The stem cell factor c-kit signaling pathway (SCF/c-kit) has been previously implicated in normal hematopoiesis, melanogenesis, and gametogenesis through the formation and migration of c-kit+ cells. These biologic functions are also determinants in epithelial–mesenchymal transitions during embryonic development governed by the Snail family of transcription factors. Here we show that the activation of c-kit by SCF specifically induces the expression of Slug, a Snail family member. Slug mutant mice have a cell-intrinsic defect with pigment deficiency, gonadal defect, and impairment of hematopoiesis. Kit+ cells derived from Slug mutant mice exhibit migratory defects similar to those of c-kit+ cells derived from SCF and c-kit mutant mice. Endogenous Slug is expressed in migratory c-kit+ cells purified from control mice but is not present in c-kit+cells derived from SCF mutant mice or in bone marrow cells from W/Wv mice, though Slug is present in spleen c-kit+ cells of W/Wv (mutants expressing c-kit with reduced surface expression and activity). SCF-induced migration was affected in primary c-kit+ cells purified from Slug−/− mice, providing evidence for a role of Slug in the acquisition of c-kit+ cells with ability to migrate. Slug may thus be considered a molecular target that contributes to the biologic specificity to the SCF/c-kit signaling pathway, opening up new avenues for stem cell mobilization.
Introduction
Hematopoiesis is a lifelong process responsible for replenishing hematopoietic progenitor cells and mature blood cells from a pool of pluripotent, long-term reconstituting stem cells.1 The daily turnover of blood cells in a normal adult is tightly regulated, involving, in part, a complex interaction between soluble and membrane-bound stimulatory and inhibitory cytokines and their corresponding receptors.2 The molecular cloning of these hematopoietic growth factors and their receptors has been instrumental in delineating the pathways that lead from a single hematopoietic stem cell to the various terminally differentiated cells in the hematopoietic system.
Although a number of cytokines have effects on progenitor and stem cells in vitro or in vivo, one cytokine discovered in the early 1990s, c-kit ligand, appears to have unique and nonredundant activities on primitive/progenitor cells.3 The in vivo roles of c-kit are well understood because of the existence of mutant mice in which genes encoding the receptor and its respective ligand are defective. Mutations in the c-kit receptor and its ligand are well represented by numerous white-spotting (W) and Steel (Sl) mutant alleles, respectively. Mice afflicted with mutations at the W locus were originally identified, as the name implies, by the presence of white spots on pigmented mice.4 Detailed examination of the mice showed that the mutation was pleiotropic. The mice also had defects in germ cell development and in hematopoiesis (characterized by macrocytic anemia). In 1988 it was shown that the W locus encoded a tyrosine kinase receptor known as c-kit.5 6
Many years after the discovery of the W locus, a mutation in mice that had a phenotype virtually identical to W mice was identified.7 Because mutations on 2 different chromosomes had the same complex phenotype that affects pigmentation, germ cells, and hematopoiesis, researchers hypothesized that there would be some relationship between the proteins encoded at these 2 loci.8 In 1990 the protein encoded at the Sl locus was identified and named as mast cell growth factor, stem cell factor (SCF), and c-kit ligand.9-13
Although the primary function of SCF in early hematopoiesis might be to induce the growth of quiescent progenitor/stem cells through synergistic interactions with other early-acting cytokines, ample evidence indicates that SCF, in the absence of other cytokines, selectively promotes viability rather than proliferation of primitive murine progenitor cells.14 Although SCF/c-kit migratory pathways and developmental fates are well documented, less is known about the molecular mechanisms that provide biologic specificity to the SCF/c-kit signaling pathway in the formation and migration of c-kit+ cells.
These biologic events controlled by the SCF/c-kit signaling pathway are reminiscent of those that take place in epithelial–mesenchymal transitions during mammalian development. Indeed, the process of mesoderm formation involves the acquisition of migratory properties and cell fate determination. These epithelial–mesenchymal transitions are controlled by a conserved family of zinc-finger proteins, the Snail family.15-17 TheDrosophila gene snail is critical for mesoderm formation and cell fate determination.18 The related murine Snail and Slug genes have also been proposed to participate in mesoderm formation and cell migration.19-21
In this study we have investigated the relationship between the SCF/c-kit signaling pathway and the Snail family of proteins. We have found that the SCF/c-kit signaling pathway specifically induces the expression of a member of the Snail gene family of zinc-finger transcription factors, Slug gene, in natural and artificially engineered c-kit+ cells. Analysis of a targeted null mutation that deleted all Slug coding sequences revealed that Slug mutant mice, like c-kit and SCF-defective mice,22 have a complex phenotype including pigmentation, gonadal defects, and hematopoietic defects. Long-term transplantation experiments demonstrated that the defect in Slug mutant mice, in which Slug−/− c-kit+ cells having a functional SCF/c-kit signaling pathway presented migratory defects similar to those of c-kit+ cells in Sl/Sld and W/Wvmice, is intrinsic to the cell. Because mutations on SCF, c-kit receptor, and Slug genes have a similar pleiotropic phenotype that affects pigmentation, germ cells, and hematopoiesis, we hypothesized there would be some relationship between them. Indeed, 3 different pieces of data demonstrate the relationship: (1) primary c-kit+ cells purified from control mice express Slug; (2) Slug is not present in primary c-kit+ marrow cells derived from W/Wv and Sl/Sld mice; and (3) SCF-induced costimulation and migration was affected in primary c-kit+ cells purified from Slug−/− mice. Taken together, these results identify Slug as a molecular target that contributes to the biologic specificity of the SCF/c-kit signaling pathway.
Materials and methods
Cell culture
Cell lines used include LAMA-84, a human megakaryocytic leukemia cell line, and Ba/F3, a mouse interleukin-3 (IL-3)–dependent pro-B cell line. Cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). When required, 10% WEHI-3B–conditioned medium was added as a source of IL-3. Ba/F3 cells expressing wild-type form of c-kit were derived as follows. The PECE-kit plasmid containing the full-length coding sequences of the mouse c-kit cDNA, a generous gift of Dr D. Martı́n-Zanca, was used to transfect the IL-3–dependent pro-B cell line. Cotransfection with a neomycin-resistant plasmid (MC1-neo) and primary selection with the neomycin analogue G418 generated a stable Ba/F3 cell line expressing the c-kit (Ba/F3 + c-kit). Ba/F3 cell populations were stained with the c-kit–specific monoclonal antibody CD117.
Mice
Animals were housed under nonsterile conditions in a conventional animal facility. Mice heterozygous and homozygous for the SlughΔ1 mutation, generated by removing the genomic sequences of the entire Slugh protein-coding region (SlughΔ1 mutant mice), have been described.19 W/Wv and Sl/Sld mice and breeding pairs were obtained from the Jackson Laboratory (Bar Harbor, ME). In experimental mice, phenylhydrazine (PHZ; 60 mg/kg body weight; Sigma Chemical, St Louis, MO) was injected intraperitoneally for 2 consecutive days.23 On each of the 3 days after the second PHZ injection, 5 mice were killed by cervical dislocation, and the bones and spleens were removed under sterile conditions for further analysis. All procedures were approved by the institutional animal care committee of Universidad de Salamanca-CSIC.
