Abstract
It has previously been shown that sera from multiparous women have increased levels of anti-idiotypic antibodies specific for anti-HLA molecules. γ-Globulins prepared from these sera may be superior to commercial preparations of intravenous γ-globulin (IVIg) for inhibiting HLA alloimmunization. To test this, F(ab′)2fragments prepared from either commercial IVIg or from the sera of men or multiparous women were coupled to CNBr-Sepharose and tested for their ability to bind F(ab′)2 fragments derived from polyspecific anti-HLA sera. As determined by flow cytometry, compared with columns coated with F(ab′)2 derived from commercial IVIg or sera from men, columns coated with F(ab′)2 prepared from the sera of multiparous women bound significantly more anti-HLA. In addition, intact IgG molecules prepared from the sera of multiparous women significantly neutralized the reactivity of the anti-HLA F(ab′)2 fragments. To determine whether the intact IgG molecules or their corresponding F(ab′)2 fragments could affect in vivo alloimmunity, they were tested for their ability to inhibit an established IgG human alloimmune response in humanized severe combined immunodeficient (SCID) mice. Compared with commercial IVIg, when intact IgG or F(ab′)2 fragments derived from multiparous women were administered to SCID mice making human anti-HLA antibodies, a significant reduction in anti-HLA reactivity was observed. The findings suggest that IgG molecules prepared from the sera of multiparous women have increased anti-idiotypic reactivity against anti-HLA antibodies, which can significantly inhibit an established human IgG alloimmune response in an Fc-independent manner.
Introduction
Intravenous γ-globulin (IVIg) is widely used to treat patients with immunoregulatory disorders, particularly chronic autoimmune thrombocytopenic purpura (AITP).1-4 Although IVIg therapy has been shown to have a benefit in raising platelet counts in autoimmune platelet disorders, it appears to be not efficacious in patients with platelet-induced HLA alloimmunization.5-8 The reasons for this are unclear but may relate to the nature of the immune response and the mechanisms of action of IVIg, that is, Fc receptor blockade/inhibition4,9 or anti-idiotypic regulation.10-12
One of the mechanisms by which the peripheral antibody repertoire is regulated is via the production of antibodies reactive with the variable regions of other antibodies, that is, anti-idiotypes.13-15 It has also been shown that the production and reactivity of anti-HLA antibodies are under the regulation of anti-idiotypic antibodies.16-18 Perhaps the most striking example of natural in vivo anti-idiotypic regulation of alloimmunization to HLA is that associated with pregnancy, both at the level of the fetus and of the mother. Phelan et al19elegantly demonstrated that normal individuals contain anti-idiotypic antibodies to anti-HLA molecules exquisitely specific for the HLA antigens encoded from the noninherited maternal allele (NIMA), but not paternal HLA alleles. Additionally, anti-HLA reactivity may be transient in patients with malignancy,20 and it has been shown that in these patients, as in pregnant women, anti-idiotypic antibodies may actively down-regulate anti-HLA antibodies.21-24
We speculated that pooling sera from multiparous women might increase the content of anti–HLA-specific anti-idiotypes to produce a more effective IVIg product for alloimmunized patients. We tested this by using affinity chromatography techniques combined with a humanized severe combined immunodeficiency (SCID) mouse model of alloimmunization. Our results indicate that, compared with commercial IVIg, IgG prepared from sera of multiparous women has higher anti-idiotypic binding capacity for anti-HLA and is superior to commercial IVIg or IgG prepared from men in inhibiting a secondary human alloimmune response.
Materials and methods
Sources and preparation of IgG
Three sources of IgG preparations were examined for anti–HLA-specific anti-idiotypic antibodies: (1) a commercial IVIg preparation (Immune Globulin Intravenous, 5%, Bayer, Etobicoke, ON, Canada), (2) the pooled sera of 34 never-transfused male volunteers (age range, 25-58 years), or (3) pooled sera from 47 multiparous women (age range, 27-47 years) who had their last pregnancy at least 1 year before blood sampling. Before pooling, anti-HLA reactivity in each serum was tested in a microlymphocytotoxicity (LCT) assay using a 30-cell panel of HLA typing cells (Canadian Blood Services, Toronto Center, Toronto, ON, Canada) and all were negative for anti-HLA antibodies. Equal amounts of serum from each individual were pooled. As a source of IgG anti-HLA antibodies, high-titered polyspecific anti-HLA human sera were obtained from the Canadian Blood Services (Dr B. Hannach, Toronto Center). For all 3 sources, IgG molecules were prepared by precipitation with 50% saturated ammonium sulfate followed by dialysis against 50 mM Tris-saline, pH 8.0. The IgG molecules were further purified by adsorption on QAE-Sephadex A-50 (Pharmacia, Mississauga, ON) to remove contaminating albumin.25
F(ab′)2 preparation
The F(ab′)2 fragments of the IgG molecules were prepared by standard methods to differentiate between idiotypic and Fc-mediated effects.25 Briefly, the IgG molecules (1%-3% wt/vol) were dialyzed against 0.2 M sodium acetate, pH 4.5, and digested with 2% (wt/wt) pepsin (Sigma, St Louis, MO) for 24 hours at 37°C. The F(ab′)2 fragments were then purified by Sephadex G150 (Pharmacia) gel filtration and protein G-Sepharose (Pharmacia) adsorption. Purity of the F(ab′)2 fragments was determined by high-performance liquid chromatography (HPLC) analysis using a Beckman Gold HPLC with an Altex TSK-3000 size exclusion column (1.5 × 30 cm) equilibrated in 0.05 M NaPO4/0.1 M NaSO4, pH 6.75. The final F(ab′)2 purity was typically more than 96%.
Anti-HLA neutralization assay
The 10-fold serial titrations (from 10−5 M) of intact IgG molecules from (1) commercial IVIg, (2) men, or (3) multiparous women were mixed with an equal volume (100 μL) of anti-HLA F(ab′)2 fragments (10−5 M final concentration) and incubated at 4°C for 18 hours. The mixtures were gently resuspended and 100 μL of protein A–conjugated Sepharose beads (Pharmacia) added for 45 minutes at 20°C to remove intact IgG. The beads were then centrifuged for 2 minutes at 800g, proteins in the supernatants quantified, and residual anti-HLA reactivity determined by flow cytometry.
Affinity chromatography
To study anti-idiotypic interactions, F(ab′)2fragments derived from either commercial IVIg, the sera of men, or multiparous women were covalently coupled to CNBr Sepharose beads (Pharmacia) and 3.0-mL beads containing 60 mg coupled protein was poured into 1 × 8-cm glass chromatography columns (Pharmacia). Each column was extensively characterized with respect to baseline protein interactions. None of the columns could retain reactivity of a 50-μg load of a goat antihuman Fc-specific polyclonal antibody but did retain the reactivity from a 50-μg load of goat antihuman H+L chain–specific antibody. Additionally, none of the columns retained a 1-mg load of human albumin. For anti-idiotypic binding studies, all the columns were subjected to the same chromatographic protocol. One milligram of anti-HLA F(ab′)2 fragments (in 1 mL) was loaded onto the columns and allowed to continuously circulate at 0.5 mL/min for 18 hours at 4°C using a pump-driven closed loop system.10 Columns were then washed with 20 column volumes of running buffer and the unbound protein collected. The columns were subsequently washed with 20 column volumes of 0.2 M glycine, pH 2.8, to elute bound proteins, which were then neutralized by adding 2 M Tris base (final concentration, 2.4% vol/vol). The eluted F(ab′)2 fragments were concentrated by membrane filtration. Both loaded and eluted proteins were made to the same concentration and anti-HLA reactivity was determined by flow cytometry.
