Abstract
We retrospectively analyzed results for 154 patients with acquired severe aplastic anemia who received bone marrow transplants between 1993 and 2000 from unrelated donors identified through the Japan Marrow Donor Program. Patients were aged between 1 and 46 years (median, 17 years). Seventy-nine donor-patient pairs matched at HLA-A, -B, and -DRB1 loci, as shown by DNA typing. Among the 75 mismatched pairs, DNA typing of 63 pairs showed that 51 were mismatched at 1 HLA locus (18 HLA-A, 11 HLA-B, 22 HLA-DRB1) and 12 were mismatched at 2 or more loci. Seventeen patients (11%) experienced either early or late graft rejection. The incidence of grade III/IV acute graft versus host disease and chronic graft versus host disease was 20% (range, 7%-33%) and 30% (range, 12%-48%), respectively. Currently, 99 patients are alive, having survived for 3 to 82 months (median, 29 months) after their transplantations. The probability of overall survival at 5 years was 56% (95% confidence interval, 34%-78%). Multivariate analysis revealed the following unfavorable factors: transplantation more than 3 years after diagnosis (relative risk [RR], 1.86; P = .02), patients older than 20 years (RR, 2.27; P = .03), preconditioning regimen without antithymocyte globulin (RR 2.28; P = .04), and HLA-A or -B locus mismatching as determined by DNA typing. Matching of HLA class I alleles and improvement of preparative regimens should result in improved outcomes in patients with severe aplastic anemia who receive transplants from unrelated donors.
Introduction
Bone marrow transplantation (BMT) from an HLA-matched family donor is the treatment of choice for children and young adults with severe aplastic anemia (SAA).1 However, this approach is limited by the availability of HLA-matched donors. Immunosuppressive therapy (IST) with a combination of antithymocyte globulin (ATG) and cyclosporine has been an alternative treatment for patients without a suitable related donor.2-4 BMT from a phenotypically HLA-matched unrelated donor is indicated as salvage therapy for patients who fail to respond to one or more courses of IST and patients who experience relapse of disease.5,6 Initial attempts to use unrelated donor BMT for patients with SAA were hampered by the high incidence of graft failure and graft versus host disease (GVHD); survival rates ranged from 20% to 54%.7-10
An optimum conditioning regimen, GVHD prophylaxis, and better donor selection are considered to be indispensable for improving outcomes for patients who receive unrelated donor BMT. Here we report a detailed analysis of outcomes for 154 SAA patients who underwent BMT from an unrelated donor identified through the Japan Marrow Donor Program (JMDP). This report represents one of the largest series of unrelated donor BMT for SAA. Moreover, results of high-resolution HLA typing were available for more than 90% of the recipient-donor pairs in this study. We attempted to clarify the effect of HLA mismatching as determined by high-resolution DNA typing. Our results suggest that higher survival rates are attained when recipients are young, transplantation is performed soon after diagnosis, an ATG-containing regimen is used, and HLA-A or -B locus–mismatched donors are not used.
Patients, materials, and methods
Patients
From February 1993 to April 2000, 154 consecutive patients with acquired SAA received bone marrow transplants from unrelated donors at 68 centers in Japan. All donors were identified through the JMDP.11 Characteristics of patients and donors are summarized in Table 1. Disease was considered severe if at least 2 of the following were noted: neutrophil count less than 0.5 × 109/L, platelet count less than 20 × 109/L, or reticulocyte count less than 20 × 109/L with a hypocellular marrow.4Patients were aged between 1 and 46 years (median 17 years). Most of the patients failed to respond to conventional ISTs and had been dependent on transfusions of red blood cells or platelets. All patients or their guardians gave informed consent for transplantation and submission of data to the JMDP.
Transplantation procedure
Characteristics of the transplantation procedures are listed in Table 1. The various preconditioning regimens used by individual centers classified into 5 categories: total body irradiation (TBI; 2-10 Gy) plus cyclophosphamide (CY; 120-200 mg/kg) plus ATG (n = 56);12 limited field irradiation (LFI; 5-8 Gy) plus CY plus ATG (n = 26); TBI plus CY (n = 51); LFI plus CY (n = 13); and others (n = 8). In 113 patients (73%), cyclosporine (CSA) and methotrexate (MTX) were used for prophylaxis against GVHD according to the Seattle protocol. Twenty-one patients (14%) received tacrolimus instead of CSA. Ex vivo T-cell depletion was not used for any of the patients. All but 5 patients received recombinant human granulocyte colony-stimulating factor to facilitate recovery of neutrophils.