Phenotypic analysis of the cells
Cell morphology was analyzed according to standard criteria. Single-cell suspensions were prepared from individual tissues, including bone marrow, spleen, thymus, and peripheral blood, by standard procedures.24 Approximately 1 × 106 cells were used for most stainings. Cells were immunophenotyped with the following antibodies: phycoerythrin (PE)–conjugated TER119 (Ly-76, a monoclonal antibody recognizing an antigen expressed on erythroid cells from erythroblasts to erythrocyte; PE-CD4, PE-Gr-1, PE-CD117; PE-CD19; PE-B220; fluorescein isothiocyanate (FITC)–conjugated CD8, FITC-IgM, FITC-MacI (all from PharMingen, San Diego, CA). Cells, suspended in Ca++-/Mg++-free phosphate-buffered saline (PBS) supplemented with 1% (vol/vol) FBS, were labeled with each antibody (approximately 1 μg/106cells) for 30 minutes on ice. Cell fluorescence was analyzed with the FACScan flow cytometer (Becton Dickinson, Bedford, MA). Cells incubated with appropriately labeled isotype controls (PharMingen) were used to gate the nonspecific fluorescence signal. Before analysis, mature red cells were depleted by hypotonic lysis (0.38% ammonium chloride for 15 minutes on ice). Background controls were treated identically except that primary antibodies were omitted. Cells were initially gated by size and by scatter to identify live cells. In some experiments, cell viability was assessed by propidium iodide (5 μg/mL; Sigma) (in flow cytometry) exclusion.
Cell purification
Mononuclear spleen suspensions were prepared by cutting the spleens into small fragments in 5 mL Ca++-/Mg++-free PBS containing 10% (vol/vol) FBS and by passing the cell suspension through progressively smaller needles. Marrow cells were flushed from the femurs with a syringe containing 2 mL PBS–10% FBS. Marrow and spleen light-density mononuclear cells were isolated by centrifugation over Ficoll-Hypaque (P = 1.077 g/mL) at 800g for 20 minutes at room temperature. For cell sorter separation, cells were incubated with c-kit–PE and c-kit+ cells sorted by fluorescence-activated cell sorting (FACS) (FACstar; Becton Dickinson). Sorted cells were then re-analyzed for purity with the cytometer.
Reverse transcription–polymerase chain reaction
To analyze the expression of Slug andSnail in cell lines and in purified c-kit+cells, reverse transcription (RT) was performed according to the manufacturer's protocol in a 20-μL reaction containing 50 ng random hexamers, 3 μg total RNA, and 200 U Superscript II RNase H. reverse transcriptase (Gibco/BRL, Paisley, United Kingdom). Thermocycling parameters for polymerase chain reaction (PCR) and the sequences of the specific primers were as follows: mSlug, 30 cycles at 94°C for 1 minute, 56°C for 1 minute, and 72°C for 2 minutes, sense primer 5′-GCCTCCAAAAAGCCAAACTA-3′, antisense primer 5′-CACAGTGATGGGGCTGTATG-3′; mSnail, 30 cycles at 95°C for 2 minutes, 60°C for 2 minutes, and 72°C for 2 minutes, sense primer 5′-CAGCTGGCCAGGCTCTCGGT-3′, antisense primer 5′-GCGAGGGCCTCCGGAGCA-3′. Amplification of β-actin RNA served as a control to assess the quality of each RNA sample. Sequences of the internal probes were as follows: mSlug, 5′-GACACACATACAGTGATTATTTCC-3′;mSnail, 5′-TGCAACCGTGCTTTTGCTGACCGCTCCAAC-3′.
In situ hybridization
RNA analysis
Total cytoplasmic RNA (10 μg) was glyoxylated and fractionated in 1.4% agarose gels in 10 mM Na2HPO4 buffer (pH 7.0). After electrophoresis, the gel was blotted onto Hybond-N (Amersham), UV cross-linked, and hybridized to 32P-labeled probes. Loading was monitored by reprobing the filters with a mouse β-actin cDNA. The Slug probe comprised the coding sequence of mouse SlughcDNA.
Bone marrow transplantation and sample collection
Recipient female C57 BL/6J mice (8-12 weeks old) were irradiated with 2 split doses of 600 cGy 2 hours apart. This dose is sufficient to eliminate endogenous hematopoiesis completely. Bone marrow (BM) cells were injected into the tail vein of the irradiated mice at 2-4 × 106 cells per mouse for long-term reconstitution. All recipients were maintained in microisolator cages on sterilized food and acidified sterile water. Animals, 5 per group, were killed, and hematopoietic tissues were collected for FACS analysis.
Hematopoietic colony assays
Bone marrow cells (0.25-1.0 × 105 cells/plate) and spleen cells (104-105 cells/plate) isolated from normal and Slug mutant mice were seeded into FBS-free semisolid culture plates (Stem Cell Technologies, Vancouver, BC, Canada). Colony growth was stimulated with the following combinations of recombinant growth factors: rat stem cell factor (100 ng/mL; Sigma), mouse IL-3 (10 ng/mL; Sigma), and human erythropoietin (2 U/mL; Roche, Barcelona, Spain) for burst-forming unit erythroid (BFU-E) growth. The growth of erythroid colony-forming unit (CFU-E)–derived colonies was stimulated with erythropoietin alone (2 U/mL). The growth of myeloid colonies (CFU-GM) was stimulated with recombinant murine granulocyte macrophage–colony-stimulating factor (GM-CSF) (10 ng/mL; Sigma) in the presence or in the absence of SCF (100 ng/mL; Sigma). Cultures were incubated at 37°C in a humidified incubator containing 5% CO2 in air and were scored either 3 days (for CFU-E–derived colonies) or 7 days (for GM-CSF– and BFU-E–derived colonies) following initiation of the culture. The frequency of the colonies was determined in triplicate cultures.
Isolation of primary bone marrow–derived mast cells, immunoprecipitation, and Western blotting
Bone marrow cells were collected by flushing the marrow cavity of femurs, and mast cells were derived by selective growth for 6 weeks in IL-3–containing medium (Opti-Mem I, Gibco-BRL; 10% FBS, 0.5 ng/mL recombinant murine IL-3; R&D Systems, Madrid, Spain). Medium was replaced daily and cells were transferred to new dishes to remove adherent cells, including macrophages and megakaryocytes. Immunoprecipitation and Western blot assays were made using extracts from 1 × 107 mast cells per lane. Briefly, cells were starved for 12 hours in Opti-Mem I medium without IL-3 and containing only 0.5% serum, before stimulation with 100 ng/mL murine SCF (R&D Systems) for 10 minutes at 37°C, where indicated. Kit was detected using affinity-purified goat antiserum against the C-terminus of mouse kit, M-14 (Santa Cruz Biotechnology, Quimigranel, Madrid, Spain). Monoclonal antibody 4G10 (UBI) was used to detect phosphotyrosine.
Histologic analysis
Tissue specimens were fixed with 10% formalin overnight, processed, and embedded in paraffin, and 6-μm sections were stained with hematoxylin and eosin, examined histologically, and photographed. All sections were taken from homogenous and viable portions of the resected tissues. Mast cells were stained with Giemsa. The number of mast cells per square millimeter was determined.
TUNEL assay
Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling (TUNEL) was performed by using the in situ cell death detection kit (Boehringer Mannheim, Mannheim, Germany), essentially following manufacturer's instructions with minor modifications, depending on the specimen preparation. In brief, sections were postfixed for 15 minutes in 4% paraformaldehyde, washed twice with PBS, and incubated in ethanol-acetic acid (2:1) for 5 minutes at −20°C. After 2 washes in PBS, sections were subjected to proteinase K digestion (10 μg/mL in 10 mM Tris HCl, pH 8.0, and 1 mM EDTA), washed twice with PBS, and counterstained with methyl green.
