Abstract
Whether measurement of ADAMTS13 activity may enable physicians to distinguish thrombotic thrombocytopenic purpura (TTP) from hemolytic uremic syndrome (HUS) is still a controversial issue. Our aim was to clarify whether patients with normal or deficient ADAMTS13 activity could be distinguished in terms of disease manifestations and multimeric patterns of plasma von Willebrand factor (VWF). ADAMTS13 activity, VWF antigen, and multimeric pattern were evaluated in patients with recurrent and familial TTP (n = 20) and HUS (n = 29). Results of the collagen-binding assay of ADAMTS13 activity were confirmed in selected samples by testing the capacity of plasma to cleave recombinant VWF A1-A2-A3. Most patients with TTP had complete or partial deficiency of ADAMTS13 activity during the acute phase, and in some the defect persisted at remission. However, complete ADAMTS13 deficiency was also found in 5 of 9 patients with HUS during the acute phase and in 5 patients during remission. HUS patients with ADAMTS13 deficiency could not be distinguished clinically from those with normal ADAMTS13. In a subgroup of patients with TTP or HUS, the ADAMTS13 defect was inherited, as documented by half-normal levels of ADAMTS13 in their asymptomatic parents, consistent with the heterozygous carrier state. In patients with TTP and HUS there was indirect evidence of increased VWF fragmentation, and this occurred also in patients with ADAMTS13 deficiency. In conclusion, deficient ADAMTS13 activity does not distinguish TTP from HUS, at least in the recurrent and familial forms, and it is not the only determinant of VWF abnormalities in these conditions.
Introduction
Thrombotic thrombocytopenic purpura (TTP) and the related condition hemolytic uremic syndrome (HUS) are diseases characterized by microangiopathic hemolytic anemia and thrombocytopenia, with signs of brain and renal involvement secondary to widespread microthrombi and reactive endothelial proliferation.1,2 The term TTP is used to identify adult cases with predominant neurologic involvement; the disease usually occurs sporadically, sometimes following the administration of certain drugs.1 On the other hand, the term HUS is used to identify the thrombotic microangiopathy that occurs in children with predominant renal failure.3 This form, mostly sporadic, is frequently associated with infection by some strains ofEscherichia coli, which produce a powerful Shiga-like toxin.3,4 The familial form of TTP and HUS is rare and occurs in more than one member of the same family; recurrence is frequent. There are also nonfamilial forms of TTP and HUS that relapse even after complete recovery of the initial episode (recurrent forms). Death and end-stage renal failure or neurologic sequelae are final outcomes in most patients with the familial and recurrent forms.5-7
Endothelial damage is a crucial event in TTP and HUS. On injury endothelial cells release von Willebrand factor (VWF), a large multimeric glycoprotein that mediates the adhesion of platelets to sites of vascular lesions.8 After immunohistology studies showed the accumulation of VWF in microvascular thrombi of patients with thrombotic microangiopathy,9 endothelial VWF secretion and processing were extensively investigated in these conditions. VWF is normally formed as ultralarge (UL) multimers because of the polymerization in endothelial cells and megakaryocytes of a native subunit of 225 kd, and it is stored as such in Weibel-Palade bodies and α-granules.8 However, UL multimers do not normally circulate because they are cleaved into smaller multimers soon after their secretion.10-13
In patients with TTP or HUS, in contrast to healthy subjects, UL multimers are sometimes detected in plasma.14,15 The relationship between UL VWF multimers and thrombotic microangiopathies remained elusive until the recent demonstration of the presence in human plasma of a metalloprotease (ADAMTS13)16-18 that cleaves VWF physiologically at the peptide bond between amino acid residues 842Thr and 843Met in the A2 domain of the subunit.11 The gene encoding this protease has been identified,19-23 and data are available that mutations in this gene lead to an inactive enzyme.21 In 2 large clinical studies,24,25 deficiency of ADAMTS13 activity was found in patients with TTP but not in those with HUS.24,25 That patients with HUS have normal ADAMTS13 activity was taken to indicate that the presence or absence of this activity could be used to classify patients as having HUS or TTP.26 However, a few recent studies27-31 have challenged this paradigm, showing that patients with a diagnosis of HUS may have complete ADAMTS13 deficiency.
In this study we measured ADAMTS13 activity in 49 patients with recurrent or familial TTP or HUS, the rarest forms of these microangiopathies. Patients were studied in the acute phase and in the remission phase of the syndromes, with the aim to clarify whether those with normal or deficient ADAMTS13 activity could be distinguished in terms of disease manifestations. We also investigated whether an antibody inhibiting the protease activity was detectable in these patients, or whether they carry an inherited defect by analyzing ADAMTS13 activity in their relatives. Finally, we explored any correlates between ADAMTS13 activity level and changes in the multimeric pattern of plasma VWF.
