Onida et al recently reported on prognostic parameters in chronic myelomonocytic leukemia (CMML) and proposed a new M. D. Anderson prognostic score (MDAPS).1 In a large independent data- base, they essentially confirmed the clinical, laboratory, cytogenetic, and prognostic findings that have been described by other groups.2-8 What is new in their paper is the finding that lymphocyte count was a variable independently associated with survival in CMML. Lymphocyte count was therefore included in the new scoring system. But the authors felt that their finding warrants confirmation, and we think that we can provide some evaluation. The large myelodysplastic syndrome (MDS) registry in Düsseldorf, which allowed us to examine prognostic and nosologic problems of CMML in the past,5,6 8 now includes 265 patients with CMML, among 2050 patients with MDS. The prognostic parameters used in the MDAPS are completely available for 212 of our CMML patients, who did not receive intensive chemotherapy or allogeneic stem cell transplantation. Onida et al studied 190 patients.
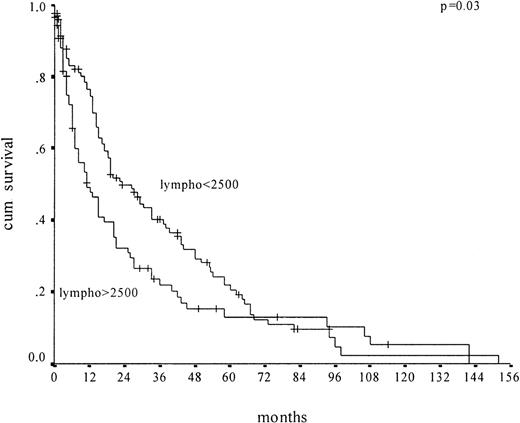
We first performed univariate and multivariate analysis. Median survival was 18 months. On univariate analysis, sex, splenomegaly, prior malignancy, hemoglobin level, platelet count, monocyte count, lactate dehydrogenase (LDH) level, medullary blast count, proportion of medullary erythroblasts, and peripheral blood lymphocyte count (Figure 1), as well as karyotype, were identified as prognostic parameters. The presence of immature myeloid precursors, identified as a prognostic marker by Onida et al, had no impact on prognosis in our analysis. On multivariate analysis, we identified as independent prognostic factors for survival: elevated LDH level (not included in the MDAPS), medullary blast count above 10% (included), male gender (not included), hemoglobin level below 12 g/dL (included), and lymphocyte count above 2500/μL (included).
Kaplan-Meier survival curves of CMML patients (n = 227) according to absolute lymphocyte count in peripheral blood.
Plots depict lymphocyte counts above 2500/μL and no more than 2500/μL.
Kaplan-Meier survival curves of CMML patients (n = 227) according to absolute lymphocyte count in peripheral blood.
Plots depict lymphocyte counts above 2500/μL and no more than 2500/μL.
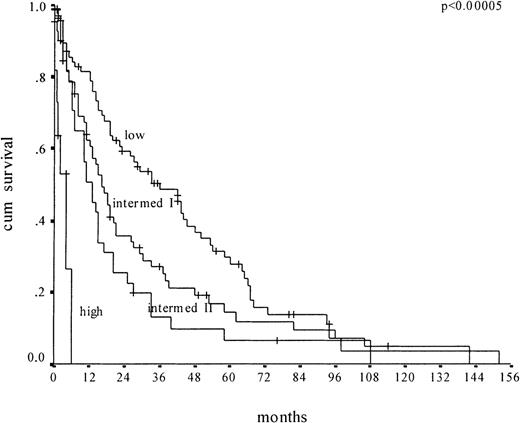
We then applied the MDAPS to our cohort of 212 CMML patients for whom all MDAPS parameters were available (Figure2). We confirmed that the MDAPS separates 4 risk groups. But there are disadvantages to the new score. First, the low-risk group, which includes 39% of the patients, has a median survival of only 36 months; it therefore does not really identify the subgroup of CMML patients who have a very good prognosis. Second, there is no significant difference in survival between the intermediate groups I and II (16 versus 13 months, respectively). Third, only 6% of the patients were classified as high risk.
Kaplan-Meier survival curves of CMML patients (n = 212) according to the MDAPS.
Plots depict low-risk, intermediate-risk 1, intermediate-risk 2, and high-risk groups.
Kaplan-Meier survival curves of CMML patients (n = 212) according to the MDAPS.
Plots depict low-risk, intermediate-risk 1, intermediate-risk 2, and high-risk groups.