Matrigel assay
Cell migration was determined in the BioCoat Matrigel Invasion Chamber assay (Becton Dickinson). Purified hematopoietic c-kit+ cells from wild-type mice (Slug+/+), Slug heterozygous mice (Slug+/−), and Slug homozygous mice (−/−) were suspended in DMEM–0.1% BSA at a concentration of 5 × 104 cells/mL, placed in the upper compartment, and incubated for 24 hours at 37°C in 5% CO2 in the absence or presence of SCF (100 ng/mL). After incubation, nonmigrating cells were removed from the upper surface of the membrane by scrubbing. Cells on the reverse side were stained with 0.1% crystal violet and were counted under a microscope at × 100 magnification. Percentage cell migration was calculated from the ratio of the number of cells recovered from the lower compartment to the total number of cells loaded in the upper compartment. Each experiment was performed using at least 4 chambers for each c-kit+ cell sample and was repeated at least twice.
Results
Induction of Slug expression by the activation of kit receptor for SCF
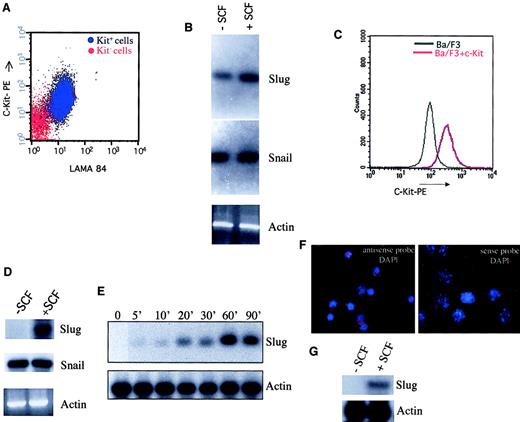
The ability of c-kit to stimulate the expression of the Snail family members was first assayed in naturally expressing c-Kit+ cells using the LAMA 84 cell line (Figure1A). As shown in Figure 1B, the expression of Slug increased rapidly in SCF-treated LAMA-84 cells. However, the level of Snail expression was not modified in the presence of SCF. To extend these previous data indicating the capacity of c-kit in SCF-treated LAMA-84 cells to specifically activate Sluggene expression, Ba/F3 cells lacking endogenous c-kit26were engineered to express a wild-type, full-length c-kit receptor (Ba/F3+ c-kit) (Figure 1C). Ba/F3 c-kit–transfected cells specifically expressed Slug on SCF stimulation (Figure 1D) and in a time-dependent-manner (Figure 1E). However, theSnail gene was expressed at similar levels in SCF-unstimulated and SCF-stimulated Ba/F3+ c-kit cells. These experiments demonstrated that activation of c-kit specifically induces expression of a member of the Snail gene family of zinc-finger transcription factors, indicating a clear relationship between c-Kit/SCF activation and Slug expression.
Activation of c-kit receptor for SCF specifically induces Slug expression.
LAMA 84 cells (A) and Ba/F3 cells engineered to express c-kit (C) were stained with a monoclonal antibody recognizing c-kit and analyzed by flow cytometry (c-kit− cells in panel A were unstained LAMA 84 cells). Expression of Slug and Snail was analyzed by RT-PCR in LAMA 84 cells (B) and in Ba/F3 cells engineered to express c-kit (D) in the absence and in the presence of SCF for 60 minutes at 37°C (100 ng/mL). PCR products were transferred to a nylon membrane and were analyzed by hybridization with end-labeled internal oligonucleotide probes specific for each gene. β-Actin was used to check cDNA integrity and loading. (E) Ba/F3 cells that express c-kit were incubated for the indicated periods of time with SCF (100 ng/mL) at 37°C. After incubation, Slug and actin expression were determined by Northern blot analysis. (F, G) Expression of Slug RNA is observed in primary c-kit+ cells purified from BM of wild-type mice, hybridized with an antisense RNA probe for Slug and subjected to DAPI (F). No Slug RNA expression is observed when a sense RNA probe is used. (G) Expression of Slug in primary c-kit+ cells in the absence and in the presence of SCF for 60 minutes at 37°C (100 ng/mL) analyzed by RT-PCR.
Activation of c-kit receptor for SCF specifically induces Slug expression.
LAMA 84 cells (A) and Ba/F3 cells engineered to express c-kit (C) were stained with a monoclonal antibody recognizing c-kit and analyzed by flow cytometry (c-kit− cells in panel A were unstained LAMA 84 cells). Expression of Slug and Snail was analyzed by RT-PCR in LAMA 84 cells (B) and in Ba/F3 cells engineered to express c-kit (D) in the absence and in the presence of SCF for 60 minutes at 37°C (100 ng/mL). PCR products were transferred to a nylon membrane and were analyzed by hybridization with end-labeled internal oligonucleotide probes specific for each gene. β-Actin was used to check cDNA integrity and loading. (E) Ba/F3 cells that express c-kit were incubated for the indicated periods of time with SCF (100 ng/mL) at 37°C. After incubation, Slug and actin expression were determined by Northern blot analysis. (F, G) Expression of Slug RNA is observed in primary c-kit+ cells purified from BM of wild-type mice, hybridized with an antisense RNA probe for Slug and subjected to DAPI (F). No Slug RNA expression is observed when a sense RNA probe is used. (G) Expression of Slug in primary c-kit+ cells in the absence and in the presence of SCF for 60 minutes at 37°C (100 ng/mL) analyzed by RT-PCR.
An important aspect of establishing that Slug acts downstream of kit is that the 2 gene products be expressed in the same cell type and at the same time in vivo. For this reason we analyzed Slug mRNA expression in primary c-kit+ cells purified from the bone marrow of wild-type mice by in situ hybridization. As shown in Figure1F, Slug expression is observed in primary c-kit+ cells, and Slug expression is induced in these cells on SCF stimulation (Figure 1G). Because mutations on 2 different genes, the c-Kit receptor and its ligand (SCF), have the same complex phenotype that affects pigmentation, germ cells, and hematopoiesis, we carefully analyzed mice lacking a Slug gene to determination in vivo which functions of the c-kit/SCF pathway are mediated by Slug.
Pigmentation, gonadal defects, and hematopoietic defects in Slug mutant mice
The most obvious phenotype of Sl and W mutants in vivo is the presence of severe runting, which is observed shortly after birth. This characteristic is also observed in mice carrying a null mutation of the Slugh gene (SlughΔ1homozygous mutant mice), which appeared significantly smaller than their littermates.19 As in c-kit– and SCF-defective mice, the growth retardation of SlughΔ1 homozygous mutant mice occurred in the first 3 weeks of life. Accordingly, we next studied whether Slug, like c-kit receptor and its ligand, is also important for dermal, gonadal, and hematopoietic development.
Pigmentation deficiencies.
Melanoblasts originate in the pluripotent neural crest and migrate along characteristic pathways. For survival and migration, they depend on numerous signaling systems.27 Heterozygous mutant mice (W/+ or Sl/+) have a characteristic white forehead blaze and additional areas of depigmentation on the ventral body, tail, and feet. Homozygous mutant mice (W/Wv or Sl/Sld) are more affected and completely lack pigmentation of skin and hair, whose melanocytes are derived from the neural crest.22
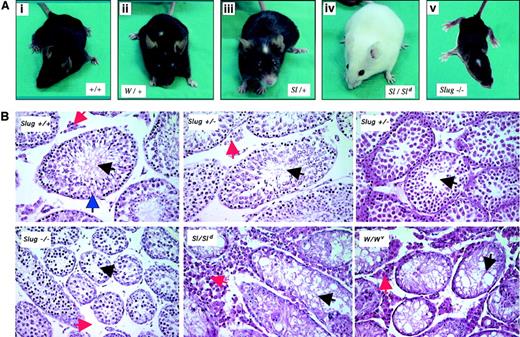
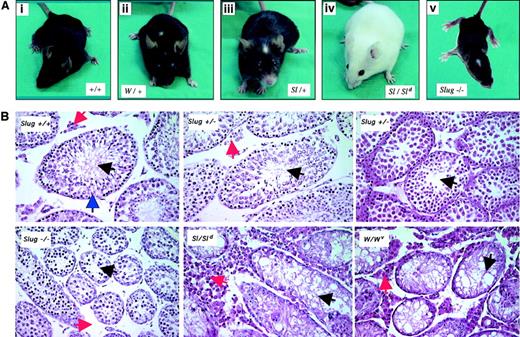
Heterozygous Slug mice did not present alterations in pigmentation. However, Slug homozygous mutant mice had a diluted coat with additional areas of depigmentation on the tails and feet and the characteristic white forehead blaze (Figure 2A). These dermal defects in Slug−/− mice consisted of various degrees of depigmentation. However, the retina and inner layer of the iris, whose melanocytes are derived from the optic cup and are independent of SCF/c-kit signaling pathway, are systematically pigmented in Slug−/− mice. These dermal defects observed in Slug−/− mice are similar to the dermal phenotype observed in W/+ and Sl/+ mice (Figure 2A) and suggest a role for Slug in the development of melanocytes derived from the neural crest.
Pigmentation and testis defects in Slug-deficient mice.
(A) Pigmentation deficiencies in Slug homozygous mutant mice. Wild-type mice (+/+) with no defective pigmentation (i); the characteristic white forehead blaze in Sl/+ and W/+ mice (ii-iii); lack of pigmentation of skin in Sl homozygous mutant mice (Sl/Sld); and Slug homozygous mutant mice with the characteristic white forehead blaze (iv). (B) Histologic analysis of testis of wild-type and Slug mutant mice. Matched testis sections from 6-week-old wild-type mice (Slug +/+), Slug heterozygous mice (Slug +/−), Slug homozygous mice (−/−), Steel-Dickie mice23(Sl/Sld), and W mutant mice (W/Wv) were stained with hematoxylin and eosin. The histologic section of Slug +/+ testis shows the presence of normal seminiferous tubules (blue arrowhead) where germ cell development begins at the periphery of the tubule and where spermatogonia reside. These cells give rise to the progressively more differentiated spermatocytes and spermatids that populate the more superficial tubular layers and ultimately yield mature spermatozoa that can normally be visualized in the tubular lumina (black arrowhead). Leydig cells are present in the interstitial space (red arrowhead). As in Sl/Sld and W/Wv mice, there is an overall reduction in size of the seminiferous tubules in Slug−/− mice, a characteristic that can be also observed in some Slug+/− mice (Slug+/− center with normal tubules versus Slug+/− right with small tubules). In the interstitial space of Slug−/− mice, there is a reduced number of Leydig cells. On the contrary, the interstitial space of Steel-Dickie and W mutant mice testis is disproportionately increased and filled with Leydig cells. Original magnification × 40.
Pigmentation and testis defects in Slug-deficient mice.
(A) Pigmentation deficiencies in Slug homozygous mutant mice. Wild-type mice (+/+) with no defective pigmentation (i); the characteristic white forehead blaze in Sl/+ and W/+ mice (ii-iii); lack of pigmentation of skin in Sl homozygous mutant mice (Sl/Sld); and Slug homozygous mutant mice with the characteristic white forehead blaze (iv). (B) Histologic analysis of testis of wild-type and Slug mutant mice. Matched testis sections from 6-week-old wild-type mice (Slug +/+), Slug heterozygous mice (Slug +/−), Slug homozygous mice (−/−), Steel-Dickie mice23(Sl/Sld), and W mutant mice (W/Wv) were stained with hematoxylin and eosin. The histologic section of Slug +/+ testis shows the presence of normal seminiferous tubules (blue arrowhead) where germ cell development begins at the periphery of the tubule and where spermatogonia reside. These cells give rise to the progressively more differentiated spermatocytes and spermatids that populate the more superficial tubular layers and ultimately yield mature spermatozoa that can normally be visualized in the tubular lumina (black arrowhead). Leydig cells are present in the interstitial space (red arrowhead). As in Sl/Sld and W/Wv mice, there is an overall reduction in size of the seminiferous tubules in Slug−/− mice, a characteristic that can be also observed in some Slug+/− mice (Slug+/− center with normal tubules versus Slug+/− right with small tubules). In the interstitial space of Slug−/− mice, there is a reduced number of Leydig cells. On the contrary, the interstitial space of Steel-Dickie and W mutant mice testis is disproportionately increased and filled with Leydig cells. Original magnification × 40.
Gonadal deficiencies.
Slug-deficient females were fertile, and the ovaries appeared normal. Most Slug−/− males were also fertile. Although they appeared to copulate normally, as evidenced by the formation of vaginal plugs, more than 15% failed to induce pregnancy in their mates. Those Slug−/− mice capable of fathering offspring usually produced small litters (3-6 pups per litter, in contrast to a normal litter of 10-12 pups). The sizes and weights of −/− testes were approximately 40% smaller than those of wild-type littermates. Histologic sections of testis from 6-week-old Slug-deficient mice revealed that testicular atrophy resulted from an overall reduction in size of the seminiferous tubules, a characteristic of W/Wv and Sl/Sldmice testis that can be also observed in some heterozygous Slug mice (Figure 2B). Spermatozoa were nonetheless visualized in the lumina, consistent with the fact that fertility was not critically compromised in these animals. Histologic analysis also revealed reduced numbers of Leydig cells in the interstitial spaces of Slug-deficient mice (Figure2B). On the contrary, the interstitial space of W/Wv and Sl/Sld mice testis is disproportionately increased and filled with Leydig cells. Therefore, Slug plays a role in male germ cell and Leydig cell development, but its loss is insufficient to completely compromise sperm cell production.
Hematopoietic deficiencies.
SCF and c-kit null mutant mice have severe hematopoietic deficiencies. SCF acts on hematopoietic progenitor cells, where it is reported to increase survival rather than recruitment into the cell cycle.14 Accordingly, we analyzed the role of Slug in normal hematopoiesis.
Macrocytic anemia in Slug−/− mice
Anemia is the most prominent hematopoietic phenotype abnormality seen in Sl and W mutants in vivo and it is responsible for growth retardation during the first weeks of life, a characteristic shared by Slug-deficient mice.19 Accordingly, we next examined blood parameters in the Slug−/− mutant mice. The hematologic parameters examined, in particular hemoglobin, mean cell volume, and mean cell hemoglobin concentration, define macrocytic anemia with normal peripheral blood cell counts (Table 1), one aspect of the mouse Sl and W, both of which are due to naturally occurring loss-of-function mutations in either SCF or c-kit receptor, respectively.
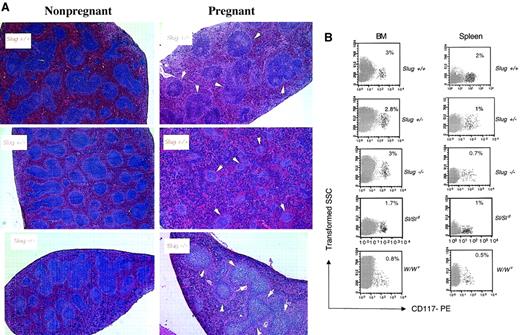
We next analyzed the capacity of expansion of erythropoiesis in Slug mutant mice on hematopoietic stress. The vast expansion of erythropoiesis that occurs in the murine spleen in response to hemolytic anemia or other hematopoietic stress (during pregnancy) is caused by the migration of BFU-E from the marrow to the spleen.28 Thus, we first examined the effects on erythropoiesis in the splenic red pulp of Slug mutant mice during pregnancy. Murine pregnancy is characterized by transient splenomegaly at mid-gestation by a dramatic increase in numbers of erythroblasts. This pregnancy-associated anemia is the major reason for the gross change in size and cell content of maternal spleen (Table2). On the contrary spleens of 12-day pregnant Slug mutant mice are smaller than spleens of control mice (Table 2). Histologic examination of spleens demonstrated that increases in splenic red pulp were less evident in Slug+/− and Slug−/− mice (Figure 3A). Flow cytometry results of the analysis of the expression of erythroid (TER-119) marker on cells from the bone marrow and spleen of normal and Slug mutant pregnant mice are shown in Table3. The frequency of TER-119+cells increased in the marrow and in the spleen during recovery from pregnancy-induced anemia in control mice. On the contrary, the increase of TER-119+ cells was compromised in Slug mutant mice in the marrow and in the spleen. These results show the poor recovery from pregnancy anemia in Slug mutant mice, indicating a defect in the generation or migration of progenitor erythroid cells in Slug mutant mice. Accordingly, we next quantitated the numbers of BFU-E, the most primitive erythroid progenitor cell, and CFU-E, which are more differentiated erythroid progenitor cells, by hematopoietic colony-forming assays in the BM and spleen of control and Slug mutant mice under physiological conditions (without erythroid stress). Numbers of BFU-E and CFU-E in Slug heterozygous mice were similar to those of control mice (Table 4). However, the numbers of BFU-E in BM and CFU-E in spleen was reduced in Slug homozygous mice in comparison with control mice (Table 4). Taken together, these results show a basal erythroid defect at the BFU-E level in Slug−/− mice. However, basal erythroid development appears normal in the Slug+/− mice, though hematopoietic stress (pregnancy) resulted in poor recovery from anemia.
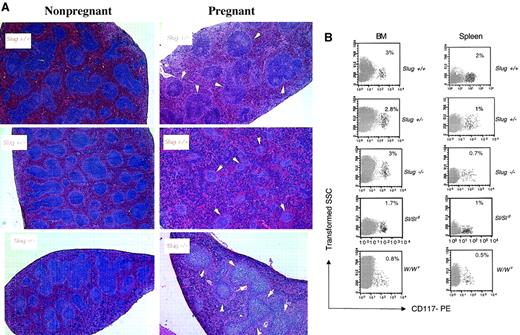
Erythroid development defects in Slug-deficient mice.
(A) Histologic examination of spleens of nonpregnant mice versus spleens of 12-day pregnant control mice (Slug+/+), Slug heterozygous mice (Slug+/−), and Slug homozygous mice (Slug−/−). Hematoxylin and eosin staining evidenced during pregnancy an enormous increase in the splenic red pulp of Slug+/+ mice in which the splenic white pulp, marked with white arrowheads, was reduced. The increase in the splenic red pulp was less evident in both Slug+/− and Slug−/− mice. (B) Representative analysis of the c-kit+ cells present in the BM and spleen of mice after induction of hemolytic anemia with PHZ. Cells isolated from a wild-type control (Slug+/+), a heterozygous mutant (Slug+/−), a homozygous mutant (Slug−/−), a Steel-Dickie mutant (Sl/Sld), and a W mutant (W/Wv) mouse were stained with the CD117-PE monoclonal antibody and analyzed by flow cytometry. The percentage of c-kit+ cells is indicated. The percentage of c-kit+ cells in spleen and BM before the induction of hemolytic anemia is as follows: 3% in BM and less than 1% in spleen of Slug+/+, Slug+/−, and Slug−/− mice and less than 1% in BM and less than 1% in spleen of W/Wv and Sl/Sld mice. Original magnification × 20.
Erythroid development defects in Slug-deficient mice.
(A) Histologic examination of spleens of nonpregnant mice versus spleens of 12-day pregnant control mice (Slug+/+), Slug heterozygous mice (Slug+/−), and Slug homozygous mice (Slug−/−). Hematoxylin and eosin staining evidenced during pregnancy an enormous increase in the splenic red pulp of Slug+/+ mice in which the splenic white pulp, marked with white arrowheads, was reduced. The increase in the splenic red pulp was less evident in both Slug+/− and Slug−/− mice. (B) Representative analysis of the c-kit+ cells present in the BM and spleen of mice after induction of hemolytic anemia with PHZ. Cells isolated from a wild-type control (Slug+/+), a heterozygous mutant (Slug+/−), a homozygous mutant (Slug−/−), a Steel-Dickie mutant (Sl/Sld), and a W mutant (W/Wv) mouse were stained with the CD117-PE monoclonal antibody and analyzed by flow cytometry. The percentage of c-kit+ cells is indicated. The percentage of c-kit+ cells in spleen and BM before the induction of hemolytic anemia is as follows: 3% in BM and less than 1% in spleen of Slug+/+, Slug+/−, and Slug−/− mice and less than 1% in BM and less than 1% in spleen of W/Wv and Sl/Sld mice. Original magnification × 20.
Next we quantitated the BFU-E and CFU-E numbers in Slug mutant mice in which we had previously induced hemolytic anemia with PHZ. Injection of PHZ causes acute red cell destruction followed by expansion of erythropoiesis.29 Accordingly, age-matched mice were injected with PHZ, and its effect was systematically monitored by day 3 in mice given PHZ by a prompt decrease in Hct and an increase in the reticulocyte count (data not shown). In Slug+/− mice with PHZ-induced hemolytic anemia, the number of CFU-E was reduced in the BM in comparison with control mice, and the expansion of BFU-E and CFU-E numbers in the spleen was affected (Table 4). Induction of hemolytic anemia with PHZ in Slug−/− mice resulted in an increase of marrow erythropoiesis, but the expected increase in splenic erythropoiesis was not entirely blocked (Table 4). These results demonstrate that the response to acute erythropoietic demand results in the expansion of erythropoiesis mainly at the BFU-E level in Slug mutant mice. A similar phenotype is seen in W/Wv mice.30 Although marrow erythropoiesis increases by day 3 in W/Wv mice given PHZ, the expected increase in splenic erythropoiesis is not produced by normal day 3.30 Flow cytometry results of the analysis of the expression of c-kit marker (CD117) on the cells from the BM and spleen of control, Slug mutant, Sl mutant, and W mutant mice after the induction of hemolytic anemia with PHZ shows that the increase in c-kit+ cells in the spleen of Slug mutant, Sl/Sld, and W/Wv mice was blocked in comparison to control mice (Figure 3B). These results show that Slug-deficient c-kit+ cells behave like Sl and W–c-kit+ cells and that the defect in erythroid development is similar between Slug mutant, Sl/Sld, and W/Wv mice.