Recipient-donor HLA matching
The HLA matching between recipient and donor was based on HLA serotyping according to the standard technique. Until 1997, the JMDP approved serologically determined 6 of 6 matched donors as suitable for corresponding patients. Since 1998, 5 patients have received transplants from donors serologically determined to be mismatched at 1 antigen. In 142 (92%) of the present 154 recipient-donor pairs, molecular analyses of HLA-A, -B, and -DRB1 loci were performed by DNA-based methods either prior to transplantation or afterward using pretransplantation samples.13
Data collection and statistical analysis
Transplantation data were collected using standardized forms provided by the JMDP. Patient baseline information and follow-up report were submitted at 100 days, 6 months, 1 year, and annually after transplantation. Analysis of patient outcome was performed using the date of last reported follow-up or the date of death. The median length of follow-up was 29 months (range, 3-82 months). Data were analyzed as of July 1, 2000.
In evaluation of engraftment, patients who died before day +22 without engraftment were not considered evaluable. Patients who failed to engraft were excluded from analyses of acute and chronic GVHD. Probability of overall survival and acute and chronic GVHD was estimated from the time of transplantation according to the Kaplan-Meier product-limit method.14 In univariate analysis, the χ2 test and log-rank statistics were used to assess significance of differences in outcome among various groups. In multivariate regression analysis of risk factors for graft rejection, acute and chronic GVHD, and 5-year survival, the Cox proportional hazards model was used. Conditioning regimens and HLA matching were each classified into 2 major categories. The following variables were evaluated: donor and recipient age, sex, disease duration before BMT, number of pretransplantation transfusions, marrow cell doses, methods of prophylaxis against GVHD, ATG-containing regimen (yes/no), TBI-containing regimen (yes/no), HLA-A or -B locus matching (yes/no), and HLA-DRB1 locus matching (yes/no). In analysis of chronic GVHD, history of grade II-IV acute GVHD was included as an additional variable.
Results
HLA matching by DNA typing
Of the 142 recipient-donor pairs evaluated by DNA typing in this study, 79 were found to be matched at HLA-A, -B, and -DRB1 loci. Among the 63 mismatched pairs, 51 were mismatched at 1 HLA locus (18 HLA-A locus, 11 HLA-B locus, 22 HLA-DRB1 locus), 11 were mismatched at 2 HLA loci, and 1 was mismatched at 3 HLA loci.
Engraftment and late graft rejection
Engraftment was defined as achievement of a peripheral blood absolute neutrophil count of more than 0.5 × 109/L for 3 consecutive days. The median time to engraftment was 18 days, ranging from 10 to 70 days. Seven (5%) of the 144 evaluable patients failed to engraft. Late graft rejection was defined as development of sustained severe neutropenia (absolute neutrophil count < 0.5 × 109/L) and hypocellular bone marrow in patients who had previously engrafted. Late graft rejection occurred in 10 (8%) of the 137 engrafted patients; thus, 17 patients (11%) experienced either early or late graft rejection. In univariate analysis, 2 variables were associated with graft failure: recipient age and conditioning regimen (Tables 2 and3). In multivariate regression analysis, relative risk was much higher in patients conditioned with non-TBI regimens than in patients conditioned with TBI-containing regimens (relative risk, 3.84; 95% confidence interval [CI], 1.26-12.4; P = .01).
