisolid media (Methocult H4230; Stem Cell Technologies, Vancouver, BC, Canada) supplemented with G-CSF (25 ng/mL), granulocyte macrophage-CSF (25 ng/mL), interleukin-3 (30 ng/mL), stem cell factor (10 ng/mL), and erythropoietin (3 U/mL). Individual colonies were plucked at day 12 of culture, lysed with proteinase K (1.2 μg in 20 μL detergent lysis buffer) for 60 minutes at 55°C and then were analyzed by PCR and RE digestion as before.
Results and discussion
Mutational analysis
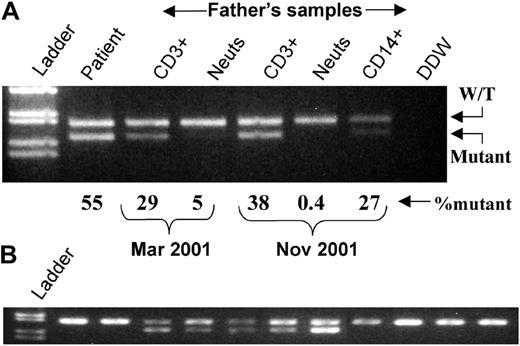
Sequencing of the ELA2 gene of the patient identified a 1929T>C mutation that would lead to a Cys42Arg substitution. PCR and RE digestion confirmed that she was heterozygous for the mutation and had approximately 50% mutant DNA in her peripheral blood (Figure1A). Her mother and 55 healthy controls had only wild-type alleles. However, preliminary analysis of the total white cells from her father detected a low level of the mutant. Semiquantitative PCR on DNA from a fresh peripheral blood sample in March demonstrated that his T cells had 28.5% ± 6.1% (mean ± SD of 4 analyses) mutant alleles, whereas his neutrophils had 4.6 ± 1.2% (n = 4) mutant alleles. These results did not change significantly with time. In November his CD3+ cells had 38.4% ± 0.9% (n = 2), neutrophils had 0.4% ± 0.7% (n = 3), and CD14+ cells had 27.3% ± 3.4% (n = 2) mutant alleles. The patient had 54.5% ± 2.3% (n = 7) mutant alleles (Figure 1A). These results suggested that the father was a somatic mosaic for his daughter's neutrophil elastase mutation and had selective loss of myeloid cells expressing the mutation.
Mismatch PCR and restriction enzyme analysis.
(A) DNA prepared from total white blood cells from the patient and purified cellular fractions from the father. Percentages of mutant DNA are derived from radioactive PCR analysis. Cell purity (November only): CD3+, greater than 98%, CD14+, 85% (by flow cytometry); neutrophils, greater than 97% (400 cell differential). (B) Representative individual erythroid colonies grown in vitro from the father's blood mononuclear cells. Neuts indicates neutrophils; W/T, wild-type; DDW, double deionized water.
Mismatch PCR and restriction enzyme analysis.
(A) DNA prepared from total white blood cells from the patient and purified cellular fractions from the father. Percentages of mutant DNA are derived from radioactive PCR analysis. Cell purity (November only): CD3+, greater than 98%, CD14+, 85% (by flow cytometry); neutrophils, greater than 97% (400 cell differential). (B) Representative individual erythroid colonies grown in vitro from the father's blood mononuclear cells. Neuts indicates neutrophils; W/T, wild-type; DDW, double deionized water.
Proof of mosaicism
To prove the probable mosaicism, 2 PCR fragments were subcloned from DNA from the father's CD3+ cells and neutrophils (March samples). One fragment covered the 1929T>C mutation identified in the affected child. The other fragment covered the previously reported 4890C>A (Ser173Ser) silent polymorphism8 9 for which the father was heterozygous but the daughter homozygous C, thus acting as an internal control. Attempts to use long-range PCR to amplify both sites within one fragment were unsuccessful, possibly because of the high GC content within that 3365-bp fragment. Analysis of individual clones showed that the C and A alleles at the polymorphic site were equally distributed in the clones from CD3+ cells and neutrophil DNA, as would be expected for a polymorphism (Table 1). However, only one fourth of clones from CD3+ cells had the mutant C1929 allele.