Patients, materials, and methods
Patients and controls
Forty-nine patients with recurrent or familial TTP or HUS were recruited from January 1995 to May 2001 among those referred to the Italian Registry of Recurrent and Familial HUS/TTP, a network of 50 Hematology or Nephrology Units established under the coordination of the Clinical Research Center for Rare Diseases Aldo e Cele Daccò. All information on the clinical history of the patients was recorded in the database of the Registry kept at the Clinical Research Center for Rare Diseases. As controls, 30 healthy subjects matched for age and sex, unrelated to any of the studied patients, were also recruited.
Patients and controls received detailed information on the purposes and design of the study and provided informed consent according to the guidelines of the Declaration of Helsinki. Protocol was approved by the institutional review board of the Mario Negri Institute.
Blood samples
For in-hospital patients, usually those studied during the acute phase of the disease, blood samples were collected by the referring clinical units and dispatched to the Clinical Research Center for Rare Diseases. During the acute phase, blood was collected before any treatment was started.
Patients in remission were summoned to the Clinical Research Center, where a detailed clinical history was obtained and blood samples were collected and processed for laboratory tests. At remission, blood was taken at least 2 weeks after the last therapeutic plasma infusion or plasma exchange. Blood samples from healthy subjects were also collected.
Diagnosis of TTP and HUS
TTP and HUS were diagnosed in patients who had one or more episodes of microangiopathic hemolytic anemia and thrombocytopenia defined on the basis of hematocrit (Ht) < 30%, hemoglobin (Hb) < 10 mg/dL, LDH > 460 IU/L, undetectable serum haptoglobin, evidence of red cell fragmentation in the peripheral blood smear, and thrombocytopenia (platelets less than 150 000/μL). Specifically, a diagnosis of TTP was made when laboratory findings of thrombotic microangiopathy occurred in patients, with or without renal involvement, in whom specific neurologic signs (ie, coma, focal or generalized convulsions, dysphasia, paresis, visual disturbances) not attributable to uremic encephalopathy dominated the clinical picture. A diagnosis of HUS was made when laboratory findings of thrombotic microangiopathy were associated with acute renal failure (defined as serum creatinine above normal ranges for several days in patients with previous normal renal function, or serum creatinine increase greater than 30% in patients with previous evidence of renal dysfunction), without evidence of specific neurologic signs except those caused by uremic encephalopathy (ie, impaired concentration, clumsiness, apathy). Patients with E coli–associated diarrhea-positive HUS and patients with sporadic forms of TTP or HUS were excluded from this study.
Familial TTP or HUS was diagnosed in patients for whom at least 2 members of the family were affected by the disease at least 6 months apart, without evidence of a common triggering agent, in particular, infection, or drugs such as cyclosporine A, ticlopidine, or clopidogrel.5-7 Recurrent TTP or HUS was diagnosed in patients who, with no family history, had one or more relapses after complete and persistent (for at least 30 days after plasma exchange or plasma infusion) remission of any sign of thrombotic microangiopathy. Patients recently (within 2 weeks) treated with plasma exchange or infusion were not included.
Remission of HUS or TTP was defined by a persistent increase in platelet count greater than 150 000/μL, normalization of the markers of hemolysis and tissue damage (LDH less than 460 IU/L, no fragmented red cell in the peripheral blood film), and normalization or near normalization of the neurologic status, for at least 1 week after plasma infusion or exchange, independently of the presence of residual renal dysfunction.
Diagnosis of TTP or HUS and of the recurrent or familial forms were made by 2 independent physicians (P.R., E.D.) according to the above criteria, masked to ADAMTS13 activity and VWF results. Their diagnoses agreed for all patients but one with acute renal failure and severe neurologic signs (coma). After consultation between the 2 physicians, the patient was later classified as having HUS because neurologic symptoms were attributed to uremic encephalopathy.
Measurement of ADAMTS13 activity
Collagen-binding assay.
Blood samples were collected into 0.1 vol 0.129 M sodium citrate, and platelet-poor plasma was prepared by centrifugation. A pool of plasmas obtained from 50 healthy women not pregnant and not taking oral contraceptives and 50 healthy men was used as reference for the assays and arbitrarily defined to contain 100% of the protease activity.32
ADAMTS13 activity was measured as previously described by Gerritsen et al.33 Pooled normal plasma was used as VWF substrate for the protease. Serial dilution of the samples to be tested and of the normal plasma pool in a buffer-containing urea were incubated with BaCl2 to achieve degradation of endogenous VWF and particularly of larger VWF multimers that might interfere with the assay by preferentially binding to type III collagen. After the first digestion, samples were incubated with the substrate, and another digestion was carried out for 2 hours at 37°C. Human collagen type III (3 μg/mL; Valter Occhiena, Milano, Italy) was used for the collagen-binding assay.33 The values of ADAMTS13 activity were read from a dose-response curve obtained for each assay run by testing serial dilutions from 1:5 to 1:320 of the reference plasma pool. The within-assay (n = 18) coefficient of variation was 8%, the between-assay (n = 100) coefficient of variation was 14%, and the lower limit of sensitivity of the method was 6% of the normal protease levels.32 Interference of endogenous VWF in test samples has been excluded by finding similar protease levels in plain plasma and in plasma after cryoprecipitation to remove a large amount of VWF, particularly of the larger multimers more reactive with type III collagen.32 Patient samples with activity levels less than 6%, which is the detection limit of the assay, were defined as having complete deficiency of ADAMTS13; activity values between 6% and 20% were considered severely reduced, values between 21% and less than 50% were considered moderately reduced, and values equal or greater than 50% were considered normal.
The presence of ADAMTS13 inhibitory antibodies was assayed by testing ADAMTS13 activity in mixtures of plasma from patients and normal pooled plasma at different dilutions (3:1,1:1,1:3) after 30-minute incubation at 37°C.33 In samples with ADAMTS13 activity 20% or lower and no inhibitor activity, to detect possible relevant ADAMTS13 inhibitors with a slower kinetics, further analysis was made by measuring protease activity in mixtures of patient plasma and normal plasma (1:1 ratio) incubated at 37°C for 1, 3, and 6 hours.
Cleavage of recombinant VWF by control and patient plasma.
To confirm the capability of normal and patient plasma to cleave VWF, recombinant VWF A1-A2-A3 (rVWF) domains were expressed inDrosophila cells (provided by Z. M. Ruggeri, La Jolla, CA; manuscript in preparation). Supernatant from Drosophilacell culture containing monomeric rVWF was incubated with control or patient plasma in the presence of Ba2+ and urea at pH 8. Mixture containing rVWF (2 μg) and plasma (35 μL), Tris saline buffer with 1.5 M urea final concentration, in the presence or not of EDTA (5 mM), was incubated for 2 hours at 37°C. Samples were electrophoresed under nonreducing conditions on 10% SDS-PAGE, and the intact rVWF A1-A2-A3 domains and their proteolytic fragments were visualized with a monoclonal antibody directed against the carboxy terminal of the rVWF (provided by Z. M. Ruggeri). In these conditions the intact rVWF of approximately 80 kd and the carboxy terminal fragment of approximately 30 kd generated by cleavage of rVWF are revealed. With this test any possible interference of endogenous VWF present in test samples is excluded.
Analysis of VWF antigen and multimers
Five milliliters blood was collected on protease inhibitors as previously described.34 Plasma VWF antigen (VWF:Ag) was measured by enzyme-linked immunosorbent assay as previously described.35
The VWF multimeric pattern was analyzed by discontinuous SDS–agarose gel electrophoresis.36,37 Gels of 1.5% high-gelling temperature agarose HGT(P) (FMC, Rockland, ME) capable of resolving high-molecular–weight (HMW) multimers were used. Low-molecular–weight (LMW), HMW, and UL multimers were classified on the basis of their electrophoretic mobility and densitometric analysis.38 For each lane, the values were expressed as ratio HMW:LMW multimers. Under our conditions the ratio for healthy subjects was 1.04 ± 0.2 (n = 13).
Data analysis
All data are presented as the mean ± SD. Data on VWF antigen, multimers, and ADAMTS13 activity were analyzed by one-way ANOVA. The correlation coefficient (r) was used to determine the relationship between ADAMTS13 activity and VWF parameters. A receiver operating characteristic curve was designed to calculate sensitivity and specificity diagnostic rates, corresponding to various cut-off values for the ADAMTS13 activity, to discriminate between TTP and HUS. The level of significance was set atP < .05 (2-tailed).
Results
Patient features
Among the 49 patients with thrombotic microangiopathy included in the study, 20 met the criteria of TTP (7 males, 13 females; mean age at onset, 32.9 years; range, 13-51 years), and 29 met those of HUS (19 males,10 females; mean age at onset,15 years; range, 0-34 years). Nonfamilial recurrent TTP and familial TTP were diagnosed in 16 and 4 patients, respectively. Eight patients with recurrent and 2 patients with familial TTP manifested one disease recurrence, at 1 to 6 years after the first event. The other 8 patients with recurrent TTP experienced 3 to 7 relapses during 2 to 10 years. One patient with recurrent TTP died during relapse, and 3 (1 recurrent, 2 familial) developed permanent neurologic sequelae (progressive neurologic deterioration, paresis).
The nonfamilial recurrent form and the familial form of HUS were diagnosed in 9 patients (onset in childhood, 6; onset in adulthood, 3) and 20 patients (onset in childhood, 10; onset in adulthood, 10), respectively. Five of the 9 patients with recurrent HUS experienced 2 relapses in 1 to 17 years; 3 experienced 4 to 10 relapses in 9 to 14 years, and another patient experienced more than 100 relapses during 30 years. Among patients with familial HUS, 12 experienced at least one disease relapse in 1 to 10 years. One HUS patient with the familial form died during a relapse. Eight patients with HUS (1 recurrent and 7 familial) developed end-stage renal disease (ESRD) just after the onset of the disease; in another 9 patients (5 recurrent, 4 familial), progressive decline of renal function leading to ESRD was observed during long-term follow-up (from 2 to 25 years). In the remaining 12 patients (3 recurrent, 9 familial) a normalization of serum creatinine was documented, though disease relapses occurred in some patients. Two patients (1 with recurrent, 1 with familial HUS) acquired neurologic sequelae in subsequent relapses.
In the TTP group 12 patients (9 with recurrent, 3 with familial TTP) were in the acute phase, and 8 (7 with recurrent, 1 with familial TTP) were in remission. For the 12 patients with active disease, plasma was obtained in 9 (6 with recurrent, 3 with familial TTP) during the acute event and at remission.
In the group of HUS patients, 9 (5 with recurrent, 4 with familial HUS) were in the active phase of disease, and 20 (4 recurrent and 16 familial HUS) were in full remission. Eight of the 9 patients with active disease (4 with recurrent, 4 with familial HUS) were studied again at remission.
For patients studied at remission, the time from the last clinical episode ranged from 1 month to 17 years. Laboratory values are shown in Tables 1 and2.
Mean VWF:Ag values during the acute phase were significantly higher (P < .05) in patients with TTP (162% ± 72%) and in patients with HUS (211% ± 108%) than in healthy subjects (84% ± 33%). In patients with HUS, VWF:Ag levels remained abnormally high during remission (152% ± 98%,P < .05) than in healthy subjects, probably because of chronic impairment of kidney function.39
ADAMTS13 activity
In the 30 healthy subjects, ADAMTS13 activity ranged from 92% to 197% (mean, 137% ± 29%). No healthy subjects with protease values lower than 50% were found in this study.
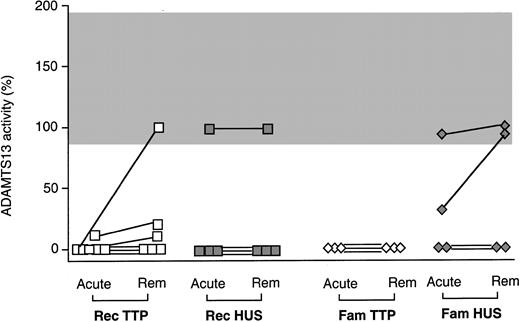
Eleven of 12 patients with acute TTP (8 recurrent, and 3 familial TTP) had no detectable ADAMTS13 activity; one patient with recurrent TTP had severe deficiency (11%) (Figure1A, Table 1). No activity of ADAMTS13 was detected at remission in 7 of 17 patients with TTP (4 with recurrent, 3 with familial TTP). Four patients with recurrent TTP had severely reduced activity, whereas in the other patients studied at remission protease activity was normal (Figure 1A, Table 1). Seven of the 12 patients with acute TTP (all with the recurrent form) and 4 patients (all with the recurrent form) of the 17 studied at remission, 2 to 6 months after the last acute episode, had a circulating inhibitor of ADAMTS13 activity (Table 1).
ADAMTS13 activity values.
ADAMTS13 activity in the plasma of patients with TTP (A) or HUS (B) according to whether they had recurrent (Rec) or familial (Fam) forms of the diseases and to phase of event. ADAMTS13 activity values were expressed as percentage activity of normal plasma pool. Horizontal gray bar indicates the range of values for plasma ADAMTS13 activity values in healthy subjects. Rem, remission.
ADAMTS13 activity values.
ADAMTS13 activity in the plasma of patients with TTP (A) or HUS (B) according to whether they had recurrent (Rec) or familial (Fam) forms of the diseases and to phase of event. ADAMTS13 activity values were expressed as percentage activity of normal plasma pool. Horizontal gray bar indicates the range of values for plasma ADAMTS13 activity values in healthy subjects. Rem, remission.
Of the 9 patients with HUS studied during the acute phase, 5 (3 with recurrent, 2 with familial HUS) had complete deficiency of the ADAMTS13 activity; of these, 2 developed ESRD and are now on chronic hemodialysis (Figure 1B, Table 2). One additional patient with familial HUS had moderately reduced values, but in the others (2 with recurrent, 1 with familial HUS) protease activity was normal (Figure 1B, Table 2). HUS patients who had complete deficiency of ADAMTS13 activity during the acute phase (n = 5, group 1) could not be clinically distinguished from those with normal ADAMTS13 activity (n = 3, group 2) in terms of incidence of ESRD, neurologic symptoms or sequelae, or relapsing microangiopathy.
Of the 28 plasma samples collected during remission from patients with HUS, 5 patients (3 with recurrent, 2 with familial HUS) had complete deficiency of the ADAMTS13 activity, one patient with the familial form had severely reduced (17%) activity, and one with the recurrent form had moderately reduced (41%) activity (Table 2, Figure 1). None of the HUS patients with deficient ADAMTS13 activity had detectable protease inhibitor in their plasma either during the acute phase or at remission (Table 2), suggesting the presence of a congenital defect.
Figure 2 shows ADAMTS13 activity values from patients with recurrent and familial TTP and HUS from whom blood samples were collected during the acute phase and at remission. Only in 1 of 13 patients with complete ADAMTS13 deficiency during the acute phase was there complete ADAMTS13 recovery at the time of remission (Figure 2). Among patients with persistently low ADAMTS13, 2 (with recurrent TTP) also had high titers of inhibitory antibodies at remission.
ADAMTS13 activity values in the plasma from patients with recurrent (Rec) or familial (Fam) TTP or HUS.
ADAMTS13 activity was tested during the acute phase and at remission. See also Figure 1.
ADAMTS13 activity values in the plasma from patients with recurrent (Rec) or familial (Fam) TTP or HUS.
ADAMTS13 activity was tested during the acute phase and at remission. See also Figure 1.
In a representative subgroup of healthy subjects (n = 6) and of TTP (n = 4) and HUS (n = 6) patients with normal or undetectable ADAMTS13 activity, values obtained by the collagen-binding assay were also evaluated by incubating plasma with rVWF A1-A2-A3 domains. Plasma from healthy subjects and plasma from patients with normal ADAMTS13 activity cleaved rVWF to form a fragment of the expected 30-kd size. Cleavage of rVWF was completely prevented by the addition of EDTA to plasma samples during incubation. By contrast, plasma from patients (n = 4) completely deficient in ADAMTS13 activity by the collagen-binding assay formed no fragment when incubated with rVWF.
Sensitivity and specificity of ADAMTS13 activity for diagnosis of TTP and HUS
Sensitivity and specificity of ADAMTS13 activity for diagnosis of recurrent and familial forms of TTP and HUS were calculated by a receiver operating characteristic curve. Complete deficiency of ADAMTS13 activity had a sensitivity of 92% and a specificity of 44% for diagnosis of TTP when measured during the acute phase. The sensitivity increased to 100% when the cut-off of ADAMTS13 activity was set at 20% or less (specificity, 44%). On remission complete deficiency of ADAMTS13 activity had a sensitivity of 41% and a specificity of 82% for diagnosis of TTP. Sensitivity increased to 65% when the cut-off was set to 20% or less, but specificity decreased to 78%.
None of the HUS patients had a detectable protease inhibitor in their plasma; hence, the specificity of detecting ADAMTS13 inhibitor for the diagnosis of TTP was 100%. All patients who had persistence of complete deficiency of the ADAMTS13 activity in remission experienced several disease relapses, indicating an association of this degree of deficiency with a predisposition for recurrences.
Distribution of ADAMTS13 activity values in relatives of TTP and HUS patients
Available asymptomatic relatives of 2 patients with familial forms of microangiopathies (2 sisters, one with HUS and one with TTP: pedigree 2) and 5 patients with the recurrent form (3 HUS: pedigrees 12, 13, 25; and 2 TTP: pedigrees 26, 57) were also investigated for ADAMTS13 activity. In all the affected family members, ADAMTS13 activity was completely deficient or severely reduced. Two patients with recurrent TTP (pedigrees 26, 57) had ADAMTS13 inhibitors. All their relatives had normal ADAMTS13 activity, confirming that in these patients the defect was acquired. As for the other 5 pedigrees, plasma levels of the protease in the asymptomatic parents of the affected patients were approximately half-normal (37% to 63%), consistent with a heterozygous carrier state. Similarly, levels for all the other healthy relatives of the patients fell into a bimodal distribution with a group of values ranging from 37% to 67%, consistent with carriership, and the others from 86% to 117% (normal values). An exception was in pedigree 2, where, in line with previously reported data,24 an asymptomatic brother of the proband had a complete deficiency of ADAMTS13 activity, and his 2 sons had half-normal levels.
Thus, in the above 5 families, the ADAMTS13 activity defect was inherited as a recessive trait and determined either TTP or HUS phenotypes. Of note, in pedigree 2 and in an additional one, pedigree 19, one patient with TTP and one patient with HUS were reported.
Relationship between ADAMTS13 activity and VWF multimeric pattern
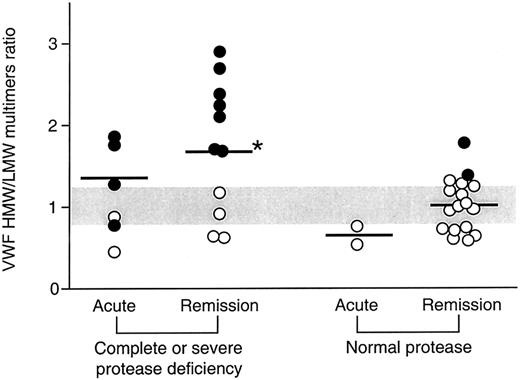
The relationship between ADAMTS13 activity and the VWF multimeric pattern was also explored in patients in whom both measurements were performed. Patients were divided in 2 groups on the basis of ADAMTS13 activity: group 1, complete or severe deficiency (acute phase, n = 6; remission, n = 11); group 2, normal activity (acute phase, n = 2; remission, n = 17); no sample from patients with moderate ADAMTS13 activity deficiency was analyzed for VWF multimers. In 7 (all with recurrent TTP and HUS) of 11 patients with complete or severe deficiency of the ADAMTS13 activity (group 1), there was a characteristic presence of UL VWF multimers during the acute phase of the disease (Figure 3), that persisted in remission, whereas the other 4 patients had no UL multimers despite complete deficiency of the ADAMTS13 activity (Figure 3). In group 1 patients during remission, densitometric analysis showed a higher HMW:LMW multimer ratio (Figure 3) compared with healthy subjects (P < .05) and with patients with normal activity studied in remission (P < .05) (group 2). In the same patients, despite the presence of UL multimers, enhanced cleavage was found during the acute phase, as shown indirectly by a lower HMW:LMW multimer ratio compared with remission (Figure 3). These results indicate that VWF is cleaved in the circulation of these patients despite the absence of the ADAMTS13 activity.
