Abstract
Using in vitro progenitor assays, serum-free in vitro cultures, and the nonobese diabetic/severe combined immune-deficient (NOD/SCID) ecotropic murine virus knockout xenotransplantation model to detect human SCID repopulating cells (SRCs) with multilineage reconstituting function, we have characterized and compared purified subpopulations harvested from the peripheral blood (PB) of patients receiving granulocyte colony-stimulating factor (G-CSF) alone or in combination with stem cell factor (SCF). Mobilized G-CSF plus SCF PB showed a 2-fold increase in total mononuclear cell content and a 5-fold increase in CD34-expressing cells depleted for lineage-marker expression (CD34+Lin−) as compared with patients treated with G-CSF alone. Functionally, G-CSF plus SCF–mobilized CD34+CD38−Lin−cells contained a 2-fold enhancement in progenitor frequency as compared with G-CSF–mobilized subsets. Despite enhanced cellularity and progenitor capacity, G-CSF plus SCF mobilization did not increase the frequency of SRCs as determined by limiting dilution analysis by means of unfractionated PB cells. Purification of SRCs from these sources demonstrated that as few as 1000 CD34+CD38−Lin− cells from G-CSF–mobilized PB contained SRC capacity while G-CSF plus SCF–mobilized CD34+CD38−Lin−cells failed to repopulate at doses up to 500 000 cells. In addition, primitive CD34−CD38−AC133+Lin−cells derived from G-CSF plus SCF–mobilized PB were capable of differentiation into CD34-expressing cells, while the identical subfractions from G-CSF PB were unable to produce CD34+cells in serum-free cultures. Our study defines qualitative and quantitative distinctions among subsets of primitive cells mobilized by means of G-CSF plus SCF versus G-CSF alone, and therefore has implications for the utility of purified repopulating cells from these sources.
Introduction
Intensive chemotherapeutic treatment together with subsequent transplantation of autologous hematopoietic support has provided beneficial outcomes for several hematopoietic and nonhematopoietic malignancies such as lymphoma and multiple myeloma.1-4 Mobilized peripheral blood (MPB) has largely replaced bone marrow (BM) as a source of hematopoietic repopulating cells for autologous transplantation, primarily owing to the rapid hematological recovery and ease of collection associated with mobilization.2,3,5 The time to hematopoietic recovery can be correlated with the absolute number of mobilized CD34+progenitor cells infused, and recent studies have established the infusion of more than 5 × 106 CD34+ cells per kilogram in consistent and rapid engraftment in a large proportion of patients.6 7
A number of hematopoietic growth factors and cytokines, including granulocyte colony-stimulating factor (G-CSF),8,9granulocyte-macrophage CSF (GM-CSF),10 and stem cell factor (SCF),11,12 used for mobilization have been shown to expand hematopoietic progenitor cells in vitro. Although recombinant human SCF alone exerts little colony-stimulating activity on human BM-derived cells in vitro, combination with other recombinant hematopoietic growth factors results in a synergistic increase in colony formation.13 In primate and mouse models, in vivo administration of SCF and G-CSF also has a synergistic effect that increases peripheral blood (PB) progenitor cell mobilization as compared with G-CSF alone.13,14 On the basis of these encouraging results, several clinical trials have been initiated; they reported the ability of the combination of SCF with G-CSF to successfully mobilize PB progenitor cells in patients.15-18 Treatment with a combination of SCF and G-CSF also resulted in significantly improved CD34+ cell yield, with a concomitant reduction in the amount of leukapheresis product required to collect an optimal harvest of 5 × 106 CD34+ cells per kilogram as compared with G-CSF mobilization alone.15 In addition, the combination of SCF and G-CSF was well tolerated and effective in achieving rapid engraftment even in heavily pretreated lymphoma and multiple myeloma patients at risk of poor mobilization.15 17
Despite the early success of clinical trials evaluating the effectiveness of mobilization of progenitor cells with the use of the combination of SCF and G-CSF, a detailed analysis of the repopulating capacity of human cells mobilized by G-CSF alone or by SCF in combination with G-CSF and their respective subsets has not been examined. With the use of an in vivo assay for primitive human hematopoietic cells capable of repopulation,19-22 previous studies have used limiting dilution analysis (LDA) to compare the frequency of repopulating cells harvested from various human hematopoietic sources such as umbilical cord blood, adult BM, MPB, and fetal tissue.23,24 Verfaillie and colleagues25 compared the short-term and long-term repopulating characteristics of G-CSF–mobilized BM and PB cells in the human–fetal sheep xenotransplantation model; they demonstrated that mobilized CD34+ cells, when serially transferred into secondary and tertiary recipients, supported earlier development of human hematopoiesis and earlier exhaustion of human hematopoiesis. These data suggested fundamental differences in the repopulating potential of MPB cells that were independent of cell phenotype used to identify human repopulating stem cells.25 Given the increased use of SCF in combination with G-CSF in patients,15-18 it is imperative to understand the effects these growth factors have on the biological function of recently characterized stem/progenitor subsets mobilized.
Here, we have used the nonobese diabetic/severe combined immune-deficient (NOD/SCID) assay for detection of human SCID repopulation cells (SRCs) and have compared the engraftment potential of both mixed and purified populations of cells harvested from patients mobilized with G-CSF alone versus G-CSF in combination with SCF. G-CSF combined with SCF effectively increased total CD34+lineage-depleted (Lin−) cell content and clonogenic progenitor capacity, as compared with MPB G-CSF treatment alone. However, when cells were transplanted into the NOD/SCID mouse, this increase in total CD34+ expression did not correlate with an increased frequency of SRCs. Furthermore, highly purified subfractions of primitive CD34− CD38−AC133+Lin−cells derived from G-CSF plus SCF–mobilized PB were capable of differentiation into CD34-expressing cells, while similar subfractions of G-CSF–mobilized cells were unable to produce CD34+cells in identical cultures. Our study is the first to show that phenotypically purified subpopulation cells harvested after treatment are functionally heterogeneous with growth factors that alter repopulation, progenitor capacity, and differentiation. These findings have important implications for future clinical transplantation of purified cell populations and for the design of in vitro cell purging and stem cell expansion strategies following patient mobilization with factors.
Patients, materials, and methods
Patient samples
Patient samples were selected as a part of a phase II open-label multicenter trial designed to optimize parameters for PB apheresis following G-CSF and SCF treatment for multiple myeloma. The study protocol was approved by the ethical review at each participating institution, and all patients gave informed written consent before study enrollment. Eligibility requirements included patients with high-risk multiple myeloma (stage I with bone lesions, stage II, or stage III) according to the Durie and Salmon classification.26 The cohort for this study included non–heavily pretreated patients, defined as having received 6 or fewer cycles of chemotherapy, with no more than 2 of these cycles containing melphalan and nitrosoureas. Laboratory requirements included an absolute neutrophil count exceeding 1.5 × 109/L; platelets exceeding 100 × 109/L, serum creatinine below 150 μM; and bilirubin, aspartate transferases, and alanine aminotransferases less than twice the upper limit of normal. Patients with multiple myeloma in persistent relapse (not responsive to second-line chemotherapy) and those with a concurrent malignancy were excluded from the study. Other exclusion criteria were uncontrolled infection, significant nonmalignant disease, being pregnant or breastfeeding, or having a known allergy to Escherichia coli–derived products.