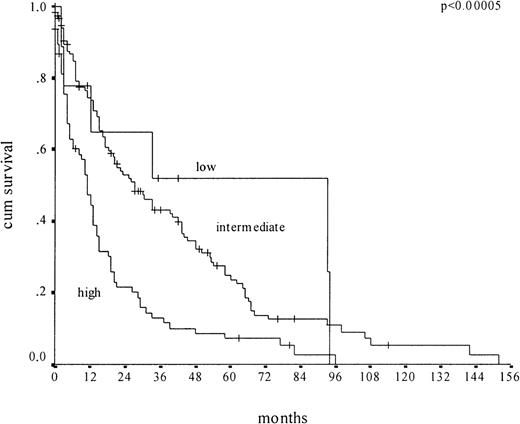
For comparison, we applied the Düsseldorf score, featuring 3 risk groups (high, intermediate, low), to the same cohort of 212 CMML patients (Figure 3). The low-risk group was much more exclusive. Comprising 6% of patients, it showed a median survival of 93 months. The high-risk group included 36% of patients and showed a median survival of 11 months. Twenty percent of low-risk patients according to the MDAPS were (appropriately) classified as high-risk patients by the Düsseldorf score. It appears that the MDAPS does not represent a major improvement in predicting the survival of CMML patients.
Kaplan-Meier survival curves of CMML patients (n = 212) according to the Düsseldorf score.
Plots depict low-risk, intermediate-risk, and high-risk groups.
Kaplan-Meier survival curves of CMML patients (n = 212) according to the Düsseldorf score.
Plots depict low-risk, intermediate-risk, and high-risk groups.
We further examined the prognostic value of lymphocyte counts in other MDS patients. In the entire group of 1082 patients with MDS (excluding CMML), the lymphocyte count could not be identified as an independent prognostic marker. Therefore, it appears that the prognostic value of lymphocyte counts is restricted to CMML patients.
Value of peripheral blood lymphocytes and MDAPS in CMML
The analysis performed by Dr Germing and colleagues in the Düsseldorf population of patients with chronic myelomonocytic leukemia (CMML) is important and appreciated, since it is the first evaluation of our findings1-1 in a population other than the one from which we derived our scoring system (MDAPS). We are pleased with the confirmation by Germing et al that survival time is independently associated with 3 of the 4 factors identified by our analysis and particularly that the absolute lymphocyte count in peripheral blood—a factor identified for the first time by our recent report—indeed has prognostic value. Like Germing and colleagues, we found no relationship between lymphocyte counts and survival time among patients diagnosed with myelodysplastic syndromes (MDSs) other than CMML. This suggests that lymphocytes may be specifically relevant to the biology of CMML.
It should be stressed that the populations of patients with CMML in the Düsseldorf and M. D. Anderson Cancer Center cohorts differ considerably. The median survival times were 18 months for patients in the Düsseldorf cohort but only 12 months for patients at our center, suggesting more advanced disease in our cohort. Furthermore, prognostic systems may account for as little as 25% of variability in patient outcome.1-2 Under these circumstances, discrepancies between the 2 systems in stratifying patients according to prognosis are not surprising.
Lack of an association between the presence of circulating immature myeloid cells (IMCs) and shorter survival times for the Düsseldorf cohort may reflect earlier stages of disease among this cohort than among the M. D. Anderson cohort; indeed, increasing percentages of circulating IMCs (including blasts) are commonly observed with disease progression. Inverse correlation between the presence of circulating IMCs and survival time was also reported by Fenaux et al1-3 (107 patients) and by del Cañizo et al1-4 (70 patients) in reports of the 2 other largest series of patients with CMML published to date. In our study, elevated serum LDH level was associated with shorter survival in univariate but not in multivariate analysis.
The Düsseldorf scoring system1-5 and the MDAPS were both derived from analyses of still relatively limited cohorts of patients, and both systems likely have advantages and disadvantages for risk stratification. However, among the current Düsseldorf cohort of 212 patients with CMML, only a small proportion (6%) was identified as low risk using the Düsseldorf scoring system, and the longer median survival time among these patients is a projection based on only 13 patients. Indeed, the survival curves provided by Germing et al show that more than 45% of the patients in their low-risk category were dead at 36 months. In contrast, the MDAPS identified 39% of the patients in the Düsseldorf cohort as low risk, with a median survival time of 36 months. Therefore, compared with the Düsseldorf scoring system, the MDAPS appears to be able to identify a larger group of patients at low risk and hence not in urgent need of experimental therapy.
Using the MDAPS, the group of patients identified as high risk within the Düsseldorf cohort (6%) is smaller than our high-risk group of patients (currently 10% of 276 assessable patients). However, the median survival time for the high-risk group is less than 6 months for both cohorts, and we believe this finding may be clinically significant for identification of patients who could benefit from new treatments based on investigational drugs.
The value of the MDAPS owes mainly to the fact that it was derived from a population composed exclusively of patients with CMML. However, considering the wide heterogeneity of the disease, both the MDAPS and the Düsseldorf scoring systems are useful and may serve as a basis for further improvements in the prognostic stratification of patients.