T-cell compartment in Slug mutant mice
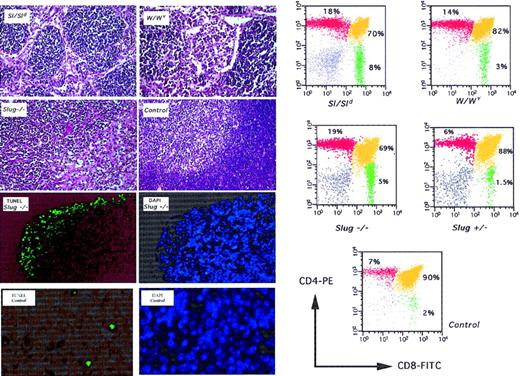
In mice lacking functional Slug expression, T-cell numbers in peripheral blood are normal, though analysis of thymus composition from 4-week-old mice shows reduced cell production and differentiation toward CD4+CD8+ cells similar to that in Sl and W mutant mice (Figure 4). This specific T-cell differentiation block was also eventually observed in Slug+/− mice. The thymus of Slug−/− mice was small and was examined on histologic sections. Morphologic differences between the thymi of −/− and +/+ animals of the same litter could be detected because the histologic appearance of the thymus of Slug−/− mice was similar to the thymus of Sl and W mutant mice (Figure 4). In thymus sections from Slug-deficient mice, we also observed many cells at the cortical level that appeared to correspond to apoptotic bodies that were not seen frequently in sections from wild-type mice (Figure 4). Consistent with this interpretation, we detected a significant increase in TUNEL-positive cells in thymus sections from Slug-deficient mice. Increased apoptosis in Slug-deficient animals correlated with thymus atrophy. These results are congruent with the idea that SCF promotes the growth of primitive mouse CD4−CD8−thymocytes, but not CD4+CD8+ cells or single CD4+ and CD8+ cells.31 32
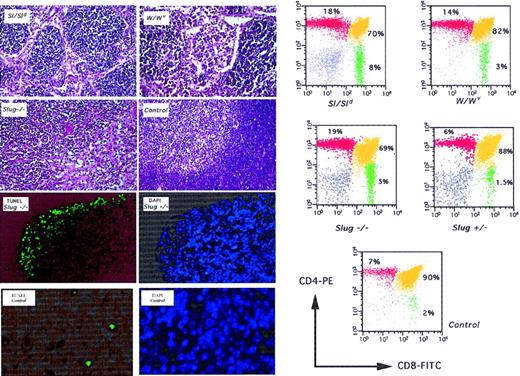
Deficient T-cell development and apoptosis in thymus of Slug-deficient mice.
Histologic examination was performed on thymus sections from 4-week-old Steel mutant (Sl/Sld), W mutant (W/Wv), wild-type (control), and homozygous mutant (Slug−/−) mice. All sections were stained with hematoxylin and eosin. The normal architecture of control thymus shows the presence of well-defined cortical and medullar regions. This organization is lost in Sl/Sld, W/Wv, and Slug−/− mice. Matched sections from Slug −/− and control mice were subjected to DAPI and TUNEL assay. Increased apoptosis in Slug-deficient animals correlated with thymus atrophy, where the weight of Slug−/− thymus was reduced by approximately 35% compared with that of wild-type mice. On the left, representative analysis of the cells present in the thymus of these mice is shown. Cells isolated from a wild-type (control), a heterozygous mutant (Slug+/−), a homozygous mutant (Slug−/−), a Steel mutant (Sl/Sld), and a W mutant (W/Wv) mouse were stained with the monoclonal antibodies and analyzed by flow cytometry. The percentage of cells is indicated.
Deficient T-cell development and apoptosis in thymus of Slug-deficient mice.
Histologic examination was performed on thymus sections from 4-week-old Steel mutant (Sl/Sld), W mutant (W/Wv), wild-type (control), and homozygous mutant (Slug−/−) mice. All sections were stained with hematoxylin and eosin. The normal architecture of control thymus shows the presence of well-defined cortical and medullar regions. This organization is lost in Sl/Sld, W/Wv, and Slug−/− mice. Matched sections from Slug −/− and control mice were subjected to DAPI and TUNEL assay. Increased apoptosis in Slug-deficient animals correlated with thymus atrophy, where the weight of Slug−/− thymus was reduced by approximately 35% compared with that of wild-type mice. On the left, representative analysis of the cells present in the thymus of these mice is shown. Cells isolated from a wild-type (control), a heterozygous mutant (Slug+/−), a homozygous mutant (Slug−/−), a Steel mutant (Sl/Sld), and a W mutant (W/Wv) mouse were stained with the monoclonal antibodies and analyzed by flow cytometry. The percentage of cells is indicated.
B-cell, myeloid, and mast cell development appear normal in Slug mutant mice
Extensive expression analysis by flow cytometry of the cell surface differentiation markers was performed on cells from spleen and from bone marrow of 5-week-old wild-type, Sl and W mutant mice, and Slug mutant mice. No reduction in cells of the myeloid and B-cell lineages was observed in the Slug mutant mice (Figure5A-B). Thus, unlike the critical role of Slug such as c-Kit/SCF interaction in the generation of erythroid and T-cell lineages, Slug does not seem to be required for normal B-cell and myeloid development in adult mice.
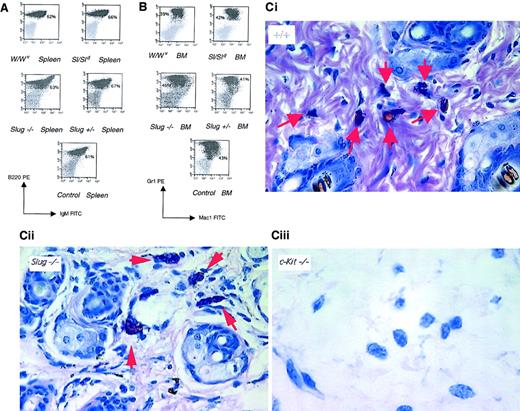
B-cell, myeloid, and mast cell development in Slug mutant mice.
Representative analysis of B cells and myeloid cells present in the spleen (A) and in the BM (B), respectively. Cells isolated from a wild-type (control), a heterozygous mutant (Slug+/−), a homozygous mutant (Slug −/−), a Steel mutant (Sl/Sld), and a W mutant (W/Wv) mouse were stained with monoclonal antibodies and were analyzed by flow cytometry. Percentage of cells is indicated. (C) Histologic examination was performed on ear sections from 4-week-old wild-type (+/+), homozygous mutant (Slug−/−), and W mutant (c-kit −/−) mice. All sections were stained with Giemsa.33 Arrowheads indicate the presence of mast cells in control and Slug−/− mice but not in W mutant mice. Original magnification × 40.
B-cell, myeloid, and mast cell development in Slug mutant mice.
Representative analysis of B cells and myeloid cells present in the spleen (A) and in the BM (B), respectively. Cells isolated from a wild-type (control), a heterozygous mutant (Slug+/−), a homozygous mutant (Slug −/−), a Steel mutant (Sl/Sld), and a W mutant (W/Wv) mouse were stained with monoclonal antibodies and were analyzed by flow cytometry. Percentage of cells is indicated. (C) Histologic examination was performed on ear sections from 4-week-old wild-type (+/+), homozygous mutant (Slug−/−), and W mutant (c-kit −/−) mice. All sections were stained with Giemsa.33 Arrowheads indicate the presence of mast cells in control and Slug−/− mice but not in W mutant mice. Original magnification × 40.
The SCF/c-kit signaling pathway is required for mast cell development.22 Thus, mast cells from 4- and 8-week-old Slug mutant mice were examined on histologic sections of different tissues.33 No morphologic differences between the mast cells of −/− and +/+ animals of the same litter could be detected (Figure 5C). Furthermore, the numbers of intestinal, skin, and peritoneal mast cells, organs known to be rich in mast cells, were equivalent in −/− and +/+ animals. Thus, the development and differentiation of mast cells does not seem to be affected by the absence of a functional Slug gene.