VWF HMW:LMW multimer ratio in the plasma from patients with TTP and HUS divided on the basis of the levels of ADAMTS13 activity and of the phase of event.
Horizontal gray bar indicates the range of values in healthy subjects. Black symbols indicate samples with the presence of UL multimers. *P < .05 versus healthy subjects and patients with normal protease activity at remission.
VWF HMW:LMW multimer ratio in the plasma from patients with TTP and HUS divided on the basis of the levels of ADAMTS13 activity and of the phase of event.
Horizontal gray bar indicates the range of values in healthy subjects. Black symbols indicate samples with the presence of UL multimers. *P < .05 versus healthy subjects and patients with normal protease activity at remission.
In patients with normal ADAMTS13 activity (group 2), the mean HMW:LMW multimer ratio at remission was comparable with that found in healthy subjects; however, 2 patients of this group had UL multimers when protease activity was measured (Figure 3). Indirect evidence of increased VWF fragmentation was also observed in patients of group 2 during the acute phase (Figure 3) which resulted in a numerically lower HMW:LMW multimer ratio than in healthy subjects, whereas the HMW:LMW ratio normalized at remission.
There was a weak inverse correlation between the ADAMTS13 activity in patients and the HMW:LMW ratio values (r = −0.42;P < .05). Altogether, these findings indicate that ADAMTS13 activity is not the main determinant of VWF multimer fragmentation in TTP and HUS.
Discussion
Most patients with a clinical diagnosis of recurrent or familial TTP had complete or severe deficiency of the ADAMTS13 activity during the acute phase of the disease, and in a subgroup the defect persisted at remission. Complete deficiency of ADAMTS13 activity was also found in plasma samples from 5 of 9 patients with recurrent and familial HUS during the acute phase and in 5 patients during remission. These results have been confirmed by evaluating the cleavage of recombinant VWF A1-A2-A3 domains, which overcomes possible artifacts of the collagen-binding assay due to the presence of endogenous undegraded VWF in test samples.25,33 40 Diagnosis of TTP or HUS was made by 2 independent physicians masked to the nature of ADAMTS13 activity and VWF results.
These observations challenge the paradigm recently put forward that “a single laboratory test may enable physicians to distinguish TTP from HUS”26 based on data24 that “patients given diagnosis of TTP had had little or no ADAMTS13 activity in plasma, whereas the plasma activity of the enzyme was normal (or nearly so) in patients considered to have familial or acquired HUS.” On the other hand, complete deficiency of the ADAMTS13 activity has recently been found in 5 children with recurrent HUS and in one with E coli–associated HUS,30 indicating that the protease defect cannot be narrowed to specific subtypes of thrombotic microangiopathies. In our series each of 2 families had one patient with TTP and one with HUS, who completely lacked ADAMTS13 activity. Because the patients within each family inherited the same genetic defect, these data can be taken to indicate that deficiency of ADAMTS13 activity may lead to either the TTP or the HUS phenotype. In a large series of patients with different forms of HUS and TTP, 6 patients with diagnosis of HUS had decreased ADAMTS13 activity.31 At variance with our data, showing a 44% specificity of the ADAMTS13 defect in distinguishing between TTP and HUS, in the above series a 91% specificity was found, which led Veyradier et al31 to conclude that “VWF-cleaving protease deficiency specifically concerns a subgroup of TMA corresponding to the TTP entity.” Differences between our patient population, including only familial and recurrent TTP and HUS, and the study of Veyradier et al,31 which mainly focused on sporadic cases, may account for discrepancies in results. In addition, Veyradier et al31 did not specify the criteria for the differential diagnosis between TTP and HUS, which was independently made by the numerous participating centers.
Two primary mechanisms for deficiency of the ADAMTS13 activity have been identified, namely a constitutive deficiency and the presence of a circulating acquired inhibitory antibody.24 25 We found an inhibitor of ADAMTS13 activity in 7 of 12 patients with acute TTP, all with the recurrent form of the disease, and in 4 patients with recurrent TTP studied at remission. Normal levels of ADAMTS13 activity were found in all the asymptomatic relatives of patients carrying the protease inhibitor.
In another group of patients (with familial or recurrent TTP or HUS), no inhibitor was ever found despite complete deficiency of the ADAMTS13 activity. The method used in this study would not detect the presence of antibodies that do not inhibit ADAMTS13 activity but do enhance the clearance of the protease in vivo, so that the prevalence of antibodies might have been underestimated in this study.