SCID mouse model of human alloimmunization
Female CB.17 SCID mice (6-8 weeks of age) were obtained from Jackson Laboratories (Bar Harbor, ME) and human alloimmunization was induced as previously described.26 Briefly, human peripheral blood mononuclear cells (PBMCs) were obtained by 1.077 g/mL Percoll fractionation from a female blood donor with a history of prior pregnancy and low levels of circulating anti-HLA class I alloantibodies; she was blood group B+, HLA A24/A34, B51/B62, Cw4, Bw4, Bw6 and had low but stable levels of circulating anti-HLA-B7 alloantibodies. The SCID mice were first engrafted with 1 × 107 of the donor's PBMCs and then challenged twice weekly for 4 weeks with 107 irradiated (2500 cGy) HLA A2/A2, Cw7+ PBMCs from healthy laboratory volunteers. Anti-HLA antibody development was monitored weekly by flow cytometry. At the fourth week, if mice developed anti-HLA, they were randomized to receive 1 g/kg intraperitoneally of either intact IgG or F(ab′)2 fragments derived from commercial IVIg or from sera from men or from multiparous women, twice weekly for 4 weeks. These IgG dosages are similar to those used for human patients with AITP.3 Serum anti-HLA reactivity after administration was compared with preadministration anti-HLA levels (week 4 of challenge).
Flow cytometry
For detection and characterization of anti-HLA, serial dilutions of the indicated test samples were incubated with 105 PBMCs from HLA-typed individuals for 45 minutes at 20°C and washed once. Fluorescein isothiocyanate (FITC)-conjugated goat antihuman IgG (H+L chain– or Fc-specific, Cedarlane Laboratories, Hornby, ON) was then added to the cells for 30 minutes at 20°C in the dark. Cells were analyzed by flow cytometry as previously described24 using a FACSort flow cytometer (Becton Dickinson, San Jose, CA) equipped with an argon laser operating at 15 mW; 10 000 events were acquired through an electronic cellular gate set on lymphocytes based on forward and side scatter and were analyzed using LYSYS II software (Becton Dickinson).
Statistical analysis
Significance between means ± SD of the flow cytometric data was determined by Student unpaired t test for analysis of means.
Results
IgG derived from sera of multiparous women contains increased anti–HLA-specific anti-idiotypes
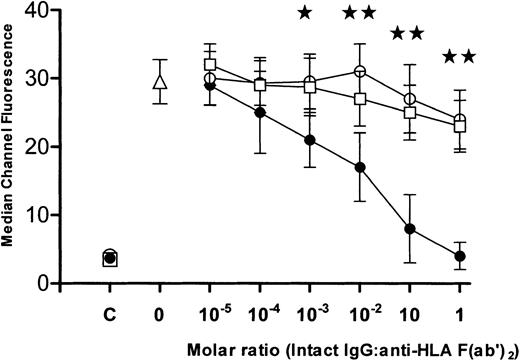
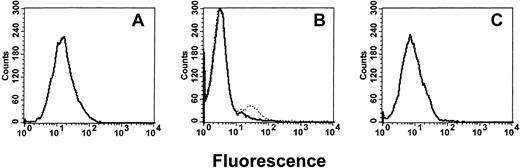
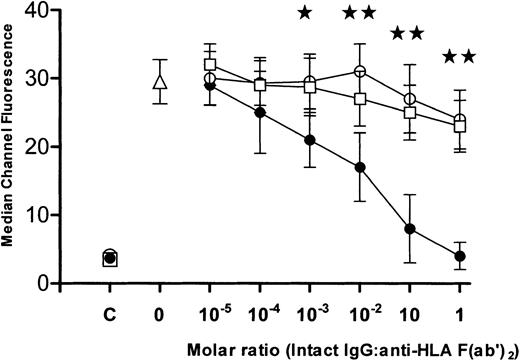
The intact IgG preparations were tested for their ability to neutralize the binding of anti-HLA antibodies. When titrations of intact IgG molecules derived from (1) commercial IVIg or from sera from (2) men or (3) multiparous women were incubated with F(ab′)2 fragments of anti-HLA, the IgG from multiparous women demonstrated significantly greater inhibition (P < .01) than was seen with IgG from either commercial IVIg or from men (Figure 1). To measure anti-HLA idiotypic binding, affinity columns coated with F(ab′)2 fragments derived from commercial IVIg, men, or multiparous women, were loaded with F(ab′)2 fragments made from anti-HLA sera and, after elution, the column-bound proteins were examined by flow cytometry. Compared with the anti-HLA reactivity of the loaded sample (Figure 2A), the eluted F(ab′)2 fragments derived from commercial or “male sera” IgG did not bind significant levels of anti-HLA (Figure 2B). In contrast, columns prepared using F(ab′)2 prepared from the sera of multiparous women (Figure 2C) yielded bound proteins with a level of anti-HLA reactivity approaching that seen with equivalent concentrations of loaded F(ab′)2 proteins (Figure 2A). Titrations of the eluted proteins showed that the affinity columns coated with F(ab′)2 made from the sera of multiparous women bound significantly higher amounts of anti-HLA than did columns coated with F(ab′)2 made from either commercial IVIg or sera from men (P < .01; Figure3).