Defect in Slug mutant mice is intrinsic to the stem cell
Because receptor signaling depends on ligand interaction, it is not surprising that mutant forms of the c-kit receptor and its ligand produce almost identical developmental defects. However, transplantation experiments reveal a critical difference between the 2 mutations: the hematopoietic stem cells of Sl mice function normally in wild-type recipients, whereas those of W mutants do not (reviewed in Fleischman22). Accordingly, we first analyzed whether Slug mutant mice have a normal SCF/c-kit signaling pathway. To ensure that we had a normal c-kit–encoded transmembrane tyrosine kinase receptor for stem cell factor (Kit/SCF-R), we examined primary mast cells from bone marrow of +/+, +/−, and −/− age-matched mice. Kit/SCF-R from −/−, +/−, and control mice was of the same size and was expressed at comparable levels (Figure6A). The Kit/SCF-R was also kinase active and autophosphorylated on tyrosine residues on stimulation with SCF (Figure 6A).
Defect in Slug mutant mice is intrinsic to the stem cell.
(A) Kit immunoprecipitations from BM-derived mast cells were probed for kit and reprobed for phosphotyrosine. Slug+/−, heterozygous mice; Slug−/−, homozygous mice; Slug+/+, wild-type mice. (B, C) Analysis of the hematopoietic system in normal recipient mice reconstituted with either Slug+/+ HSC or Slug−/− HSC by FACS. BM, spleen, and thymus samples were stained with monoclonal antibodies and were analyzed by flow cytometry. The hematopoietic composition of mice reconstituted with Slug−/− HSC is similar to that for Slug−/−mice. A representative analysis of the c-kit+ cells present in the BM and spleen of normal recipient mice reconstituted with either Slug+/+ HSC or Slug−/− HSC after the induction of hemolytic anemia with PHZ is shown.
Defect in Slug mutant mice is intrinsic to the stem cell.
(A) Kit immunoprecipitations from BM-derived mast cells were probed for kit and reprobed for phosphotyrosine. Slug+/−, heterozygous mice; Slug−/−, homozygous mice; Slug+/+, wild-type mice. (B, C) Analysis of the hematopoietic system in normal recipient mice reconstituted with either Slug+/+ HSC or Slug−/− HSC by FACS. BM, spleen, and thymus samples were stained with monoclonal antibodies and were analyzed by flow cytometry. The hematopoietic composition of mice reconstituted with Slug−/− HSC is similar to that for Slug−/−mice. A representative analysis of the c-kit+ cells present in the BM and spleen of normal recipient mice reconstituted with either Slug+/+ HSC or Slug−/− HSC after the induction of hemolytic anemia with PHZ is shown.
To define whether the nature of the defect was either extrinsic or intrinsic to the stem cell, we analyzed the ability of Slug mutant hematopoietic stem cells to reconstitute permanent hematopoiesis in irradiated hosts. Engraftment of bone marrow cells from a healthy donor cures the hematopoietic phenotype seen in Slug−/− mice. On the other hand, wild-type recipients lethally irradiated and reconstituted with the hematopoietic stem cells of Slug−/− mice presented macrocytic anemia, and the composition of the hematopoietic system was similar to Slug−/− mice (Figure 6B). These mice had normal B-cell and myeloid development and a block in T-cell differentiation toward CD4+CD8+ cells. In addition, when treated with PHZ, c-kit+ cells were unable to migrate to the spleen. Moreover, the frequencies of BFU-E and CFU-E in the BM and in the spleen of PHZ-treated mice were similar between Slug−/− mice and wild-type mice reconstituted with Slug−/− BM. These findings indicate that the defect in Slug mutants is intrinsic to the stem cell.
Primary BM c-kit+ cells do not express Slug in W and Sl mutant mice
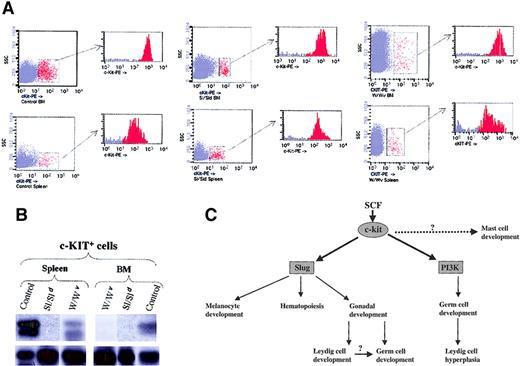
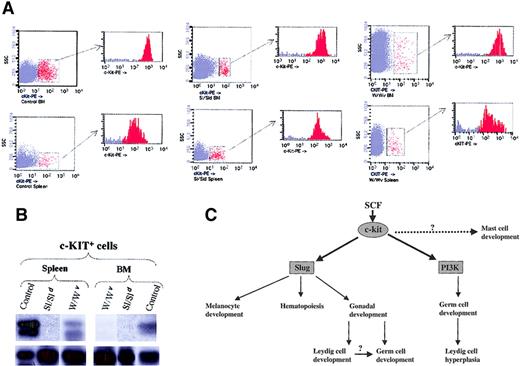
Slug subserves pivotal functions in promoting the development, survival, and proliferation of hematopoietic progenitor cells, neural crest–derived cells, and germ cells, a role well illustrated by the depletion of erythroid precursors and associated macrocytic anemia, gonadal defects, and hypopigmentation manifested by Slug-deficient mice. The findings that activation of c-kit specifically induces the expression of Slug and that Slug-deficient mice have phenotypes similar to those of Sl and W mutant mice prompted us to test whether Slug expression levels are up-regulated as a consequence of SCF/c-kit activation in control versus Sl and W primary c-kit+ cells. Accordingly, we induced hemolytic anemia with PHZ in control and Sl and W mutant mice. By day 3 c-kit+ cells from bone marrow and spleen were purified by sorting in control and Sl and W mutant mice (Figure 7A). Then we tested whether Slug was also present in c-kit+ cells purified from these mice. Examination of Slug expression by RT-PCR revealed that Slug was present in primary c-kit+ cells derived from bone marrow and spleen of control mice (Figure 7B). β-Actin expression was used to assess the integrity and loading of each RT-PCR reaction (Figure 7B, bottom section). The expression of Slug was higher within migratory cells seen in the spleen than in the c-kit+ cells that remained in the BM. In contrast and using the same experimental conditions, we could not detect the expression of Slug in primary c-kit+ cells purified from the bone marrow of W and Sl mutant mice. Only we observed Slug expression in primary c-kit+ cells derived from the spleen (migratory cells) of W mutant mice.
SCF/c-kit signaling pathway in development.
(A) c-kit+ cells present in the BM and spleen of wild-type (control), Steel mutant (Sl/Sld), and W mutant (W/Wv) after the induction of hemolytic anemia with PHZ. For cell sorter separation, cells were incubated with c-kit-PE and c-kit+ cells sorted by FACS. Sorted cells were then re-analyzed for purity with the cytometer; purity was 95% or greater. (B) Expression of Slug was analyzed by RT-PCR in the purified c-kit+ cells of BM and spleen from control, Sl/Sld, and W/Wv mice. PCR products were transferred to a nylon membrane and analyzed by hybridization with end-labeled internal oligonucleotide probe specific for theSlug gene (upper panel). β-Actin was used to check cDNA integrity and loading (bottom panel). (C) Model of the function of the SCF/c-kit signaling pathway in development. The SCF/c-kit signaling pathway impairs the development of 3 stem populations: melanoblasts, hematopoietic stem cells, and germ cells. Data presented here indicate that Slug contributes to the functions of SCF/c-kit in melanoblasts and hematopoietic cells, though there are Slug-independent aspects of melanocyte development and hematopoiesis. Identification of the molecular mechanism mediating the mast cell defect is still pending. Within the germ cells, SCF/c-kit function is mediated by Slug (this paper) and PI 3-kinase.35 36 The impairment of Leydig cell development in the absence of Slug could secondarily affect germ cell maturation.