Human cells
The mobilization regimen consisted of 2.5 g/m2cyclophosphamide administered intravenously on day 0, followed by 20 μg/kg/d SCF (Amgen, Mississauga, ON, Canada) plus 10 mg μg/kg/d G-CSF (Amgen) administered subcutaneously, from day 1 to completion of apheresis (day 11). Owing to the possibility of allergic reactions, patients were premedicated with ranitidine and salbutamol before each administration of SCF. Leukapheresis was performed on day 11 after treatment. Approximately 3 to 5 mL concentrated leukapheresis product was diluted in phosphate-buffered saline (PBS) (1:10), and mononuclear cells (MNCs) were collected by centrifugation on Ficoll-Paque (Pharmacia, Piscataway, NJ) as described previously.27Cells were harvested from patients who achieved a total cell dose exceeding 5 × 106 CD34+ cells per kilogram. Mobilized MNCs were cryopreserved in 90% fetal bovine serum (FBS) with 10% dimethylsulfoxide (Gibco BRL, Burlington, ON, Canada) added dropwise; they were stored at a concentration of 50 × 106 cells per milliliter in liquid nitrogen. Frozen cells were thawed on the day of experimentation and washed in serum-free Iscoves modified Dulbecco medium (IMDM) (Gibco BRL). Cell viability was assessed by means of trypan-dye exclusion and 7-AAD staining by fluorescence-activated cell sorting (FACS) analysis. Clinical follow-up of individual patients after transplantation is currently ongoing.
Purification of primitive cell populations
Lin− cells were isolated from mobilized MNCs by means of a standard immunomagnetic protocol.19 Briefly, MNCs were stained with a cocktail of 9 lineage-specific antibodies (CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, and glycophorin A) followed by a secondary antibody conjugated to metal colloid. For cell culture experiments in which CD34+CD38−Lin− or CD34−CD38−AC133+Lin−cells were to be isolated, CD33 and CD38 antibodies were added to the lineage-depletion cocktail. Lin− cells were purified by negative selection by means of a StemSep device as described by the manufacturer (Stem Cell Technologies, Vancouver, BC, Canada).19 Lin− cells were stained with fluoroscein isothiocyanate (FITC)–conjugated human anti-CD34, allophycocyanin-conjugated human anti-CD38 (Becton Dickinson, San Jose, CA), and phycoerythrin (PE)–conjugated human anti-AC133 (Miltenyi Biotechnology, Auburn, CA). Primitive CD34+Lin−, CD34+CD38−Lin−, or CD34−CD38−AC133+Lin−cells were isolated by flow cytometry by means of a Vantage SE cell sorter (Becton Dickinson).20,28 Sorting gates were established on Lin− cells stained with isotype immunoglobulin G1 (IgG1) conjugated to the appropriate fluorochromes (Becton Dickinson) as shown previously.28
Transplantation of human cells into NOD/SCID mice
MPB MNCs or selected subpopulations from Lin−populations were transplanted into sublethally irradiated (340 to 375 cGy with the use of a 137Cs γ-irradiator) 8- to 10-week-old NOD/LtSz-scid/scid (NOD/SCID) ecotropic murine virus knockout (EMVnull) (Jackson Laboratories, Bar Harbor, MA) mice according to our standard protocol.19 Mice that received transplants of purified cell populations were supplemented with 105 irradiated (1500 cGy with the use of a cobalt source) Lin+ accessory cells as shown previously.29 NOD/SCID EMVnull mice were bred in the defined flora barrier facility at the Robarts Research Institute (London, ON, Canada). The Animal Care committee at the Robarts Research Institute and at the University of Western Ontario (London, ON, Canada) approved animal experiments. Mice were maintained under sterile conditions in microisolator cages in a ventilated rack and were killed 6 to 8 weeks after transplantation. BM cells were harvested from the femurs, tibiae, and iliac crests of animals and suspended in IMDM according to our standard protocols.28 30
Flow cytometric analysis of mouse BM
To prepare mouse BM cells for flow cytometric analysis, red cells were lysed by means of 0.8% ammonium chloride solution, and the remaining cells were washed in PBS containing 5% FBS. Approximately 106 cells were incubated for 30 minutes at 4°C with human panleukocyte-specific marker anti-CD45–FITC in combination with human anti-CD38–PE (Becton Dickinson) or isotype controls. Cells were then washed 3 times in PBS plus 5% FCS and analyzed by flow cytometry on a FACSCalibur with CellQuest software (Becton Dickinson). Analysis of multilineage engraftment was performed on mouse BM that demonstrated a high level of human engraftment (greater than 10% human). Briefly, 105 cells from mouse BM were stained and gated for human cells (anti-CD45–peridinin chlorophyll protein (PerCP) and analyzed for B-lymphoid cells (anti-CD20–FITC, anti-CD19–PE); myeloid cells (anti-CD33–FITC, anti-CD15–PE); primitive cells (anti-CD34–FITC, anti-CD38–PE); and T-lymphoid cells (anti-CD4–FITC, anti-CD8–PE) (all antibodies from Becton Dickinson).
Analysis of human cell engraftment
High–molecular-weight DNA was also isolated from the BM of mice that received transplants by means of phenol/chloroform extraction or DNAzol reagent (Gibco BRL) according to the manufacturer's specifications. The proportion of human cells in the mouse BM was determined by Southern blot analysis with the use of a human chromosome 17–specific α-satellite probe (p17H8)31 as described previously.22 The level of human cell engraftment was quantified by analysis of Southern blots with the use of a phosphoimager and ImageQuant software (Molecular Dynamics, Sunnyvale, CA) by comparing the characteristic 2.7-kilobase (kb) band with human-to-mouse DNA mixture controls (limit of detection, approximately 0.1% human DNA).
Colony-forming unit assays
Human clonogenic progenitor cell assays were performed by plating human cells into Methocult H4434 (Stem Cell Technologies) containing 50 ng/mL recombinant human SCF (rhSCF) (Amgen), 10 ng/mL rhGM-CSF, 10 ng/mL rh–interleukin-3 (rhIL-3), and 3 U/mL rh-erythropoietin (all from R&D Systems, Minneapolis, MN). Differential colony counts were assessed following incubation for 10 to 14 days at 37°C and 5% CO2 in a humidified atmosphere as shown previously.20 28
Serum-free in vitro cultures
De novo isolated CD34+CD38−Lin− and CD34−CD38−AC133+Lin−cell populations (200 to 1500 cells) were directly isolated by FACS analysis prior to seeding in serum-free liquid culture. Serum-free cultures contained medium consisting of 9500 bovine serum albumin, insulin, and transferrin (BIT) (Stem Cell Technologies) supplemented with 10−4 M β-mercaptoethanol and 2 mM l-glutamine (Gibco BRL) in combination 300 ng/mL rhSCF, 50 ng/mL rhG-SCF (Amgen), 300 ng/mL rhFlt-3, 10 ng/mL rhIL-3, and 10 ng/mL rhIL-6 (R&D Systems) (complete growth factor media) as optimized previously for both CD34+ and CD34−primitive human cells.20,28,32 Cells were plated in fibronectin-coated 96-well plates (Becton Dickinson) at 37°C and 5% CO2. Fresh complete growth factor medium was replenished every 2 to 3 days. After 10 days of culture, viable cells were counted and analyzed for CD34, CD38, and AC133 expression by flow cytometry as described previously.28 30
Statistics
Levels of human engraftment were shown as the mean ± SEM for mice grouped according to the number of transplanted cells. The frequency of SRCs was determined by LDA as described previously.19,24 Briefly, a mouse that had received transplants was scored as positive (engrafted) if any human cells were detectable by Southern blot. The data from several transplanted doses were grouped and analyzed by applying Poisson statistics with the use of the single-hit model. The frequency of SRCs in a population of cells was calculated by means of the maximum likelihood estimator, with the assumption of a normal distribution.19 24 Analysis of statistical significance for colony-forming unit (CFU) data (frequency of colonies ± SEM) was performed by a 2-tailed Studentt test with the use of Microsoft Excel software.
Results
G-CSF plus SCF mobilization increased the frequency and total number of CD34+ cells
Clinical trials have reported the ability of the combination of SCF with G-CSF to successfully mobilize PB, resulting in significantly improved CD34+ cell yield with a concomitant reduction in the number of leukapheresis procedures required to collect a harvest of 5 × 106 CD34+ cells per kilogram as compared with G-CSF mobilization alone.15 Direct comparison of MPB cells from 4 patients for each mobilization regime confirmed that G-CSF plus SCF mobilization was more effective at mobilizing leukocytes into the periphery and that it improved leukapheresis cell harvests for autologous transplantation (Table 1), results that are consistent with those shown in previous studies.3,15,33 The total number of leukocytes collected per leukapheresis were increased in G-CSF plus SCF–mobilized PB (12.6 ± 4.3 × 1010 cells) when compared with G-CSF–mobilized PB (8.8 ± 2.2 × 1010 cells). The increase in total cellularity was observed despite a decrease in the mean volume of leukapheresis product collected from G-CSF plus SCF–mobilized PB (Table 1). G-CSF plus SCF leukapheresis product contained increased total MNCs (P < .05) and higher numbers of MNCs per milliliter of leukapheresis product (P < .01) when compared with G-CSF treatment (Table 1). G-CSF plus SCF leukapheresis product also contained an increased frequency of total CD34+ cells (4.1% ± 1.2%) when compared with G-CSF–mobilized MNCs (1.2% ± 0.5%) (P < .05) (Table 1), resulting in a significant increase in the total number of CD34+ cells per milliliter of leukapheresis product (P < .05). The increase in overall cellularity and increased frequency of CD34+ cells resulted in a 5-fold increase in the total number of CD34+ cells obtained for reinfusion after G-CSF plus SCF mobilization as compared with G-CSF alone (Table 1).
SRC function of transplanted MNCs obtained from G-CSF plus SCF–mobilized PB is similar to that of G-CSF–mobilized PB
Although total CD34+ cell content has been suggested as a predictor for short-term engraftment and hematologic recovery,6,15,34 this measure is unable to quantify the long-term repopulating function of human stem cell populations mobilized under different conditions. Accordingly, we performed a quantitative analysis of human stem cells capable of multilineage reconstitution in NOD/SCID EMVnull mice and compared the frequency and total number of these repopulating cells within the G-CSF– and G-CSF plus SCF–mobilized PB samples. In this context, transplantation of human sources of hematopoietic stem cells into the NOD/SCID EMVnull mouse provides an in vivo assay for long-term human hematopoietic stem cell repopulating function.19,21,22 24 To quantify the frequency of SRCs isolated from patients mobilized with G-CSF plus SCF or with G-CSF alone, we employed the NOD/SCID EMVnull mouse and LDA. The two mobilization regimes demonstrated remarkably similar reconstituting capacity when dose ranges between 5 × 106and 25 × 106 MNCs were intravenously transplanted into NOD/SCID EMVnull mice. Transplantation of fewer than 10 × 106 cells allowed 7 out of 10 mice to engraft, at an average of 0.4% ± 0.2% for G-CSF–mobilized PB cells; 5 out of 9 mice engrafted at an average level of 0.7% ± 0.3% G-CSF plus SCF–mobilized PB cells (Figure 1A-B). Higher doses of cells led to increased average levels of engraftment (3.1% ± 1.6% and 6.1% ± 3.3% for G-CSF– or G-CSF plus SCF–mobilized PB, respectively) with populations from either mobilization regime. Statistical analysis of in vivo repopulating data demonstrated comparable frequencies of SRCs for both mobilization regimes, with 1 SRC in 8.2 × 106 (range, 4.3-14.8 × 106 MNCs) G-CSF–mobilized MNCs, and 1 SRC in 8.1 × 106 (range, 4.6-14.0 × 106 MNCs) G-CSF plus SCF–mobilized MNCs (Figure 1A-B).
Analysis of the repopulating capacity of human MNCs isolated from G-CSF– or G-CSF plus SCF–mobilized PB.
(A-B) Summary of the level of human engraftment in NOD/SCID EMVnull mice that received transplants of PB MNCs isolated following (A) G-CSF or (B) G-CSF plus SCF mobilization. Mouse BM was extracted 6 to 8 weeks after transplantation and analyzed for human engraftment by flow cytometry and Southern blot analysis. Each symbol (▪) represents a single mouse recipient of transplanted MNCs (5 to 50 × 106 cells) derived from a total of 6 independent G-CSF–mobilized samples (n = 18) and 9 independent G-CSF plus SCF–mobilized samples (n = 27). Horizontal lines represent the average level of engraftment for each cell dose and did not show a statistical difference between mobilization regimes. LDA showed a similar frequency of SCID repopulating cells (1 SRC in approximately 8 × 106 MNCs) for both G-CSF– and G-CSF plus SCF–mobilized PB. (C-D) Representative analysis of multilineage stem cell repopulation assessed in human-engrafted mice receiving transplants of 25 × 106 G-CSF– (C) or G-CSF plus SCF–mobilized PB MNCs (D). Mouse BM cells were stained with monoclonal antibody combinations for human-specific antibody against the panleukocyte marker CD45 (gated R1) (Ci,Di), and human cells were assayed for presence of mature B lymphoid cells (CD20, CD19) (Ciii,Diii), mature myeloid cells (CD33, CD15) (Civ,Div), primitive cells (CD34, CD38) (Cv,Dv), and mature T-lymphoid cells (CD4 and CD38) (Cvi,Dvi). Limits for quadrants were established with the use of FITC isotype and PE isotype controls (Cii,Dii), and statistics display the frequency of subsets representative of human hematopoietic differentiation in the BM of NOD/SCID recipients of transplants of either G-CSF– or G-CSF plus SCF–mobilized PB MNCs.
Analysis of the repopulating capacity of human MNCs isolated from G-CSF– or G-CSF plus SCF–mobilized PB.
(A-B) Summary of the level of human engraftment in NOD/SCID EMVnull mice that received transplants of PB MNCs isolated following (A) G-CSF or (B) G-CSF plus SCF mobilization. Mouse BM was extracted 6 to 8 weeks after transplantation and analyzed for human engraftment by flow cytometry and Southern blot analysis. Each symbol (▪) represents a single mouse recipient of transplanted MNCs (5 to 50 × 106 cells) derived from a total of 6 independent G-CSF–mobilized samples (n = 18) and 9 independent G-CSF plus SCF–mobilized samples (n = 27). Horizontal lines represent the average level of engraftment for each cell dose and did not show a statistical difference between mobilization regimes. LDA showed a similar frequency of SCID repopulating cells (1 SRC in approximately 8 × 106 MNCs) for both G-CSF– and G-CSF plus SCF–mobilized PB. (C-D) Representative analysis of multilineage stem cell repopulation assessed in human-engrafted mice receiving transplants of 25 × 106 G-CSF– (C) or G-CSF plus SCF–mobilized PB MNCs (D). Mouse BM cells were stained with monoclonal antibody combinations for human-specific antibody against the panleukocyte marker CD45 (gated R1) (Ci,Di), and human cells were assayed for presence of mature B lymphoid cells (CD20, CD19) (Ciii,Diii), mature myeloid cells (CD33, CD15) (Civ,Div), primitive cells (CD34, CD38) (Cv,Dv), and mature T-lymphoid cells (CD4 and CD38) (Cvi,Dvi). Limits for quadrants were established with the use of FITC isotype and PE isotype controls (Cii,Dii), and statistics display the frequency of subsets representative of human hematopoietic differentiation in the BM of NOD/SCID recipients of transplants of either G-CSF– or G-CSF plus SCF–mobilized PB MNCs.
Repopulating human (CD45+) stem cells from either mobilization regime were analyzed for mature leukocyte markers; they demonstrated multilineage potential in vivo (Figure 1C-D) with similar representation of human B-cells (Figure 1Ciii,Diii), human myeloid cells (Figure 1Civ,Div), and primitive cells (Figure Cv,Dv). Quadrant frequencies were established with the use of isotype controls (Figure Cii,Dii) and are representative of human cell maturation observed previously in the NOD/SCID mouse.35 High levels of myeloid and B-lymphoid development were observed under both mobilization regimes with a concurrent absence of human T-lymphocytes (Figure Cvi,Dvi); these results are not supported in the NOD/SCID model.35 In addition, analysis of primitive human hematopoietic markers demonstrated the presence of primitive repopulating CD34+CD38− cells and more mature CD34+CD38+ cells, demonstrating the retention of a significant frequency of primitive progenitor cells.19 24 These data demonstrate that multilineage engraftment could be detected with the use of MPB cells isolated from distinct mobilization regimes and that these samples possessed a propensity to proliferate and differentiate equally in vivo.
Total harvested SRCs from G-CSF plus SCF–mobilized PB were greater than 2-fold higher than the total number of SRCs derived from the use of G-CSF alone (Table 1). A total of 7220 ± 1320 SRCs within G-CSF plus SCF–mobilized PB could be isolated in comparison with 3130 ± 936 SRCs mobilized with the use of G-CSF alone (P < .05). The increase in total SRCs from G-CSF plus SCF–mobilized PB samples was also accompanied by a significant increase in the number of CD34+ cells per milliliter of leukapheresis product (Table 1; P < .01) when compared with G-CSF–mobilized PB. However, the frequency of SRCs calculated per 1 million CD34+ cells of leukapheresis product was approximately 4-fold lower in G-CSF plus SCF–mobilized PB MNCs as compared with G-CSF–mobilized PB (Table 1). Although combined G-CSF and SCF mobilization provided a phenotypic increase in the frequency of CD34+ cells per milliliter leukapheresis (Table 1), this increase did not correlate with an enhanced frequency of cells with NOD/SCID repopulating function. In comparison with G-CSF–mobilized PB, the inability to correlate enhanced CD34+ cell number with SRC function in G-CSF plus SCF–mobilized PB samples, prompted the purification of subsets from G-CSF– and G-CSF plus SCF treated–mobilized PB for direct comparison of in vivo repopulating capacity and in vitro progenitor activity.
CD34 expression correlates with increased in vitro progenitor content of Lin− cells isolated from G-CSF plus SCF– versus G-CSF–mobilized PB
We have previously described the isolation of Lin− BM and umbilical cord blood (CB) samples for the isolation of primitive CD34+ and CD34− subpopulations with primitive hematopoietic function.20,28 36 Using a similar isolation strategy, we have purified primitive hematopoietic cells from growth factor–mobilized PB samples. Comparison of Lin− cells from G-CSF– or G-CSF plus SCF–mobilized PB analyzed for the primitive stem cell markers CD34 and CD38 are represented in Figure2A-B. Live cells were selected with the use of forward- and side-scatter properties and 7-AAD staining (data not shown); quadrant statistics (based on isotype controls [Figure inset] performed on the day of analysis) were used to isolate CD34+CD38−Lin− cells for functional analysis (gated R1, Figure 2A-B). The frequency of cells expressing CD34 (Figure 2A-B) from G-CSF plus SCF–mobilized PB were increased as compared with samples mobilized with G-CSF alone. Upon analysis of G-CSF (n = 4) and G-CSF plus SCF (n = 5) Lin− samples, the average frequency of primitive CD34+CD38−Lin− cells was 5.2% ± 1.2% for G-CSF plus SCF–mobilized and 1.1% ± 0.6% G-CSF–mobilized samples (P < .05). Therefore, the increase in the frequency of CD34+ cells from G-CSF plus SCF–mobilized PB leukapheresis products (Table 1) was reflected in an increased frequency of primitive CD34+CD38−Lin− cells as compared with G-CSF–mobilized PB. These results indicate that G-CSF plus SCF–mobilized PB samples contain a higher frequency of primitive CD34+ and CD34+CD38−Lin−, as compared with G-CSF–mobilized PB.
Functional progenitor capacity of primitive subsets isolated by lineage depletion and FACS sorting from G-CSF– or G-CSF plus SCF–mobilized PB.
(A-B) Primitive subsets were purified by depletion of mature cells by means of a cocktail of 9 lineage-specific antibodies (CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, and glycophorin A) and a magnetic column. The resulting Lin− fractions from both G-CSF– and G-CSF plus SCF–mobilized PB were stained with CD34 and CD38 antibodies conjugated with the fluorochromes indicated and sorted for CD34+CD38− cells. The data represent a characteristic dot plot illustrating the abundance of CD34- and CD38-expressing cells isolated from G-CSF–mobilized (A) or G-CSF plus SCF–mobilized (B) PB. R1 represents CD34+CD38−Lin− cells selected to be assayed for progenitor activity and for transplantation into NOD/SCID mice. Quadrant statistics represent the frequency (mean ± SEM) of expression of CD34 and CD38 isolated from 4 G-CSF– and 5 G-CSF plus SCF–mobilized PB Lin− samples. A significant increase in the frequency of primitive CD34+CD38−cells was observed when G-CSF plus SCF Lin− cells were compared with G-CSF Lin−cells (P < .05). (C) Purified CD34+CD38−Lin− cells were assayed for progenitor function by means of in vitro colony-forming cell assays by plating 1000 CD34+CD38−Lin−cells in methylcellulose and were scored at 12 to 14 days. CFUs were also assessed in additional experiments at days 21 and 28 and showed similar CFU number and type regardless of the mobilization regime used. (Ci) The data represent the number of colonies per 1000 CD34+CD38−Lin− cells plated (mean ± SEM) from 4 G-CSF– and 5 G-CSF plus SCF–mobilized PB samples. A significant increase in the progenitor content of CD34+CD38−Lin− cells was observed when G-CSF plus SCF–mobilized PB was compared with G-SCF–mobilized PB (***P < .001). (Cii) Summary of the proportion of total progenitor colony types produced by G-CSF–mobilized PB (open bars) and G-CSF plus SCF–mobilized PB (shaded bars) CD34+CD38−Lin− cells. G-CSF plus SCF–mobilized PB CD34+CD38−Lin−cells demonstrated a significant increase in the frequency of erythroid burst-forming units (BFU-E) (*P < .05) and a significant decrease in the frequency of granulocyte colony-forming units (CFUs-G) (**P < .01) when compared with CD34+CD38−Lin− cells from G-CSF mobilization alone.
Functional progenitor capacity of primitive subsets isolated by lineage depletion and FACS sorting from G-CSF– or G-CSF plus SCF–mobilized PB.
(A-B) Primitive subsets were purified by depletion of mature cells by means of a cocktail of 9 lineage-specific antibodies (CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, and glycophorin A) and a magnetic column. The resulting Lin− fractions from both G-CSF– and G-CSF plus SCF–mobilized PB were stained with CD34 and CD38 antibodies conjugated with the fluorochromes indicated and sorted for CD34+CD38− cells. The data represent a characteristic dot plot illustrating the abundance of CD34- and CD38-expressing cells isolated from G-CSF–mobilized (A) or G-CSF plus SCF–mobilized (B) PB. R1 represents CD34+CD38−Lin− cells selected to be assayed for progenitor activity and for transplantation into NOD/SCID mice. Quadrant statistics represent the frequency (mean ± SEM) of expression of CD34 and CD38 isolated from 4 G-CSF– and 5 G-CSF plus SCF–mobilized PB Lin− samples. A significant increase in the frequency of primitive CD34+CD38−cells was observed when G-CSF plus SCF Lin− cells were compared with G-CSF Lin−cells (P < .05). (C) Purified CD34+CD38−Lin− cells were assayed for progenitor function by means of in vitro colony-forming cell assays by plating 1000 CD34+CD38−Lin−cells in methylcellulose and were scored at 12 to 14 days. CFUs were also assessed in additional experiments at days 21 and 28 and showed similar CFU number and type regardless of the mobilization regime used. (Ci) The data represent the number of colonies per 1000 CD34+CD38−Lin− cells plated (mean ± SEM) from 4 G-CSF– and 5 G-CSF plus SCF–mobilized PB samples. A significant increase in the progenitor content of CD34+CD38−Lin− cells was observed when G-CSF plus SCF–mobilized PB was compared with G-SCF–mobilized PB (***P < .001). (Cii) Summary of the proportion of total progenitor colony types produced by G-CSF–mobilized PB (open bars) and G-CSF plus SCF–mobilized PB (shaded bars) CD34+CD38−Lin− cells. G-CSF plus SCF–mobilized PB CD34+CD38−Lin−cells demonstrated a significant increase in the frequency of erythroid burst-forming units (BFU-E) (*P < .05) and a significant decrease in the frequency of granulocyte colony-forming units (CFUs-G) (**P < .01) when compared with CD34+CD38−Lin− cells from G-CSF mobilization alone.
To characterize the function of purified cell populations from MPB sources, purified subsets were assayed for in vitro progenitor and in vivo repopulating function. CD34+CD38−Lin− cells were plated in methylcellulose for 12 to 14 days to compare short-term progenitor function by means of an in vitro CFU assay (Figure 2C). Progenitor function was expressed as the number of hematopoietic colonies per 1000 CD34+CD38−Lin− cells initially plated (mean ± SEM) from 4 independent G-CSF– and 5 independent G-CSF plus SCF–mobilized PB samples. G-CSF plus SCF–mobilized CD34+CD38−Lin− cells demonstrated increased progenitor activity, with 1000 purified cells generating as many as 78 total CFUs (mean clonogenic efficiency of 1 colony per 20 cells plated) whereas G-CSF mobilization alone generated half the number of colonies (mean clonogenic efficiency, 1 colony per 41 cells plated) (Figure 2Ci). Furthermore, analysis of the frequency of individual colony types demonstrated significantly increased erythroid progenitors (P < .05), with lowered granulocyte colonies in G-CSF plus SCF–mobilized PB as compared with CD34+CD38−Lin− cells mobilized by G-CSF (P < .01) (Figure 2Cii). CD34+CD38+Lin− cells from the mobilized sources demonstrated similar progenitor activity with as few as 1000 purified CD34+CD38+Lin−cells from G-CSF plus SCF samples generating as many as 121 total CFUs (mean clonogenic efficiency, 1 colony per 17 cells plated) whereas fewer CFUs were generated with 1000 G-CSF–mobilized CD34+CD38+Lin− cells (mean clonogenic efficiency, 1 colony per 28 cells plated).
Long-term assessment of CFUs was also performed both on CD34+CD38−Lin− and on CD34+CD38+Lin− subsets derived from G-CSF– and G-CSF and SCF–mobilized samples. The number of CFUs were scored on day 21 and day 28 after seeding and showed no change in the progenitor frequency in either purified subfraction, regardless of mobilization treatment used to harvest the cells. In addition, macroscopic colonies of greater that 0.5 mm in diameter, indicative of high-proliferative progenitor colony-forming cells,37 38 were not detected at days 14, 21, or 28. Taken together, these data demonstrate that human primitive CD34+CD38−Lin− and CD34+CD38+Lin− cells exposed to G-CSF plus SCF in vivo possess increased progenitor potential in comparison with CD34+CD38−Lin−and CD34+CD38+Lin− cells isolated from G-CSF–mobilized PB.
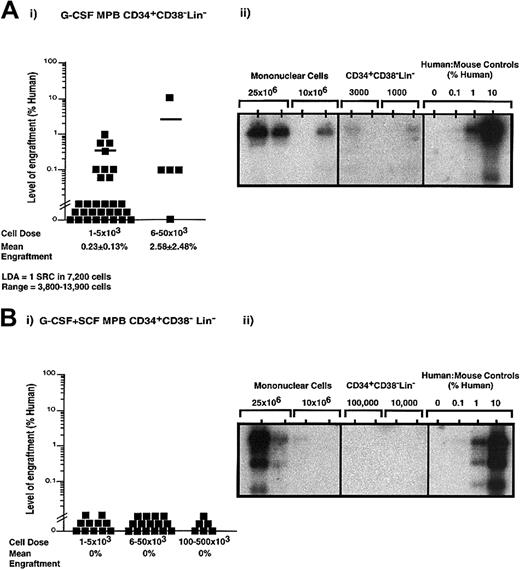
Characterization of human SRCs derived from Lin−CD34+ cells isolated from G-CSF versus G-CSF plus SCF mobilization
Despite an increased frequency of primitive CD34+Lin− cells and increased clonogenic progenitor capacity seen with G-CSF plus SCF–mobilized PB (Figure 2C), the NOD/SCID repopulating capacity of MPB MNCs isolated following G-CSF plus SCF mobilization was equivalent to that of MNCs isolated from G-CSF mobilization alone. To further examine the repopulating characteristics of purified cells derived from a total of 11 independent G-CSF–mobilized PB samples (n = 35) and 10 independent G-CSF plus SCF–mobilized PB samples (n = 32), purified CD34+CD38−Lin− cells were transplanted into recipient NOD/SCID EMVnull mice (Figure3). Human engraftment was observed with as few as 1000 G-CSF–mobilized CD34+CD38−Lin− cells (Figure 3Ai-Aii). LDA revealed a frequency of 1 SRC in 7200 (range, 1 SRC in 3800-13 900 cells) G-CSF–mobilized CD34+CD38−Lin− cells. Although human engraftment was consistently observed after transplantation of fewer than 10 000 CD34+CD38−Lin−cells isolated from G-CSF–mobilized PB, human SRC function could not be observed from the purified G-CSF plus SCF CD34+CD38−Lin− cells at doses up to 500 000 cells (Figure 3Bi). Remarkably, the addition of SCF to the mobilization regime increased the number of CD34+CD38−Lin− progenitor cells harvested by leukapheresis (Figure 2C); however, the in vivo repopulating function of these cells was reduced in comparison with G-CSF–mobilized PB. Furthermore, both G-CSF– and G-CSF plus SCF–mobilized PB CD34+CD38−Lin−cells expressed CXCR4 (data not shown), the receptor for the chemokine stromal derived factor–1 implicated in the migration of primitive human hematopoietic cells.39 Taken together, these data demonstrate that the addition of SCF during mobilization produced altered functional characteristics in the CD34+CD38−Lin− subset that enhanced in vitro proliferation of progenitors (Figure 2C) at the potential expense of SRC function (Figure 3).
Quantitative analysis of SRCs derived from CD34+CD38−Lin− cells isolated from G-CSF– or G-CSF plus SCF–mobilized PB.
Summary of human cell engraftment in NOD/SCID EMVnull mice that received transplants of G-CSF–mobilized CD34+CD38−Lin− cells (Ai) or G-CSF plus SCF–mobilized CD34+CD38−Lin− cells (Bi). Cells were transplanted into the tail vein of sublethally irradiated NOD/SCID mice at the dose ranges indicated. Mouse BM cells were extracted 6 to 8 weeks after transplantation, filtered, and stained with human CD45 and CD38 antibodies conjugated to fluorochromes to detect the presence or absence of human cells by flow cytometry. Each symbol (▪) represents a single mouse recipient with CD34+CD38−Lin− transplanted cells derived from a total of 11 independent G-CSF–mobilized samples (n = 35) and 10 independent G-CSF plus SCF–mobilized samples (n = 32). Sorting gates and quadrants were established by means of isotype controls for each experiment. Human engraftment was observed with as few as 1000 G-CSF–mobilized CD34+CD38−Lin− cells. LDA revealed that 1 SRC was present in approximately 7200 G-CSF–mobilized CD34+CD38−Lin− cells (range, 3800-13 900 cells). Human engraftment was not detectable from G-CSF plus SCF CD34+CD38−Lin− cells at doses up to 500 000 cells. Representative Southern blot analysis is depicted as G-CSF–mobilized MNCs and CD34+CD38−Lin− cells (Aii), and G-CSF plus SCF–mobilized MNCs and CD34+CD38−Lin− cells (Bii). Human engraftment exceeding 0.1% human DNA was compared with human-to-mouse DNA controls.
Quantitative analysis of SRCs derived from CD34+CD38−Lin− cells isolated from G-CSF– or G-CSF plus SCF–mobilized PB.
Summary of human cell engraftment in NOD/SCID EMVnull mice that received transplants of G-CSF–mobilized CD34+CD38−Lin− cells (Ai) or G-CSF plus SCF–mobilized CD34+CD38−Lin− cells (Bi). Cells were transplanted into the tail vein of sublethally irradiated NOD/SCID mice at the dose ranges indicated. Mouse BM cells were extracted 6 to 8 weeks after transplantation, filtered, and stained with human CD45 and CD38 antibodies conjugated to fluorochromes to detect the presence or absence of human cells by flow cytometry. Each symbol (▪) represents a single mouse recipient with CD34+CD38−Lin− transplanted cells derived from a total of 11 independent G-CSF–mobilized samples (n = 35) and 10 independent G-CSF plus SCF–mobilized samples (n = 32). Sorting gates and quadrants were established by means of isotype controls for each experiment. Human engraftment was observed with as few as 1000 G-CSF–mobilized CD34+CD38−Lin− cells. LDA revealed that 1 SRC was present in approximately 7200 G-CSF–mobilized CD34+CD38−Lin− cells (range, 3800-13 900 cells). Human engraftment was not detectable from G-CSF plus SCF CD34+CD38−Lin− cells at doses up to 500 000 cells. Representative Southern blot analysis is depicted as G-CSF–mobilized MNCs and CD34+CD38−Lin− cells (Aii), and G-CSF plus SCF–mobilized MNCs and CD34+CD38−Lin− cells (Bii). Human engraftment exceeding 0.1% human DNA was compared with human-to-mouse DNA controls.
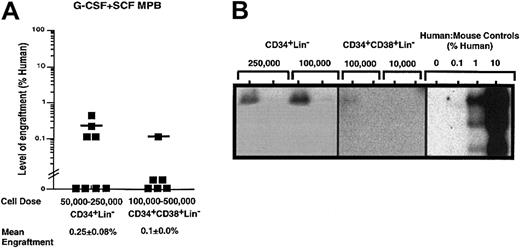
On the basis of the detection of SRC function with G-CSF plus SCF–mobilized PB MNCs (Figure 1B) and the lack of SRC function upon transplantation of CD34+CD38−Lin−cells (Figure 3B), we isolated alternatively purified populations of CD34+Lin− cells and CD34+CD38+Lin− cells from G-CSF plus SCF–mobilized PB samples to further characterize the nature of SRCs from this source. CD34+CD38+Lin− cells are considered more mature than CD34+CD38−Lin−cells19 and can be produced by CD34+CD38−precursors.22Transplanted doses of 50 000 to 250 000 G-CSF plus SCF CD34+Lin− cells showed low levels of repopulating capacity detectable by Southern blot analysis in 4 out of 8 mice receiving transplants (Figure4Aii), but confirmed that the CD34+Lin− population contained cells with SCID repopulating function. Transplantation of purified CD34+CD38+Lin− cells isolated from G-CSF plus SCF–mobilized PB did not demonstrate significant repopulating ability in vivo, with only 1 out of 6 mice showing 0.1% human cells in mouse BM after transplantation of high cell doses (Figure 4). Furthermore, no repopulating ability was observed for CD34+CD38+Lin− cells from G-CSF mobilization alone (data not shown). Taken together, these data indicate that although G-CSF plus SCF–treated CD34+Lin− cells possessed only modest SRC function, SCF treatment considerably reduced the SCID repopulating potential of further purified CD34+Lin− cells.
Quantitative analysis of the repopulating capacity of CD34+Lin− and CD34+CD38+Lin− cell populations isolated from G-CSF plus SCF–mobilized PB.
Summary of human cell engraftment in NOD/SCID EMVnull mice that received transplants of (A) G-CSF plus SCF–mobilized CD34+Lin− or CD34+CD38+Lin− cells. Cells were injected into the tail vein of sublethally irradiated NOD/SCID EMVnull mice at the dose ranges indicated. Mouse BM cells were extracted 6 to 8 weeks after transplantation to detect the presence of human cells by flow cytometry. Each symbol (▪) represents a single mouse with CD34+Lin− or CD34+CD38+Lin+ transplanted cells derived from a total of 5 independent G-CSF plus SCF–mobilized samples (n = 14). Human engraftment was observed with 100 000 G-CSF plus SCF–mobilized CD34+Lin− and CD34+CD38+Lin− cells. Representative Southern blot analysis of human cell engraftment after transplantation with (B) G-CSF plus SCF–mobilized CD34+Lin− and CD34+CD38−Lin− cells. The level of human engraftment was determined by comparing the characteristic 2.7-kb band to human-to-mouse DNA mixture controls.
Quantitative analysis of the repopulating capacity of CD34+Lin− and CD34+CD38+Lin− cell populations isolated from G-CSF plus SCF–mobilized PB.
Summary of human cell engraftment in NOD/SCID EMVnull mice that received transplants of (A) G-CSF plus SCF–mobilized CD34+Lin− or CD34+CD38+Lin− cells. Cells were injected into the tail vein of sublethally irradiated NOD/SCID EMVnull mice at the dose ranges indicated. Mouse BM cells were extracted 6 to 8 weeks after transplantation to detect the presence of human cells by flow cytometry. Each symbol (▪) represents a single mouse with CD34+Lin− or CD34+CD38+Lin+ transplanted cells derived from a total of 5 independent G-CSF plus SCF–mobilized samples (n = 14). Human engraftment was observed with 100 000 G-CSF plus SCF–mobilized CD34+Lin− and CD34+CD38+Lin− cells. Representative Southern blot analysis of human cell engraftment after transplantation with (B) G-CSF plus SCF–mobilized CD34+Lin− and CD34+CD38−Lin− cells. The level of human engraftment was determined by comparing the characteristic 2.7-kb band to human-to-mouse DNA mixture controls.
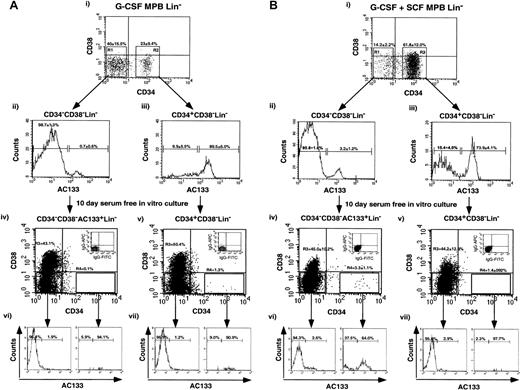
G-CSF plus SCF–mobilized CD34−CD38−AC133+Lin−cells differentiate into CD34-expressing cells in contrast to G-CSF–mobilized CD34−CD38−AC133+Lin−cells
Recently, several groups have reported primitive hematopoietic repopulating potential from human CD34−Lin−cells.20,40-42 Our laboratory has demonstrated enhanced primitive hematopoietic function in a subfraction of the CD34−Lin− population in cells that express the cell surface receptor AC133 derived from full-term CB.28 CB-derived CD34−CD38−AC133+Lin−cells were shown to proliferate in serum-free culture with a percentage of the cells acquiring CD34 expression, whereas CD34−CD38−AC133−Lin−cells possessed limited differentiating potential over 4 days of culture.28 In addition, CB CD34+CD38−Lin− cells have been previously shown to proliferate under identical culture conditions, with the majority of cells retaining CD34 expression with a concomitant up-regulation of CD38 on the cell surface.32 By means of a similar purification strategy optimized for the selection of primitive populations, adult CD34+CD38−Lin− and CD34−CD38−AC133+Lin−cells were isolated from G-CSF– and G-CSF plus SCF–mobilized samples. Figure 5Ai,Bi shows a representative example and the average frequencies of these subsets after depletion of lineage marker and CD38-expressing cells. In addition to an increase in the percentage CD34+CD38−Lin−within the G-CSF plus SCF–mobilized PB cells, this mobilization regime also showed a relative increase in the proportion of CD34−CD38−AC133+Lin−human cells (3.2% ± 1.2%) when compared with G-CSF–mobilized PB (0.7% ± 0.6%; P = 0.17). The frequency of CD34−CD38−AC133+Lin−cells from G-CSF plus SCF samples was also increased more than 5-fold in comparison with the frequency detected in identically processed CB sources.28 It is important to note that G-CSF–mobilized PB provided such low numbers of primitive CD34 cells that, in the majority of samples, the number of CD34−CD38−AC133+Lin−cells that could be isolated from G-CSF–mobilized PB was insufficient (fewer than 300 cells) to analyze cell surface phenotype of cultured cells after 10 days.
Phenotypic analysis of G-CSF– or G-CSF plus SCF–mobilized PB primitive CD34+(CD34+CD38−Lin−) or CD34−(CD34−CD38−AC133+Lin−) cells after 10 days in in vitro culture.
G-CSF–mobilized (A) or G-CSF plus SCF–mobilized (B) PB Lin− cells were sorted by means of 3-color flow cytometry with the use of antibodies for human CD34, CD38, and AC133. Lin− cells were sorted for CD34 expression (Ai,Bi), with sorting gates established to select for CD34−CD38− (R1) and CD34+CD38− (R2) populations. Cells were re-sorted to exclude any potential contaminating cells, and each population was reanalyzed for AC133 expression. CD34−CD38−AC133+Lin−(Aii,Bii) and CD34+CD38−Lin−(Aiii, Biii) cells were purified for in vitro culture. Equal numbers of purified CD34−CD38−AC133+Lin−and CD34+CD38−Lin− cells were cultured in serum-free conditions containing hematopoietic growth factors. After 10 days of culture, the contents of the wells were analyzed for CD34, CD38, and AC133 expression. The CD34 and CD38 expression is shown for 10-day–cultured CD34+CD38−Lin− (Aiv,Biv) and CD34−CD38−AC133+Lin−(Av,Bv) cells for G-CSF–mobilized (A) and G-CSF plus SCF–mobilized (B) PB samples. Insets illustrate isotype (IgG1) controls of stained cultured cells used to establish quadrant statistics for CD34−CD38− (R3) and CD34+CD38−cells (R4) gating. Approximately 3.3% ± 1.1% of the G-CSF plus SCF–mobilized CD34−CD38−AC133+Lin−cells acquired CD34 expression after 10 days of culture, while G-CSF–mobilized CD34−CD38−AC133+Lin−cells did not (0.1%). The AC133 expression after 10 days in vitro culture is shown for CD34−CD38−AC133+Lin−(Avi,Bvi) and CD34+CD38−Lin−(Avi,Bvi) cells. Data shown are representative of independent experiments with the use of a G-CSF sample and the mean of 3 additional G-CSF plus SCF–mobilized PB samples.
Phenotypic analysis of G-CSF– or G-CSF plus SCF–mobilized PB primitive CD34+(CD34+CD38−Lin−) or CD34−(CD34−CD38−AC133+Lin−) cells after 10 days in in vitro culture.
G-CSF–mobilized (A) or G-CSF plus SCF–mobilized (B) PB Lin− cells were sorted by means of 3-color flow cytometry with the use of antibodies for human CD34, CD38, and AC133. Lin− cells were sorted for CD34 expression (Ai,Bi), with sorting gates established to select for CD34−CD38− (R1) and CD34+CD38− (R2) populations. Cells were re-sorted to exclude any potential contaminating cells, and each population was reanalyzed for AC133 expression. CD34−CD38−AC133+Lin−(Aii,Bii) and CD34+CD38−Lin−(Aiii, Biii) cells were purified for in vitro culture. Equal numbers of purified CD34−CD38−AC133+Lin−and CD34+CD38−Lin− cells were cultured in serum-free conditions containing hematopoietic growth factors. After 10 days of culture, the contents of the wells were analyzed for CD34, CD38, and AC133 expression. The CD34 and CD38 expression is shown for 10-day–cultured CD34+CD38−Lin− (Aiv,Biv) and CD34−CD38−AC133+Lin−(Av,Bv) cells for G-CSF–mobilized (A) and G-CSF plus SCF–mobilized (B) PB samples. Insets illustrate isotype (IgG1) controls of stained cultured cells used to establish quadrant statistics for CD34−CD38− (R3) and CD34+CD38−cells (R4) gating. Approximately 3.3% ± 1.1% of the G-CSF plus SCF–mobilized CD34−CD38−AC133+Lin−cells acquired CD34 expression after 10 days of culture, while G-CSF–mobilized CD34−CD38−AC133+Lin−cells did not (0.1%). The AC133 expression after 10 days in vitro culture is shown for CD34−CD38−AC133+Lin−(Avi,Bvi) and CD34+CD38−Lin−(Avi,Bvi) cells. Data shown are representative of independent experiments with the use of a G-CSF sample and the mean of 3 additional G-CSF plus SCF–mobilized PB samples.
We examined and compared the survival and the proliferative and developmental potential of CD34+CD38−Lin− and CD34−CD38−AC133+Lin−cells in vitro for G-CSF– and G-CSF plus SCF–mobilized PB. After 10 days of culture in serum-free conditions previously optimized for these subfractions derived from CB,27,30,32 43 cell surface expression of CD34, CD38, and AC133 was analyzed and compared. The increase in cell number with G-CSF–mobilized PB was approximately 140-fold for CD34+CD38−Lin−cells while CD34−CD38−AC133+Lin−cells increased a modest 17-fold in the experiment shown. The average increase in cell number with G-CSF plus SCF–mobilized PB was 83-fold and 24-fold for CD34+CD38−Lin−and CD34−CD38−AC133+Lin−cells, respectively, demonstrating that cell proliferation in culture was comparatively reduced for CD34−CD38−AC133+Lin−cell population from MPB.
Within G-CSF–mobilized PB samples, both CD34−CD38− AC133+Lin−cells and CD34+CD38−Lin− cells demonstrated similar phenotypic profiles after 10 days in vitro culture (Figure 5Aiv-Av). Only a small number of cells expressed CD34 at the end of culture, and most cells were found to be CD34−CD38− and CD34−CD38+. Likewise, CD34+CD38−Lin− cells from G-CSF plus SCF–mobilized PB also demonstrated the loss of CD34 expression during the period of in vitro culture, and only a small percentage of G-CSF–mobilized PB cells (less than 2%) retained CD34 expression. The loss of CD34 expression during culture is unique to the mature mobilized cells since the majority of CD34+CD38−Lin− cells isolated from CB retain CD34 expression after 10 days of culture.32The loss of CD34 expression and up-regulation of CD38 expression were supported by our culture conditions and observed for both G-CSF– and G-CSF plus SCF–mobilized CD34+CD38−Lin− cells after 10 days of culture.23,27,32 These findings correlate with the progression of primitive populations toward a more mature phenotype indicative of cell differentiation.23,27 32
In contrast to cultured CD34−CD38−AC133+Lin−cells from the G-CSF–mobilized PB, a portion of CD34−CD38−AC133+Lin−cells from G-CSF plus SCF–mobilized PB retained phenotypic markers characteristic of primitive cells with 3.3% ± 1.1% (n = 3) of cells acquiring CD34 expression (Figure 5Biv), and the majority (64.0% ± 14.9%) of these CD34+ cells retained AC133 expression (Figure 5Bvi). Statistical analysis of G-CSF plus SCF–mobilized PB CD34−CD38−AC133+Lin−cells (n = 3) cultured for 10 days in vitro demonstrated the consistent acquisition of CD34 (3.3% ± 1.1% CD34+) whereas the majority of CD34+CD38−Lin− cells lost CD34 expression (1.4% ± 0.9% CD34+). We suggest that mature G-CSF plus SCF–mobilized CD34−CD38−AC133+Lin−cells share functional similarity to CB CD34−CD38−AC133+Lin−cells in their ability to differentiate into CD34+ cells in vitro, in contrast to G-CSF–mobilized fractions, which did not contain CD34+ precursors. G-CSF plus SCF–mobilized PB CD34+CD38− Lin− cells also retained a more primitive phenotype after 10 days in vitro culture, thereby indicating a fundamental difference in response to SCF treatment when compared with phenotypically identical populations derived from G-CSF–mobilized PB cells. Our observations indicate that the addition of SCF to mobilization protocols stimulates the production of functionally distinct CD34-expressing cells with altered developmental capacity as compared with G-CSF mobilization alone.