However, in the available asymptomatic parents of the above patients, levels of ADAMTS13 activity were consistent with carriership (approximately 50% activity), indicating that the ADAMTS13 defect was inherited. These results are in agreement with previous reports21,24 25 and support the view that the deficiency of ADAMTS13 activity in thrombotic microangiopathies may be acquired as a result of an autoimmune mechanism or may be inherited.
The discovery of deficient ADAMTS13 activity in TTP has been rapidly integrated into the prevailing model of the pathophysiology of VWF-mediated thrombotic microangiopathies. According to this model, congenital or autoimmune dysfunction of ADAMTS13 prevents the normal proteolysis of large VWF multimers as they are secreted from injured endothelial cells.41 This ultimately causes the development of circulating UL VWF multimers, which, in conditions of high shear stress, are capable of supporting platelet aggregation more efficiently than normal multimers.42 This condition would predispose patients to microvascular thrombosis at sites of endothelial cell injury. However, direct proof of this was never provided.
Results of our analysis of the relationship between VWF multimeric pattern and ADAMTS13 activity in TTP and HUS patients challenge the above hypothesis. On one hand, confirming previously reported data,38,43 we found UL multimers in 2 patients with recurrent TTP with normal ADAMTS13 activity. On the other hand, many patients with either TTP or HUS, with complete or severe deficiency of the ADAMTS13 activity, did not have UL multimers in their circulation. In addition, patients with deficiency of ADAMTS13 activity showed increased VWF fragmentation during the acute phase, as indirectly documented by a lower HMW:LMW multimer ratio, than at remission. The same results were reported by Veyradier et al,31 who found no correlation between ADAMTS13 activity levels and UL multimers in their large series. As previously reported,38 we also found that the normal 189-, 176-, and 140-kd fragments were present in the blood of all patients with deficient ADAMTS13 activity, and the percentage of the native intact VWF subunit was even lower than in healthy subjects, at least in the acute phase (data not shown). These results indicate that besides ADAMTS13, other protease(s) are present in the blood of patients with TTP and HUS that cleave VWF to the normal fragments and support the possibility that deficiency of the ADAMTS13 activity is not the only determinant of VWF abnormalities in these diseases. Consistent with the present findings, previous studies from our and other groups have documented that in sporadic,35recurrent,35,38,44 and familial TTP and HUS38and in the acute phase of HUS resistant to plasma therapy,35 45 rather than the presence of UL VWF multimers, there is a loss of such multimers and an increase of LMW forms that indicate enhanced proteolytic fragmentation of the molecule.
In summary, this large study has attempted to clarify the complex and controversial issue of the pathogenesis of TTP and HUS. We found that low values of ADAMTS13 activity may not be specific for TTP, at least in recurrent and familial forms. In addition, deficient ADAMTS13 activity is not the only determinant of the presence of ultralarge VWF multimers in some phases of the clinical disease of these patients. Whether there is a correlation between ADAMTS13 activity and response to treatment is a crucial issue that requires a specially designed prospective study.
Investigation into the etiology and genetics of TTP and HUS represents a rapidly advancing field that is outpacing the previous disease nomenclature. Thus, instead of using the terms TTP and HUS, it should be now more appropriate and clinically relevant to classify patients with thrombotic microangiopathies on the basis of the underlying specific defect and to consider patients with ADAMTS13 deficiency as a separate subset.
We thank Prof Zaverio Ruggeri for providing rVWF and the anti-rVWF–specific antibody and Dr Annalisa Perna for her valuable help in statistical analysis. We also thank the Comitato 30 ore per la vita for supporting the study.
The Italian Registry of Recurrent and Familial HUS/TTP
Coordinators.
P. Ruggenenti, MD, G. Remuzzi, MD (Clinical Research for Rare Diseases “Aldo e Cele Daccò,” Ranica, and Division of Nephrology and Dialysis, “Ospedali Riuniti” Azienda Ospedaliera, Bergamo); M. Noris, ChemPharmD (Clinical Research for Rare Diseases “Aldo e Cele Daccò,” Ranica).
Investigators.