Inhibition of anti-HLA F(ab′)2 fragments.
Ability of intact IgG derived from commercial IVIg (○), sera from men (■), or sera from multiparous women (●), to inhibit reactivity of anti-HLA F(ab′)2 fragments. Anti-HLA reactivity is expressed as median channel fluorescence (mean ± SD, n = 6) at the indicated intact IgG/anti-HLA F(ab′)2molar ratios. As controls, the anti-HLA reactivity of the 3 intact IgG preparations (x-axis = C) and of anti-HLA F(ab′)2fragments (x-axis = 0, ▵) incubated only with phosphate-buffered saline is shown. The stars indicate significance (★:P < .05, ★★: P < .01) between data points for intact IgG from multiparous women versus intact IgG derived from commercial IVIg.
Inhibition of anti-HLA F(ab′)2 fragments.
Ability of intact IgG derived from commercial IVIg (○), sera from men (■), or sera from multiparous women (●), to inhibit reactivity of anti-HLA F(ab′)2 fragments. Anti-HLA reactivity is expressed as median channel fluorescence (mean ± SD, n = 6) at the indicated intact IgG/anti-HLA F(ab′)2molar ratios. As controls, the anti-HLA reactivity of the 3 intact IgG preparations (x-axis = C) and of anti-HLA F(ab′)2fragments (x-axis = 0, ▵) incubated only with phosphate-buffered saline is shown. The stars indicate significance (★:P < .05, ★★: P < .01) between data points for intact IgG from multiparous women versus intact IgG derived from commercial IVIg.
Flow cytometric histograms.
Representative examples of flow cytometric histograms of the anti-HLA reactivities of 1 μg of (A) anti-HLA F(ab′)2fragments loaded onto affinity columns, (B) protein eluates from the affinity columns coated with F(ab′)2 fragments from commercial IVIg (_______) or from sera of men (… … …), and (C) protein eluates from the affinity columns coated with F(ab′)2 fragments derived from the sera of multiparous women.
Flow cytometric histograms.
Representative examples of flow cytometric histograms of the anti-HLA reactivities of 1 μg of (A) anti-HLA F(ab′)2fragments loaded onto affinity columns, (B) protein eluates from the affinity columns coated with F(ab′)2 fragments from commercial IVIg (_______) or from sera of men (… … …), and (C) protein eluates from the affinity columns coated with F(ab′)2 fragments derived from the sera of multiparous women.
Anti-HLA reactivity.
Anti-HLA reactivity (mean ± SD) of the median channel fluorescence values (n = 5) of the anti-HLA F(ab′)2fragments loaded onto the affinity columns (▵), and of bound proteins eluted from the affinity columns coated with F(ab′)2derived from (1) commercial IVIg (○), (2) sera from males (■), or (3) sera from multiparous women (●). The median channel fluorescence of phosphate-buffered saline only is shown (at x-axis = 0 [■]). The stars indicate significance (★: P < .01) between data points comparing proteins eluted from the affinity columns coated with F(ab′)2 derived from commercial IVIg versus from sera of multiparous women.
Anti-HLA reactivity.
Anti-HLA reactivity (mean ± SD) of the median channel fluorescence values (n = 5) of the anti-HLA F(ab′)2fragments loaded onto the affinity columns (▵), and of bound proteins eluted from the affinity columns coated with F(ab′)2derived from (1) commercial IVIg (○), (2) sera from males (■), or (3) sera from multiparous women (●). The median channel fluorescence of phosphate-buffered saline only is shown (at x-axis = 0 [■]). The stars indicate significance (★: P < .01) between data points comparing proteins eluted from the affinity columns coated with F(ab′)2 derived from commercial IVIg versus from sera of multiparous women.
The LCT assays were used to determine the specificities of eluted anti-HLA antibodies. Antibodies of several anti-HLA specificities (HLA-A2, -A3, -A24, -A32, -B8, -B27, and -B39) were retained by the columns prepared from F(ab′)2 fragments from the sera of multiparous women, whereas only a weak anti–HLA-A2 reactivity could be detected in eluates from the columns prepared from either commercial IVIg or the sera of men.
Intact IgG and F(ab′)2 fragments derived from the sera of multiparous women can inhibit human alloimmunization
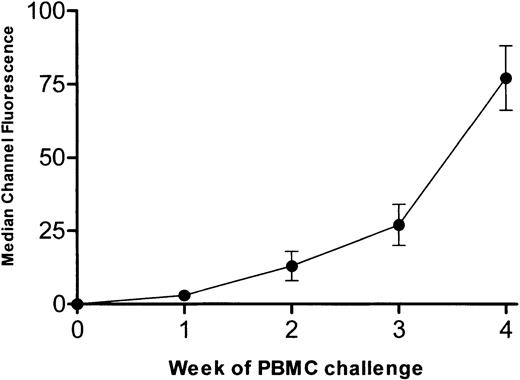
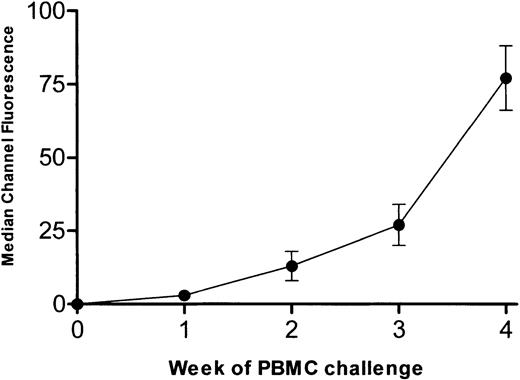
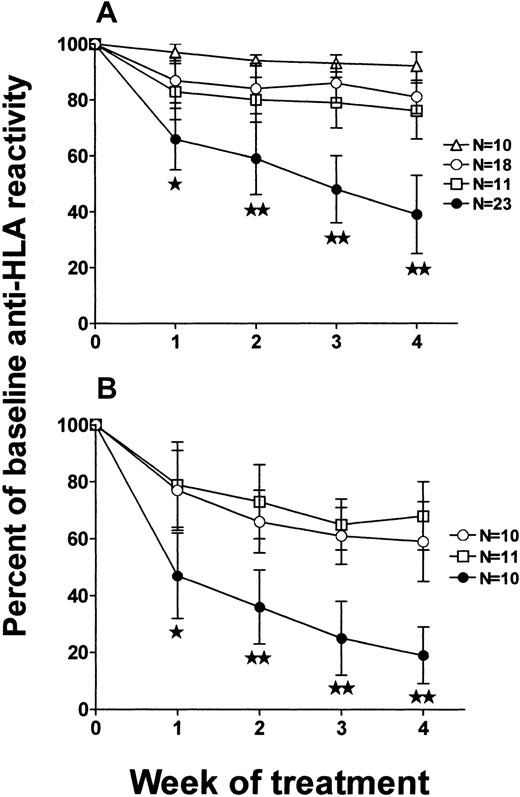
To determine the effect of intact IgG and corresponding F(ab′)2 fragments derived from multiparous women on a human alloimmune response, a SCID mouse model of human alloimmunization was used. When SCID mice were engrafted with PBMCs from an alloimmunized donor and challenged twice weekly with allogeneic PBMCs, human IgG anti-HLA antibodies developed in the sera of 93 of 102 (91%) mice by the second week of antigenic challenges. Figure4 shows the level of reactivity of human IgG anti-HLA in the sera of the 93 SCID mice. At the fourth week of PBMC challenges, mice were randomized to receive twice weekly injections (1 g/kg) of intact IgG or F(ab′)2 fragments derived from either commercial IVIg or from the sera of men or multiparous women. Compared with the mice that received F(ab′)2 fragments derived from either commercial IVIg or sera from men (Figure 5A), the F(ab′)2 fragments from multiparous women significantly inhibited the reactivity of serum anti-HLA antibodies by the second week of administration (Figure 5B; P < .01). When intact IgG derived from either commercial IVIg or from sera of multiparous women was injected into alloimmunized (anti-HLA+) SCID mice, a greater degree of inhibition was also observed compared with that seen with the corresponding F(ab′)2 fragments (Figure5B versus 5A). Hence, IgG prepared from the sera of multiparous women was superior to IgG from commercial IVIg or the sera of men for the inhibition of an ongoing secondary anti-HLA immune response.