Acute GVHD
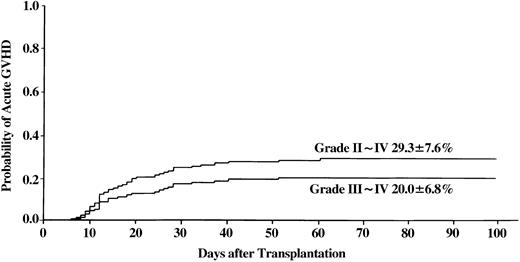
Figure 1 shows the incidence at 100 days of grade II-IV acute GVHD (29%, range 14%-44%) and grade III/IV acute GVHD (20%, range 7%-33%). In univariate analysis, none of the covariates were statistically significant. Multivariate analysis revealed that significantly higher incidence of grade III/IV acute GVHD was associated with conditioning regimens without ATG and use of an HLA-A or -B locus–mismatched donor (Table4).
Incidence of acute GVHD in patients with SAA who received transplants from unrelated donors.
Incidence of acute GVHD in patients with SAA who received transplants from unrelated donors.
Chronic GVHD
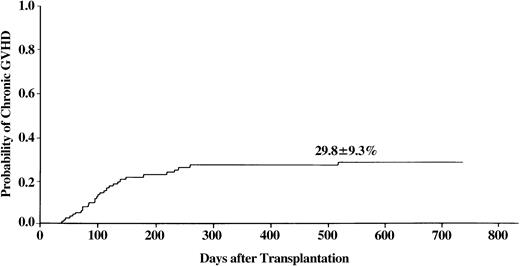
At 2 years after BMT, overall incidence of chronic GVHD was 30% (range, 12%-48%) (Figure 2). Of the patients with chronic GVHD, 51% had extensive type. In univariate analysis, a history of prior grade II-IV acute GVHD was associated with higher incidence of chronic GVHD (53% vs 18%, P = .001). This finding was confirmed by multivariate analysis. Mismatch at the HLA-A or -B locus was another statistically significant risk factor (Table 4).
Incidence of chronic GVHD in patients with SAA who received transplants from unrelated donors.
Incidence of chronic GVHD in patients with SAA who received transplants from unrelated donors.
Survival
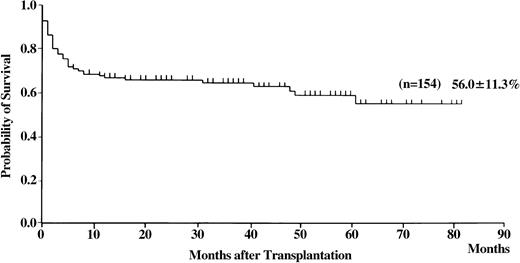
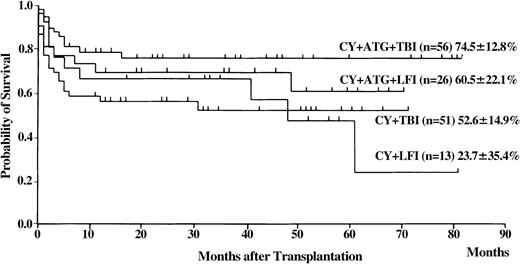
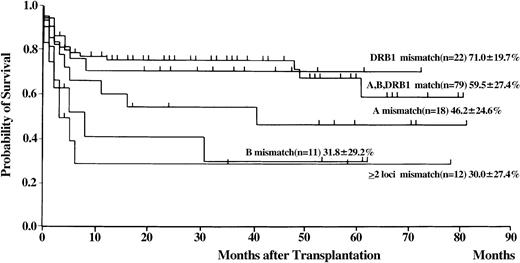
Of the 154 patients, 99 are presently alive, 3 to 82 months (median 29 months) after transplantation. The probability of overall survival at 5 years was 56% (Figure3; 95% CI, 34%-78%). Univariate analysis revealed the following statistically significant variables: recipient and donor age, donor sex, recipient/donor sex, disease duration before BMT, and HLA matching (Tables 2 and 3). The conditioning regimen that included CY, ATG, and TBI resulted in the highest probability of survival (Figure4). Probability of survival did not differ between recipients of transplants from donors that matched at HLA-A, -B, and -DRB1 loci and those who received transplants from HLA-DRB1–mismatched donors (Figure 5). Multivariate analysis revealed the following unfavorable variables: older recipient, late BMT, non-ATG–containing regimen, and HLA-A or -B locus mismatching, as determined by DNA typing (Table 4). Mismatching of HLA-DRB1 did not result in increased mortality.
Probability of survival in patients with SAA who received transplants from unrelated donors.
Probability of survival in patients with SAA who received transplants from unrelated donors.
Probability of survival for various conditioning regimens in patients with SAA who received transplants from unrelated donors.
Probability of survival for various conditioning regimens in patients with SAA who received transplants from unrelated donors.
Probability of survival according to HLA matching on the basis of DNA typing in patients with SAA who received transplants from unrelated donors.
Probability of survival according to HLA matching on the basis of DNA typing in patients with SAA who received transplants from unrelated donors.
Causes of death are summarized in Table5. Graft failure, bacterial/fungal infections, and acute GVHD were major causes of death. The performance status at the time of the last follow-up was measured using the Karnofsky activity score. Of the 94 evaluable patients, 85 (90%) had a normal or nearly normal activity score (Karnofsky assessment, 90%-100%). The scores of the remaining 9 patients ranged from 40% to 80%.
Discussion
The survival rate for BMT from an HLA-matched sibling has increased substantially over the past 20 years for patients with SAA. Five-year survival rates as high as 90% have recently been reported.15-17 In contrast, results of unrelated donor BMT have generally been unsatisfactory for SAA.7-10
In this large series of unrelated donor BMT for SAA, we attempted to identify prognostic factors that may be useful for improving outcomes of patients. Better donor selection based on molecular HLA typing appears to be one of the most important variables for improvement of survival rate.18,19 HLA-A, -B, and -DRB1 genotypic mismatches were found in 63 (44%) of the 142 donor-recipient pairs that were matched for HLA-A, -B, and -DR by serology in our series. The results of the current study are consistent with those of a previous JMDP study in which only 10% of the patients had SAA. In the latter study, mismatching of class I HLA alleles between donor and recipient was found to be a strong risk factor for GVHD and survival,13 whereas mismatching of HLA class II alleles did not have a significant effect on outcomes of the patients. In the National Marrow Donor Program (NMDP) study, mismatching of HLA-DRB1 was found to be the most crucial risk factor for survival.20,21 Patients who received transplants from donors with matching DRB1 alleles had significantly better outcomes than DRB1-mismatched patients: 60% versus 15% survival at 3 years. In the current JMDP study, the 5-year survival rate was 56% for the 79 patients who received transplants from HLA-A–, -B–, and -DRB1–matched donors, compared with 71% for the 22 patients who received transplants from donors matched at HLA-A and -B and mismatched at HLA-DRB1 (P = .95). The differences between the results of the JMDP study and those of the NMDP study may be due to differences in the roles of individual HLA loci in unrelated donor BMT in different ethnic groups.22 Restricting BMT to donor-recipient pairs perfectly matched at molecular levels will exclude many patients from candidacy for unrelated donor BMT. The current study demonstrated that mismatching of DRB1 alleles is acceptable for Japanese patients, and this finding could increase the number of possible unrelated BMT donor-recipient pairs.
Large registration studies have shown that the inclusion of irradiation in preparative conditioning can reduce graft rejection.23Both LFI and TBI are used for patients with SAA. Our results clearly show a lower risk of graft rejection for transplantation patients who received a TBI regimen, compared with those who received a non-TBI regimen. In the present study, CY (120 mg/kg) plus TBI (10 Gy) plus ATG and, also, CY (200 mg/kg) plus LFI (7.5 Gy) plus ATG were the most frequently used regimens, although other doses were also used. Only 1 (3%) of the 30 patients (17 HLA-matched, 13 HLA-mismatched) experienced graft rejection in the CY/TBI/ATG group, whereas 5 (21%) of the 24 patients in the CY/LFI/ATG group (15 HLA-matched, 6 HLA-mismatched, 3 HLA not tested by molecular techniques) failed to engraft. A high-dose TBI-containing regimen is effective in ensuring engraftment, but there is growing concern about regimen-related morbidity and mortality. Moreover, long-term complications such as cataracts and endocrine dysfunction are frequent in patients who received TBI.24 In light of these concerns, the NMDP conducted a prospective study to determine the minimal dose of TBI sufficient to achieve sustained engraftment when it was used in combination with CY (200 mg/kg) and ATG.25 The doses of TBI were as follows: first cohort of patients, 2 Gy × 3; second cohort, 2 Gy × 2; subsequent cohorts, 2 Gy × 1. All 30 patients who received 6 Gy or 4 Gy of TBI achieved engraftment, and 17 of those patients are presently alive. Of the 13 patients who received 2 Gy of TBI, 1 failed to engraft and 8 are presently alive. Pulmonary toxicity occurred in 8 of the 30 patients who received 6 Gy or 4 Gy of TBI and 2 of the 13 patients who received 2 Gy of TBI. The authors of the NMDP study concluded that a TBI dose of 2 Gy was sufficient to allow engraftment without increasing toxicities for perfectly HLA-matched pairs.