C. Cascone, MD, G. Delfino, MD (Division of Nephrology and Dialysis, “S. Giacomo” Hospital, Castelfranco Veneto, Treviso); F. Casucci, MD, F. Cazzato, MD (Division of Nephrology, “Miulli” Hospital, Acquaviva delle Fonti, Bari); G. F. Rizzoni, MD, A. Gianviti, MD (Division of Nephrology and Dialysis, “Bambino Gesù” Pediatric Hospital, Roma); C. Grimaldi, MD, M. Salvadori, MD (Division of Internal Medicine and Division of Nephrology, “S. Giovanni di Dio” Hospital, Firenze); G. C. Barbano, MD (Division of Pediatric Nephrology, “G. Gaslini” Institute, Genova); A. Edefonti, MD (Division of Pediatric Dialysis, “De Marchi” Pediatric Clinic, Milano); E. Rossi, MD, A. Lattuada, BiolSciD (Transfusional Center, “L. Sacco” Hospital, Milano); G. B. Haycock, MD (Pediatric Renal Unit, “Guy's Hospital,” London, United Kingdom); A. Indovina, MD, R. Marcenò, MD (Bone Marrow Transplantion Unit, “V. Cervello” Hospital, Palermo); E. Daina, MD, E. Bresin, MD, S. Gamba, Research Nurse, A. Schieppati, MD (Information Center for Rare Diseases, Ranica, Bergamo); C. Zoccali, MD, M. Garozzo, MD, G.Enia, MD (Division of Nephrology and Dialysis, Bianchi-Melacrino-Morelli Hospital, Reggio Calabria); T. Cicchetti, MD, G. Putortı̀, MD (Division of Nephrology and Dialysis, “N. Giannettasio” Hospital, Rossano Calabro, Cosenza); D. Landau, MD (Division of Pediatric Nephrology, Soroka Medical Center, Beer-Sheba, Israel); O. Amatruda, MD (Division of Nephrology, “Fondazione Macchi” Hospital, Varese); E. Pogliani, MD, D. Belotti, BiolSciD (Division of Hematology and Transfusional Center, “S. Gerardo” Hospital, Monza, Milano); M. Sanna, MD (Division of Medical Pathology University Hospital, Sassari); R. Coppo, MD, A. Amore, MD (Division of Nephrology and Dialysis, “Regina Margherita” Pediatric Hospital, Torino); T. Ring, MD (Department of Nephrology, Aalborg Hospital, Aalborg, Denmark); P. Ponce, MD (Hospital “Garcia de Orta,” Almada, Portugal); R. Bellantuono, MD, T. De Palo, MD (Division of Nephrology and Dialysis, Pediatric Hospital “Giovanni XXIII,” Bari); T. Barbui, MD (Division of Hematology, “Ospedali Riuniti, Azienda Ospedaliera,” Bergamo); R. Wens, MD (Clinique de Nephrologie-Dialyse “CHU-Brugmann,” Bruxelles, Belgium); J. Ferraris, MD (Division of Nephrology, “Hospital Italiano de Buenos Aires,” Buenos Aires, Argentina); A. Cao, MD (Istituto di Clinica e Biologia dell'Età Evolutiva, Cagliari); V. Kimonis, MD (Department of Pediatrics, SIU School of Medicine, Springfield, IL); A. Bettinelli, MD (Pediatric Unit, “San Leopoldo Mandic” Hospital, Merate, Lecco); V. Toschi, MD (Transfusional Center, “San Carlo Borromeo” Hospital, Milano); E. Trabassi, MD (Division of Nephrology and Dialysis, “San Massimo” Hospital, Penne, Pescara); F. Mandelli, MD, A. Amendola, MD (Hematology Unit, Department of Human Biopathology, “LaSapienza” Univer-sity, Roma); A. Pinto, MD (Division of Nephrology and Dialysis, “S.G. di Dio e Ruggi d'Aragona” Hospital, Salerno); E. Nesti, MD (Division of Nephrology and Dialysis, “S Miniato” Hospital, Firenze); A. Khaled, MD (Division of Nephrology, “S. Chiara” Hospital, Trento); L. Tavecchia, MD (Division of Hematology, “Borgo Roma” Hospital, Verona).
Laboratory analysis.
F. Gaspari, ChemD (Clinical Research for Rare Diseases “Aldo e Cele Daccò,” Ranica, Bergamo); C. Ottomano, MD, A. Vernocchi, MD, A. Crippa, MD (Division of Laboratory Analysis, “Ospedali Riuniti, Azienda Ospedaliera,” Bergamo).
Biochemical studies.
M. Galbusera, BiolSciD, S. Gastoldi, Chemist, S. Contaretti, BiolSciD, J. Caprioli, BiolSciD, P. Bettinaglio, BiolSciD, B. Amadei, BiolSciD, D. Macconi, BiolSciD (“M. Negri” Institute for Pharmacological Research, Bergamo, Italy); M. T. Canciani, BiolSciD (“A. Bianchi Bonomi” Hemophilia and Thrombosis Center, IRRCS Maggiore Hospital and University of Milan, Italy); P. F. Zipfel, MD (Hans Knoell Institute for Natural Products Research, Jena, Germany).
Statistical analysis.
A. Perna, StatSciD (Clinical Research for Rare Diseases “Aldo e Cele Daccò,” Ranica, Bergamo).
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2001-12-0166.
Supported in part by Orphan Europe s.r.l. S.C. is the recipient of a fellowship from Associazione Ricerca Malattie Rare (ARMR, Bergamo). J.C. is the recipient of the Nando Peretti fellowship through the Associazione Ricerca Malattie Rare (ARMR, Bergamo).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Miriam Galbusera, Mario Negri Institute for Pharmacological Research, Via Gavazzeni 11, 24125 Bergamo, Italy; e-mail: galbusera@marionegri.it.