Anti-HLA alloimmune response in SCID mice.
Development of human anti-HLA alloimmune response in SCID mice (n = 93) engrafted with PBMCs from an alloimmunized donor and challenged twice weekly for 4 weeks with allogeneic PBMCs. Data are expressed as the median channel fluorescence (mean ± SD) of SCID mouse sera at a 1:100 dilution.
Anti-HLA alloimmune response in SCID mice.
Development of human anti-HLA alloimmune response in SCID mice (n = 93) engrafted with PBMCs from an alloimmunized donor and challenged twice weekly for 4 weeks with allogeneic PBMCs. Data are expressed as the median channel fluorescence (mean ± SD) of SCID mouse sera at a 1:100 dilution.
Inhibition of anti-HLA immunity in alloimmune SCID mice.
Ability of (A) F(ab′)2 fragments or (B) intact IgG molecules to affect anti-HLA immunity in alloimmune SCID mice. The results of inhibition are expressed as the percent of baseline (100%) anti-HLA reactivity, that is, calculated from the median channel fluorescence values of each SCID mouse serum at each week after administration of proteins derived from commercial IVIg (○), the sera of men (■), or the sera of multiparous women (●), compared with the preadministration reactivity. The stars indicate significance (★:P < .05; ★★: P < .01) between data points (means ± SD) obtained using proteins derived from commercial IVIg versus the sera of multiparous women. The N values of each group of treated mice are as shown. Control mice that received no treatment with intact IgG or F(ab′)2 fragments are shown in panel A (▵).
Inhibition of anti-HLA immunity in alloimmune SCID mice.
Ability of (A) F(ab′)2 fragments or (B) intact IgG molecules to affect anti-HLA immunity in alloimmune SCID mice. The results of inhibition are expressed as the percent of baseline (100%) anti-HLA reactivity, that is, calculated from the median channel fluorescence values of each SCID mouse serum at each week after administration of proteins derived from commercial IVIg (○), the sera of men (■), or the sera of multiparous women (●), compared with the preadministration reactivity. The stars indicate significance (★:P < .05; ★★: P < .01) between data points (means ± SD) obtained using proteins derived from commercial IVIg versus the sera of multiparous women. The N values of each group of treated mice are as shown. Control mice that received no treatment with intact IgG or F(ab′)2 fragments are shown in panel A (▵).
Discussion
Therapy with IVIg is effective in treating immunodeficiency states, bacterial/viral infections, and immunoregulatory disorders, particularly immunohematologic disorders such as autoimmune thrombocytopenia, autoimmune neutropenia, and autoimmune hemolytic anemia.1,3,27 Although the mechanisms of action of IVIg in immune regulation are complex and not yet fully elucidated, several theories have been postulated. In autoimmune thrombocytopenic disorders, for example, several experimentally supported theories of the mechanism of action of IVIg have been proposed. These include reticuloendothelial Fc receptor blockade,4 down-regulation of FcγRIIIa via FcγRIIb,9 anti-idiotypic regulation,10-12 and cytokine alterations.3In contrast to the recognized efficacy of IVIg therapy inautoimmune disorders, there is controversy regarding its benefit in transfusion-induced HLAalloimmunization.5-8 Although several investigators have demonstrated that commercial IVIg preparations can inhibit anti-HLA in vitro,28-35 the inhibition has been incomplete and may be the result of absence of the necessary anti–HLA-specific anti-idiotypes in commercial IVIg.36 37Our results indicate that, compared with commercial IVIg or IgG prepared from the sera of men, the IgG derived from multiparous women has higher anti-idiotypic binding capacity for anti-HLA and can significantly inhibit an established human anti-HLA immune response in humanized SCID mice. Overall, these results support the hypothesis that IgG molecules prepared from multiparous women may be an effective γ-globulin product for the treatment of alloimmune platelet disorders.
Compared with the commercial IVIg or IgG derived from men, intact IgG molecules derived from multiparous women bound and neutralized significantly more anti-HLA F(ab′)2 fragments (Figure 1). Furthermore, compared with F(ab′)2-coupled affinity columns derived from either commercial IVIg or the sera from men, affinity columns coated with F(ab′)2 fragments from the sera of multiparous women bound significantly more anti-HLA in an idiotypic-dependent fashion (Figures 2 and 3). It is, however, important to note that IgG prepared from commercial IVIg or male donors was prepared in an identical fashion and used at the same concentration as the IgG derived from multiparous women; nonetheless, bound anti-HLA reactivity was different. The fact that IgG from a relatively small pool of male donors (n = 34) behaved similarly to the commercial IVIg (pooled from thousands of donors) suggests that pool size is not likely the reason for the lower anti-HLA binding capabilities (eg, due to dilution of anti-idiotypes on large-scale pooling). However, whether increasing the pool size of sera from multiparous women will change their anti-idiotypic binding patterns toward anti-HLA has not yet been established; we are currently studying this.
The probable explanation for higher anti-HLA idiotypic reactivity in the IgG preparations from multiparous women is likely because of the women's prior multiple exposures to paternal HLA antigens during pregnancy, resulting in anti-HLA alloimmunization and subsequent development of cross-reactive anti-HLA idiotypes. This contention is supported, in part at least, by the observation that despite exposures to paternal HLA antigens, none of the sera collected after at least 1 year from the last pregnancy contained anti-HLA reactivity. Similarly, 94% of 109 multiparous women (different from those used in this study) screened by LCT had no detectible anti-HLA reactivity (B. Hannach, Canadian Blood Services, Toronto Center, personal communication). The need to screen the sera of multiparous women for anti-HLA reactivity before being used as a source of IgG production may be important because the presence of these antibodies may, when transfused, lead to the development of severe side effects such as transfusion-related acute lung injury (TRALI).38 For example, it was recently demonstrated that administration of plasma from multiparous women to patients in intensive care units had a higher incidence of TRALI reactions compared to patients receiving control plasma units,39 although donor plasma anti-HLA reactivity was not specifically determined.
To determine whether the IgG purified from the sera of multiparous women could inhibit an established human alloimmune response, we used a SCID mouse model of human alloimmunization.26 When the F(ab′)2 preparations were administered to SCID mice already anti-HLA alloimmunized, F(ab′)2 preparations derived from the sera of multiparous women significantly inhibited SCID mouse serum anti-HLA reactivity. This supports the conclusions based on the affinity chromatography experiments that sera from multiparous women contain more anti–HLA-specific anti-idiotypes and suggests that these anti-idiotypes can more effectively inhibit a human anti-HLA response than does commercial IVIg. The time kinetics of in vivo HLA alloimmune inhibition within the first week after the initial F(ab′)2 administration have not been determined; however, the data are in agreement with those reported in several transplant studies showing that anti–HLA-specific anti-idiotypes are correlated with lower alloimmunization rates.16-18
Intact IgG preparations from multiparous women or commercial IVIg caused a greater inhibition of anti-HLA reactivity in the SCID mice than did their corresponding F(ab′)2 fragments (Figure 5). Although this may suggest that the presence of the Fc region has an additive effect to anti-idiotypic regulation in reducing HLA alloimmunization, it could not be ruled out that the differences may be also due to different in vivo half-lives of intact IgG and F(ab′)2 fragments in this mouse model.
Studies in several animal models have recently shown that the mechanism of IVIg in the reversal of either autoimmune- or xenoimmune-mediated thrombocytopenia is primarily due to Fc-dependent inhibition of the reticuloendothelial system.9,40 41 Our results suggest that, with respect to HLA alloimmunization, an IgG product can be produced from the sera of multiparous women that mediates its effects via anti-idiotypic interactions. These apparent discrepant results underscore that γ-globulins may have multiple mechanisms of action, which may be reflected by differing methodologies or type of immune phenomenon studied.