Analysis of individual subclones derived from paternal peripheral blood cell DNA and in vitro colonies for the presence of the 1929T>C mutation and 4890C>A polymorphism
| . | 1929T>C mutation . | 4890C>A polymorphism . | ||
|---|---|---|---|---|
| C1929 clones (colonies)/total analyzed . | A4890 clones/total clones analyzed . | |||
| No. . | % . | No. . | % . | |
| Peripheral blood cells | ||||
| CD3+ | 13 of 54 | 24* | 27 of 53 | 48 |
| Neutrophils | 6 of 84 | 7* | 16 of 36 | 44 |
| In vitro colonies | ||||
| BFU-E | 33 of 58 | 57 | — | — |
| CFU-GM | 14 of 34 | 41 | — | — |
| . | 1929T>C mutation . | 4890C>A polymorphism . | ||
|---|---|---|---|---|
| C1929 clones (colonies)/total analyzed . | A4890 clones/total clones analyzed . | |||
| No. . | % . | No. . | % . | |
| Peripheral blood cells | ||||
| CD3+ | 13 of 54 | 24* | 27 of 53 | 48 |
| Neutrophils | 6 of 84 | 7* | 16 of 36 | 44 |
| In vitro colonies | ||||
| BFU-E | 33 of 58 | 57 | — | — |
| CFU-GM | 14 of 34 | 41 | — | — |
Difference in the percentage of mutant clones derived from CD3+ and neutrophil DNA is statistically significant (P < .001).
Expression of neutrophil elastase is exclusively limited to cells of the myeloid lineage and thus would not exert a functional selection pressure on T cells.10 11 These results indicated that one half of the father's nonmyeloid cells contained the mutation; therefore, the mutation arose at the first mitotic division after fertilization on his allele with C at nucleotide 4890. Furthermore, the low proportion of C1929 clones from neutrophils (approximately 7%) suggested that cells expressing the elastase mutation had been selectively lost at an earlier stage in myelopoiesis.
Further proof of mosaicism was obtained from analysis of individual colonies cultured in vitro from the father's peripheral blood mononuclear cells. Normal numbers of colonies were obtained—608 erythroid burst-forming units (BFU-E) and 231 granulocyte macrophage colony-forming units (CFU-GM) per milliliter peripheral blood (median ± SD for 20 healthy controls analyzed on 4 separate occasions = 356 ± 280 and 95 ± 127/mL, respectively). PCR analysis confirmed the presence of wild-type and heterozygous colonies in both lineages (Table 1, Figure 1B). This apparently normal myeloid growth (by colony count) is consistent with growth factor–supplemented in vitro studies previously reported in SCN patients12,13and may be explained by the increased proportion of macrophages in colonies from such patients. Cells of the monocyte-macrophage lineage express much lower levels (approximately 6% that of neutrophils)14 of neutrophil elastase and thus would be less susceptible to any potential toxic effects of the mutant enzyme.
Recent studies have demonstrated that mosaicism is a relatively common event in several genetic disorders.15-17 For example, in a recent study of hemophilia A, evidence of mosaicism was found in 8 of 61 (13%) of families studied.15 In the case presented here, a neutrophil elastase mutation acquired in the father at the embryonic 2-cell stage had been passed on to his daughter, who was heterozygous for the mutation and had classical SCN. The phenotype of the child was unusually severe in that she failed to respond to G-CSF therapy. Mutations in the extracellular domain of the G-CSF receptor have been reported in 2 SCN patients who similarly had no response to G-CSF,18 19 but no evidence for such a mutation was found in the present case (data not shown). The hematologically normal phenotype of the father could be explained by the almost complete absence of mutation-expressing neutrophils in his blood, presumably because neutrophils never developed from stem cells expressing the ELA2 mutant or were destroyed before entering the circulation. This result not only provides the first in vivo confirmation of the pathogenic role of mutant neutrophil elastase in humans, it also argues against a paracrine mechanism for the detrimental activity of the mutant enzyme.
Prepublished online as Blood First Edition Paper, May 24, 2002; DOI 10.1182/blood-2002-01-0060.
Supported by the Roald Dahl Foundation (P.J.A.), and Amgen Ltd (unrestricted educational grant) (P.J.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Phil Ancliff, Department of Haematology, University College London, 98 Chenies Mews, London, WC1E 6HX, United Kingdom; e-mail: p.ancliff@ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal