Ten percent of newly diagnosed myeloma patients treated with any type of chemotherapy develop deep venous thrombosis (DVT). Thalidomide has proven activity in refractory multiple myeloma (MM), and although single-agent thalidomide has minimal prothrombogenic activity, its combination with cytotoxic chemotherapy is associated with a significantly increased risk of DVT. We analyzed the incidence of DVT in 232 MM patients who received a combination of chemotherapy and thalidomide on 2 protocols that differed only by the inclusion of doxorubicin in one. DT-PACE (dexamethasone/thalidomide/cisplatin/doxorubicin/cyclophos- phanide/etoposide) was offered to patients with preceding standard dose therapy, but no prior autotransplantation, while DCEP-T (dexamethasone/cyclophosphamide/etoposide/cisplatin/thalidomide) was administered for relapse after transplantation. If there were signs or symptoms suggestive of DVT, patients received additional investigations, including Doppler ultrasonography, followed by venography if indicated. Only patients on DT-PACE but not DCEP-T experienced an increased incidence of DVT. A statistical association between the incidence of DVT and combination chemotherapy including doxorubicin (P = .02) was observed; this association was confirmed on multivariate analysis. MM patients treated with thalidomide and doxorubicin have a high risk of developing DVT.
Introduction
Deep venous thrombosis (DVT) is observed in different types of cancer,1 including multiple myeloma (MM).2 This hypercoagulability may be related to an impaired fibrinolytic system, to the presence of lupuslike anticoagulant antibodies,3-7 or to increased levels of proinflammatory procoagulant cytokines (interleukin 6, tumor necrosis factor-α).8 Thalidomide is an active agent in refractory MM9 and is associated with a very low incidence of DVT (2%) when used as a single agent.10 However, when thalidomide is part of a combination that includes other cytotoxic agents, the incidence of DVT increases substantially.11 12To identify potential risk factors for the increased thrombogenicity, we have analyzed the incidence of DVT in different treatment protocols combining thalidomide and cytotoxic agents.
Patients, materials, and methods
Patients analyzed in this study had symptomatic MM and were enrolled in 2 different phase III studies. Patients previously treated with conventional chemotherapy but not with high-dose therapy (HDT) and stem cell support received a combination of dexamethasone, thalidomide, cisplatinum, doxorubicin, cyclophosphamide, and etoposide (DT-PACE).13 Patients relapsing after HDT were given the same combination of agents at similar doses but without doxorubicin (DCEP-T) (Table 1). Patients were evaluated at well-defined intervals by medical staff and underwent additional studies if signs or symptoms suggestive of DVT were observed. Patients with such manifestations were assessed by Doppler ultrasonography followed by venography if indicated. An informed consent form approved by the University of Arkansas for Medical Sciences Institutional Review Board was obtained prior to enrollment.
All statistical analyses were performed using SAS software (SAS version 8.0, SAS Institute, Cary, NC). Only cases of DVT confirmed by Doppler ultrasonography were considered, and the date of the DVT was defined as the date of the Doppler sonogram. The doxorubicin-DVT association was tested using Fisher exact test. This test, in conjunction with the Kruskal-Wallis test,14 was also applied in univariate analysis to patient baseline characteristics to detect clinical covariates showing imbalance with respect to DVT or protocol. Cumulative DVT incidence curves were estimated by the method of Kaplan and Meier15and analyzed for homogeneity with the log-rank test. Logistic regression and proportional-hazards regression16 were employed for multivariate analysis as follows: doxorubicin and unbalanced (P < .1) covariates were entered into multivariate regression models with variable selection. Covariates studied in the multivariate regression model included age, chromosome 11, platelet count, prior therapy, serum M, and race. Covariates significant (P < .05) under multivariate analysis were declared independently significant, and multivariate final models were then used to examine further the doxorubicin-DVT association after adjusting for independently significant covariates. To examine the same association while controlling for independently significant covariates through stratification, the methods of Cochran, Mantel, and Haenszel17,18 were employed in conjunction with the Breslow-Day test for homogeneity of odds ratios across strata.19 Cytogenetic abnormalities were studied by standard karyotyping, which showed mitoses in 216 of 232 patients prior to the start of therapy (34 of 36 DCEP-T and 182 of 196 DT PACE patients).
Results
All patients included were enrolled in DT-PACE or DCEP-T studies and received their first cycle of chemotherapy on or before January 1, 2001, to allow for a risk period of at least 9 months of observation to develop DVT. At the time of this analysis, 196 patients on DT-PACE and 36 patients on DCEP-T fulfilled these criteria. Four patients enrolled in DT-PACE did not receive doxorubicin because of pre-existing cardiac abnormalities or prior exposure to a total cumulative dose of doxorubicin > 400 mg/M2 (therefore, n = 192). These 4 patients were included in the DCEP-T group (therefore, n = 40). None of these 4 patients developed DVT. Patient characteristics are shown in Table 2. The median age was 58 years (range, 39 to 72 years) for DCEP-T–treated patients and 60 years (range, 31 to 88 years) for the DT-PACE group (P = .38). Not unexpectedly, a significantly longer duration of prior cytotoxic therapy was observed in patients receiving DCEP-T (46 months vs 6 months, P < .0001), and the median platelet count, although within normal limits in both groups, was significantly lower in the DCEP-T group (158 000/μL vs 226 000/μL,P = .0005). Among patients with measurable paraprotein levels, those on DT-PACE had higher median serum M protein levels (70 g/dL vs 0.01 g/dL, P = .0004). There was no difference, however, in median platelet count or serum M protein between patients who did and did not develop DVT (P = .47 and .52, respectively).
Levels of β2M (P = .85) and bone marrow plasmacytosis (P = .59) were similar. Known risk factors for DVT, such as central venous catheters (CVC) (present in all patients), performance status, and hormonal therapy were not significantly different between the 2 study groups (all Pvalues > .1). Prior history of DVT was comparable between the 2 study groups (10% vs 12%), and no patient was receiving anticoagulation at the time of enrollment.
DVT developed in 31 of 192 (16%) patients treated with the doxorubicin-containing regimen (DT-PACE) compared with only 1 of 40 (2.5%) patients in the control arm (DCEP-T) (P = .02). Eleven patients developed DVT at the site of CVC, and 21 at distant sites: 5 in the upper and 16 in the lower extremities. In the latter cases, 13 involved the femoral/popliteal vein, and DVT was bilateral in 2. One of these patients developed nonfatal pulmonary embolism. Isolated popliteal and calf vein involvement was documented in 2 cases. Thrombosis was limited to a deep vein below the knee in one patient.
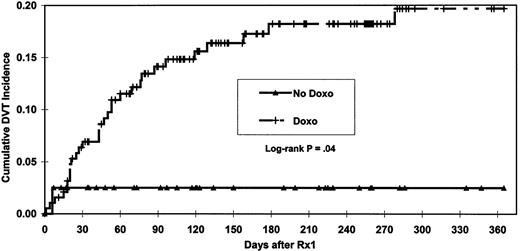
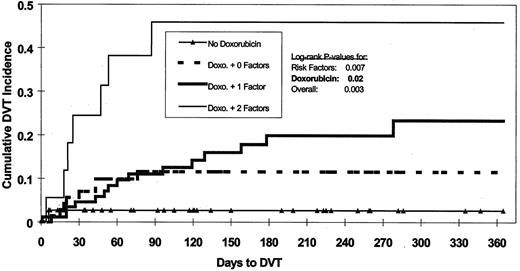
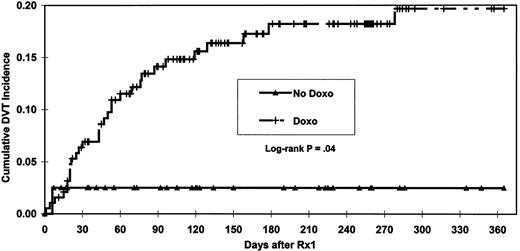
A significantly shorter (P = .04) time to DVT was observed among patients exposed to doxorubicin (Figure1). Patients who developed DVT were older than those without DVT (P = .01, with median ages 63 vs 59 years, respectively). Patients with chromosome 11 abnormalities developed DVT more frequently than those without (23% vs 11%,P = .04). Multivariate analysis indicated that doxorubicin, age, and chromosome 11 abnormalities were the only independent factors statistically significant for increased risk of DVT. Age of more than 60 years and chromosome 11 abnormality were defined as risk factors for DVT and used, along with doxorubicin exposure, to analyze cumulative DVT incidence in more detail (Figure2). Both doxorubicin exposure (P = .02) and number of these risk factors (P = .007) were independently significant for time to DVT. Without doxorubicin administration, the 365-day cumulative incidence of DVT was 2.6% irrespective of other risk factors. In the presence of doxorubicin and 0, 1, or 2 risk factors, the cumulative incidence of DVT increased to 11.6%, 23.4%, and 45.9%, respectively. Examination of risk of DVT by age quartiles showed that the DVT rate for patients in the oldest 3 quartiles was comparable (9/58, 11/58, and 10/58; average 17%) and much higher than for patients in the youngest age quartile (2/58, 3%). Age quartiles and chromosome 11 status were used to stratify patients in order to analyze the doxorubicin-DVT association using Cochran-Mantel-Haenszel17 18 methods to control for these 2 independently significant covariates. After conditioning on the strata, the statistical significance of the doxorubicin-DVT association persisted (P = .01, odds ratio = 11.3). Examination for homogeneity across strata revealed no evidence (P = .7) that the strength of the doxorubicin-DVT association varied with age or chromosome 11 abnormality. Inspection of the chromosome 11 abnormalities revealed that DVT was associated only with trisomy or tetrasomy (30% DVT vs 11% for absence of this trait [P = .01]) and not with either deletion or translocation of chromosome 11. Restratifying patients on age quartiles and status for polysomy 11 and conditioning on the new strata did not change the statistical significance (P = .01) nor the homogeneity (P = .6) of the doxorubicin-DVT association. For both DVT occurrence (P = .3) and time to DVT ( = 0.9), length of prior therapy was not statistically significant in multivariate analysis. The median duration of prior therapy tended to be longer for patients who developed DVT (P = .18). A marginally significant trend toward a higher incidence of DVT was seen in patients with more than 6 months of prior therapy (P = .06). However, under multivariate analysis, this trend lost significance (P = .13).
Effect on DVT of doxorubicin and risk factors.
Risk factors are age > 60 and chromosome 11 abnormality.
Effect on DVT of doxorubicin and risk factors.
Risk factors are age > 60 and chromosome 11 abnormality.
Discussion
Our study documents a strong association between DVT and exposure to doxorubicin in patients receiving thalidomide. When added to our previous observations of a low incidence of DVT (2%) with single-agent thalidomide10 and a much higher risk of thrombosis (14%) when thalidomide was added to a single course of vincristine, doxorubicin, and dexamethasone (VAD),11 our study indicates that a strong association exists between DVT and exposure to the doxorubicin-thalidomide combination.
The statistical significance of the DVT-doxorubicin association in our study persisted after accounting for 2 independently significant confounding factors, namely, age and chromosome 11, through both multivariate modeling and Cochran-Mantel-Haenszel17 18methods. As expected, older age was also associated with an increased risk of DVT. The apparent role of chromosome 11 dosage remains unexplained. No known genes coding for factors predisposing to increased risk of DVT are found on this chromosome. However, polysomy 11 is almost invariably associated with polysomy of other chromosomes, such as 3, 5, and 19, which code for one or more thrombogenic factors. It is also possible that, because multiple clinical covariates were tested for their potential confounding influence, the observed association between DVT and polysomy 11 is incidental and will not hold in a larger study.
The development of DVT early in the course of therapy (22/32 or 69%) within the first 60 days (Figure 1) suggests that this complication may be related to the release of thrombogenic factors from apoptotic myeloma cells rather than to cumulative thalidomide exposure. However, when patients evaluable for paraprotein response were analyzed, no statistically significant association was seen between DVT and response > 50% to the first 2 cycles of chemotherapy.
An increased incidence of DVT also has been observed when thalidomide was used in combination with gemcitabine and 5-fluorouracil for the treatment of metastatic renal cell carcinoma20 or in combination with bis-chloro-ethyl nitrosourea (BCNU) for patients with high-grade glioma.21 In the latter study, DVT developed in 27% of patients (n = 11); half of the DVTs were associated with pulmonary embolism.
Similar increases in DVT are now reported with other antiangiogenesis drugs used in combination with chemotherapy. Compound SU5416, a specific inhibitor of FLK-1 (VEGF receptor on endothelial cells), resulted in a dose-related vascular toxicity (DVT, stroke, and transient ischemic attacks) in up to 50% of patients when given in combination with cisplatin and gemcitabine.22 Such high toxicity was not observed previously when SU5416 was used alone or in combination with 5-fluorouracil and leucovorin.23
Our study indicates that drugs such as cyclophosphamide, etoposide, cisplatinum, and dexamethasone at these doses and schedules combined with thalidomide do not produce significant cardiovascular toxicity in MM patients. We have previously reported that doxorubicin-containing chemotherapy without thalidomide has a lower cardiovascular profile compared with that to which thalidomide is added.11
The observation of a strong link between DVT and therapy with doxorubicin plus thalidomide in myeloma patients should raise a high index of suspicion in all cancer patients treated with such combinations. No information is yet available to document if sequential treatment with these 2 agents increases the risk of DVT to the same extent as observed when they are given simultaneously. We are carefully evaluating if screening for factors generally associated with increased risk of DVT, such as acquired activated protein C (APC) resistance, can identify patients at higher risk for DVT in this setting. Until we have a better understanding of these risk factors, the combination of thalidomide and doxorubicin should probably be limited to patients entered in carefully designed and properly monitored clinical investigational studies.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2002-01-0335.
Supported in part by a grant from the National Cancer Institute (CA 55819).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Maurizio Zangari, 4301 W Markham, Slot 776, Little Rock, AR 72205; e-mail: zangarimaurizio@uams.edu.