In summary, sera from multiparous women have an increased content of anti-idiotypic antibodies specific for anti-HLA alloantibodies. These purified anti-idiotypic antibodies can significantly inhibit an established IgG anti-HLA immune response in a humanized SCID mouse model. The results suggest a new and relatively simple approach to producing a superior γ-globulin product for the treatment of platelet alloimmunization.
The authors would like to thank Mr Andrew R. Crow (Senior Research Assistant, Canadian Blood Service) for his excellent technical assistance.
Supported by a grant from the Bayer Blood Partnership Fund (no. 0005R).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John W. Semple, Department of Laboratory Medicine and Pathobiology, St Michael's Hospital, 30 Bond St, Toronto, ON, Canada, M5B 1W8; e-mail: semplej@smh.toronto.on.ca.

![Fig. 3. Anti-HLA reactivity. / Anti-HLA reactivity (mean ± SD) of the median channel fluorescence values (n = 5) of the anti-HLA F(ab′)2fragments loaded onto the affinity columns (▵), and of bound proteins eluted from the affinity columns coated with F(ab′)2derived from (1) commercial IVIg (○), (2) sera from males (■), or (3) sera from multiparous women (●). The median channel fluorescence of phosphate-buffered saline only is shown (at x-axis = 0 [■]). The stars indicate significance (★: P < .01) between data points comparing proteins eluted from the affinity columns coated with F(ab′)2 derived from commercial IVIg versus from sera of multiparous women.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/3/10.1182_blood.v100.3.1055/5/m_h81522920003.jpeg?Expires=1769288707&Signature=0fEoVmVwzoGIZ6fTlq0VRMiT5Sd~sgJODINU9nxwOWnEeMjs2rglhiSNLn9CQI4dq6RmtaUEwKrbhKoO7VKv0Y4n61OF82m-U~v9rZ5H~GUIUT4c0usGRaNvdFptO3eLgMxO5rQRwCmcjL9HO4IqRx3yXrOPIwsISIOeN7PkpUagEKRUS2FOvI4xA5onnj81kCXMFGwP~JDaXyaQaLL1QMjGHcR7IfBH-HDfKvbKh-cgE-PfAWpYuN7JbLzJDV7hgvKwIobGnAnEEAMODjDIT46qNgvrd0eHO-AU7QN~FelhhyGMSFbHhLl34QNDonMGkNv-Uo~8ZjrY0qAjh2c3iA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 3. Anti-HLA reactivity. / Anti-HLA reactivity (mean ± SD) of the median channel fluorescence values (n = 5) of the anti-HLA F(ab′)2fragments loaded onto the affinity columns (▵), and of bound proteins eluted from the affinity columns coated with F(ab′)2derived from (1) commercial IVIg (○), (2) sera from males (■), or (3) sera from multiparous women (●). The median channel fluorescence of phosphate-buffered saline only is shown (at x-axis = 0 [■]). The stars indicate significance (★: P < .01) between data points comparing proteins eluted from the affinity columns coated with F(ab′)2 derived from commercial IVIg versus from sera of multiparous women.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/3/10.1182_blood.v100.3.1055/5/m_h81522920003.jpeg?Expires=1769288708&Signature=Vk1FilOhHk~vsHzJgcjAzQE~JMCELEtJkauptBm6E-zObFL46EVrrpgkfTlNxhpCkYVWQlOrbkzhoSiLf33CecGmiQKkgQEXrbvQWahvUxTS~vaaoXGD1Pkdct-aW8fMD7mwv5RzG6FsSoRQbII8Unp~41uViijKlfyXgauQKIxXjW0DN0zhd9Z4bbXQZzCIZZ-vY0~CdlsUi2HnFd20zxzEFPOYEKh8stpw65YnsPcV89s6qLKxsZUWGLbL3lDK1~g6l45ypbIIo22yszg~2K5vWB-4dsfpPsJfQGrzDvlsP4sPk8G2HuRdb8WlchgoRjrqj9JGegOJOx8PI4OiCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)