In evaluating the effects and toxicities of TBI, we must be mindful that observed effects and toxicities could be related to interactions among CY, TBI, and ATG. In our study, 2 of the 3 patients (2 HLA-matched, 1 HLA-mismatched) who were conditioned with CY (120 mg/kg) plus low-dose TBI (5 or 8 Gy) failed to engraft, and 3 patients died. In contrast, 10 patients (6 HLA-matched, 4 HLA-mismatched) who were conditioned with (200 mg/kg) plus low-dose TBI (2 to 5 Gy) plus ATG engrafted, and 9 are presently alive. These results extended the findings of the NMDP TBI study by demonstrating that low-dose TBI can be sufficient to allow engraftment in patients who receive a transplant even from an HLA-nonidentical donor.
The combined effects of these favorable prognostic factors result in a 5-year survival rate of 88%, which is comparable to the best results achieved by SAA patients who received transplants from HLA-matched siblings. Given the present encouraging results, we recommend unrelated donor transplantation as a salvage therapy in nonresponders to IST in children and young adults.
The following centers participated in the bone marrow transplantations for SAA facilitated by the JMDP: Hokkaido University School of Medicine; Sapporo Medical University; Sapporo Hokuyu Hospital; Tohoku University School of Medicine; Hirosaki University School of Medicine; Iwate Medical University School of Medicine; Yamagata University Hospital; Akita University School of Medicine; Fukushima Medical College; Toho University School of Medicine, Omori Hospital; Tokyo University Hospital; Tokyo Metropolitan Komagome Hospital; Nihon University School of Medicine, Itabashi Hospital; Keio University; Tokyo Medical College Hospital; Tokyo Medical and Dental University Hospital, Faculty of Medicine; Yokohama City University Hospital; Kanagawa Children's Medical Center; Tokai University Hospital; Chiba University School of Medicine; Chiba Municipal Hospital; Chiba Children's Hospital; Saitama Children's Medical Center; Saitama Cancer Center Hospital; Saitama Medical School; Ibaraki Children's Hospital; Saiseikai Maebashi Hospital; Shinshu University School of Medicine; Saku Central Hospital; Shizuoka General Hospital; Shizuoka Children's Hospital; Japanese Red Cross Nagoya First Hospital; Meitetsu Hospital; Nagoya University Hospital; Nagoya Ekisaikai Hospital; Kouseiren Showa Hospital; Mie University Hospital; Kanazawa University School of Medicine; Toyama Prefectural Central Hospital; Shiga University of Medical Science; Kinki University School of Medicine; Osaka University School of Medicine; Osaka Medical Center and Research Institute for Maternal and Child Health; Matsushita Memorial Hospital; Hyogo College of Medicine; Hyogo Medical Center for Adults; Kobe University School of Medicine; Kyoto University Hospital; Kyoto Municipal Hospital; Okayama University Medical School; Tottori University Hospital; Hiroshima Red Cross Hospital and Atomic-bomb Survivors Hospital; Ehime Prefectural Central Hospital; Kurashiki Central Hospital; Hamanomachi General Hospital; National Kyushu Cancer Center; Kokura Memorial Hospital; Saga Prefectural Hospital; Miyazaki Prefectural Hospital; and Kagoshima University Hospital.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Seiji Kojima, Dept of Developmental Pediatrics, Nagoya University School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya 466-8550, Japan; e-mail: kojimas@med.nagoya-u.ac.jp.