Discussion
Hematopoietic cells treated in vivo with growth factors (G-CSF alone or G-CSF plus SCF) administered to mobilize stem/progenitor cells into the periphery produced several fundamental differences in the repopulating properties and progenitor capacity of treated adults. Phenotypic analysis of MPB depleted for mature cell lineage markers (ie, Lin− cells) demonstrated that the addition of SCF to the mobilization regime produced a significant increase in total cellularity and the number of CD34-expressing cells and was consistent with previous clinical studies achieving rapid engraftment after autologous transplantation of G-CSF plus SCF–mobilized PB.15-17 Transplantation of human MNCs from G-CSF– or G-CSF plus SCF–mobilized PB samples demonstrated equivalent repopulating capacity (ie, SRCs) by limited dilution analysis, despite a 4-fold increase in the frequency of CD34+ cells harvested and a 2-fold increase in the number of CD34+CD38−Lin− cells with progenitor capacity isolated from G-CSF plus SCF–mobilized PB. The frequency of repopulating cells was 1 SRC in approximately 8 × 106 MNCs for both mobilized patient cohorts; this frequency correlates closely with the previously reported frequency of 1 SRC in 6.0 × 106 MNCs from G-CSF–mobilized volunteers.24 G-CSF plus SCF treatment also increased the total number of human MNCs capable of pluripotent repopulating function in vivo, as compared with G-CSF treatment alone. However since the frequency of SRCs within MNCs were the same for G-CSF and for G-CSF plus SCF–mobilized samples, the increase in total number of SRCs was due to an increase in the total number of cells mobilized to the periphery.
Purified CD34+CD38−Lin− cells from G-CSF plus SCF–mobilized PB demonstrated no repopulating ability, whereas G-CSF–mobilized CD34+CD38−Lin− cells consistently demonstrated in vivo repopulating function. Transplantation of the entire CD34+CD38−Lin− population isolated from the entire sample of G-CSF plus SCF–mobilized PB leukapheresis (up to 500 000 CD34+CD38−Lin− cells) resulted in no observable human engraftment by Southern blot analysis (level of detection = 0.1% human DNA). The lack of detectable repopulating ability in the G-CSF plus SCF–mobilized CD34+CD38−Lin− cells could not be attributed to a reduction in cell viability that was due to processing or cell isolation, since purified cells from these samples demonstrated progenitor capacity with the use of in vitro clonogenic assays, and this capacity was in fact enhanced as compared with purified CD34+CD38−Lin− cells mobilized by G-CSF alone. The lack of in vivo repopulating ability from purified CD34+CD38−Lin− G-CSF plus SCF–mobilized PB was unexpected and suggests that SCF treatment alters expression of traditional phenotypic markers found on the surface of human cells and/or alters the in vivo repopulating ability of this subset upon purification. On the basis of these observations, we suggest that monitoring CD34 expression from G-CSF plus SCF–mobilized PB may not accurately predict long-term repopulating capacity.44 45 Our data caution against assuming equivalent functional properties of CD34+ subsets generated by in vivo administration of different growth factors.
Autologous transplantation protocols for MBP were designed to employ the transfer of the entire leukapheresis product. Our data confirm that MNCs from the two mobilization protocols were equally capable of multilineage repopulation and suggested that the injected mixed-cell populations contained functional SRCs. Given the absence of repopulating capacity with CD34+CD38−Lin− cells, SCF plus G-CSF MNCs were purified by removing lineage-restricted (mature) cells and selection of CD34-expressing cells to identify the nature of the SRCs found within this unique source. CD34+Lin− populations isolated after coadministration with G-CSF and SCF possessed low repopulating capacity. The purified CD34+Lin− cells achieved engraftment in 4 out of 8 mice receiving cell transplants at doses more than 10-fold higher than required for engraftment with G-CSF–mobilized CD34+CD38−Lin−cells. The reduced repopulating capacity observed by purified populations from G-CSF and SCF treatment may also be attributed to the differentiation of CD34-expressing cells from SRCs to CFUs after exposure to SCF. The processing of Lin− cells from G-CSF plus SCF–mobilized PB samples may have resulted in the removal of repopulating function that may require mature cells expressing lineage-commitment markers such as CD38.46,47 Therefore, the ability to engraft animals with MNCs alone and the inability to engraft animals with cells purified on CD34 expression from G-CSF plus SCF–mobilized samples may be due to the lack of mature cells and subsequent removal of accessory cells that facilitate engraftment.46,47 SRCs from G-CSF plus SCF samples may require further cellular support that untreated adult BM and G-CSF–mobilized sources do not.19 35
Our study indicates that G-CSF versus G-CSF plus SCF administration in humans causes phenotypically and functionally distinct cellular subsets to be mobilized into the circulation. Although the cellular basis for the inability to enrich for repopulating cells in G-CSF plus SCF–mobilized PB is unknown, this property seems to be distinct from G-CSF–mobilized products. We favor the hypothesis that G-CSF plus SCF–mobilized PB is predisposed toward differentiation. This was supported by analysis of the maintenance of primitive cell phenotypes when directly comparing G-CSF versus G-CSF plus SCF–mobilized cells in identical serum-free cultures. Primitive CD34+CD38−Lin− cells isolated after mobilization with G-CSF plus SCF, differentiated into more committed cell phenotypes and no longer expressed CD34 after 10 days in culture, suggesting that these cells are primed to differentiate,32 whereas G-CSF CD34+CD38−Lin− cells retained a small fraction of CD34+ cells. In the case of G-CSF plus SCF–mobilized PB, this may be countered by the fact that primitive CD34−CD38−AC133+Lin−cells28 were relatively abundant in comparison with G-CSF–mobilized PB (3.2% ± 1.2% for G-CSF plus SCF–mobilized PB cells versus 0.7% ± 0.6% for G-CSF–mobilized PB CD34−CD38−Lin− cells) and were capable of differentiation into CD34+CD38−cells after 10 days in vitro, suggesting that G-CSF plus SCF–mobilized PB provides a superior source of CD34+ precursors (CD34−CD38+AC133+Lin−cells). These unique characteristics of G-CSF plus SCF–mobilized subsets may be exploited for the future development of clinical methods for in vitro stem cell expansion or gene therapy procedures by providing unique sources of primitive hematopoietic subsets.
Supported by funding from Amgen Inc, Thousand Oaks, CA, and Mississauga, Ontario; an operating grant from the Multi-organ Transplant Group, London Health Sciences Center, Ontario; and the Canadian Institutes for Health Research, Ottawa, Ontario; and by scientist scholarship award no. MSH-35681 (M.B.), the Canadian Research Chair in Stem Cell Biology (M.B.), and a fellowship award (D.H) provided by the Canadian Institutes for Health Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mickie Bhatia, The John P. Robarts Research Institute, 100 Perth Dr, London N6A 5K8, ON, Canada; e-mail:mbhatia@rri.on.ca.