SCF/c-kit signaling pathway in development.
(A) c-kit+ cells present in the BM and spleen of wild-type (control), Steel mutant (Sl/Sld), and W mutant (W/Wv) after the induction of hemolytic anemia with PHZ. For cell sorter separation, cells were incubated with c-kit-PE and c-kit+ cells sorted by FACS. Sorted cells were then re-analyzed for purity with the cytometer; purity was 95% or greater. (B) Expression of Slug was analyzed by RT-PCR in the purified c-kit+ cells of BM and spleen from control, Sl/Sld, and W/Wv mice. PCR products were transferred to a nylon membrane and analyzed by hybridization with end-labeled internal oligonucleotide probe specific for theSlug gene (upper panel). β-Actin was used to check cDNA integrity and loading (bottom panel). (C) Model of the function of the SCF/c-kit signaling pathway in development. The SCF/c-kit signaling pathway impairs the development of 3 stem populations: melanoblasts, hematopoietic stem cells, and germ cells. Data presented here indicate that Slug contributes to the functions of SCF/c-kit in melanoblasts and hematopoietic cells, though there are Slug-independent aspects of melanocyte development and hematopoiesis. Identification of the molecular mechanism mediating the mast cell defect is still pending. Within the germ cells, SCF/c-kit function is mediated by Slug (this paper) and PI 3-kinase.35 36 The impairment of Leydig cell development in the absence of Slug could secondarily affect germ cell maturation.
Effects of SCF of costimulation and migration of c-kit+cells in Slug−/− mice
Our observations raised the question of whether Slug affected the SCF functions on hematopoietic progenitor cells. It is well known that SCF in combination with other cytokines significantly enhance the growth of clonogenic cells in vitro. In Slug−/− mice, SCF did not increase the numbers of BFU-E and CFU-GM in combination with erythropoietin and GM-CSF, respectively (Table5). Moreover, to functionally test the effect of Slug on the migration of c-kit+ cells on SCF stimulation, we performed Matrigel assays with purified hematopoietic c-kit+ cells from BM of wild-type mice (Slug+/+), Slug heterozygous mice (Slug+/−), and Slug homozygous mice (Slug−/−) (Table 6). Control Slug+/+ c-kit cells traversed the reconstituted basement membrane at high cell frequency (28%) on SCF stimulation. In contrast, Slug−/− c-kit cells migrated through Matrigel at significantly lower rates on SCF stimulation (3%). These findings, together with the discovery that the activation of c-kit specifically induces the expression of Slug and Slug-deficient mice have a phenotype similar to that of Sl and W mutant mice, indicate that Slug is a molecular target that contributes to the biologic specificity of the SCF/c-kit signaling pathway.
Discussion
Developmental defects of SCF/c-kit signaling pathway mediated by Slug
Since the 1990s the in vivo SCF/c-kit migratory pathway and developmental fates are well known because of the existence of mutant mice in which genes encoding the receptor and its respective ligand are defective.5,6,9-13 Less is known about the mechanisms that provide biologic specificity to the SCF/c-kit signaling pathway in the formation and migration of c-kit+ cells. A key issue is the identity of the targets of c-kit signaling that underlie the migratory behavior of c-kit+ cells. In this regard, the biologic events controlled by the SCF/c-kit signaling pathway are similar to those that take place in epithelial–mesenchymal transitions during mammalian development, which are controlled by the Snail family of zinc-finger transcription factors.15-17 These proteins, which share an evolutionarily conserved role in invertebrates and vertebrates,15 are implicated in the generation and migration of mesoderm and neural crest cells in several vertebrate species.15-17 In this study we have investigated whether the biologic functions governed by the SCF/c-kit signaling pathway are mediated by the Snail family of proteins. Data presented here show that the activation of c-kit by SCF specifically induces the expression of a specific member of the Snail family of zinc-finger transcription factors, Slug, indicating a clear relationship between SCF/c-kit activation and Slug expression.
Because mice with mutations on the c-Kit receptor and its ligand (SCF) have the same complex phenotype that affects pigmentation, germ cells, and hematopoiesis, we carefully analyzed mice lacking a Sluggene to determine in vivo whether Slug mediates some functions of c-kit/SCF pathway. Mice carrying a loss-of-function mutation at the Slug locus were generated.19 The expression pattern of theSlug gene in migratory neural crest cells had suggested a function for this gene in the development of the nervous system.19 Accordingly, analysis of the mutant mice were focused on this system. Now, the analysis of the mutant mice focused on those developmental aspects dependent on the SCF/c-kit pathway. Our results demonstrated the presence of dermal, gonadal, and hematopoietic developmental defects in Slug mutant mice.
Only Slug−/− mice showed alterations in pigmentation, indicating that the absence of Slug impairs the migration or survival of pigmented stem cells derived from the neural crest in such a way that the failure of melanoblasts in Slug−/− mice occurs at sites farther from the neural crest—that is, the forehead. This function of Slug is congruent with the fact that in the mouse, the Slug gene is not expressed in premigratory neural crest cells but is expressed in migratory neural crest cells.19 These alterations in the pigmentation observed in Slug−/− mice are similar to those described in W and Sl mutant mice, explaining why some areas in W and Sl heterozygous mutant mice are completely depigmented whereas others remain normal. These data also indicate that intracellular signaling mediated by c-kit must exceed a critical threshold for Slug to become activated and melanoblasts to migrate and survive. Sl and W heterozygous mice do not seem to reach this threshold. In fact, melanoblasts migrating to the forehead and other affected areas may be at the low end of an SCF gradient.34
In addition to this deficiency of melanocytes, homozygous-mutant Slug animals showed evidence of testis defects. These defects involved spermatogonia and Leydig cells. The spermatogonia defect is well known in W and Sl homozygous mice, which are sterile, and it is specifically controlled by kit-mediated PI 3-kinase activation.35,36However, the interstitial space in W and Sl homozygous mice and in KitY719F/KitY719F testis is disproportionately increased and filled out with Leydig cells. One possible explanation for these observations is that balancing mechanisms exist within the Leydig lineage, such as soluble SCF produced within the testis37 and other signaling pathways such as FSH and insulinlike growth factor-1,37 that try to compensate for deficiencies in primitive germ cell compartments in W, Sl, and in KitY719F/KitY719F mutant mice by stimulating proliferation or survival of Leydig cells. On the contrary, in Slug−/− mice, the Leydig cell compartment, which derives from the neural crest and is c-kit+,37 is reduced. The impairment of Leydig cell development observed in Slug−/− mice indicates that the balancing mechanisms37 would need Slug. This defect of Leydig cell development could secondarily affect germ cell maturation. Thus, the SCF/c-kit signaling pathway would have a dual function within the testis: germ cell development mainly governed by kit-mediated PI 3-kinase activation35 36 and Leydig development controlled by Slug (Figure 7C).
Analysis of hematopoietic development in Slug mutant mice demonstrated a phenotype similar to that of W and Sl defective mice. Sl, W, and Slug homozygous mutant mice had macrocytic anemia. These mutations impaired the developmental capacity of progenitor cells of the erythroid and T-cell lineages and followed normal B-cell development. The defect in hematopoietic cell development in Slug mutant mice was cell intrinsic. Slug mutant mice presented identical phenotypes, irrespective of whether the hematopoietic cells were isolated directly from the mutant mice or recovered from transplant recipients. Therefore, the phenotype of Slug mutant mice is not caused by insufficiencies in the microenvironment (as in Sl mutant mice) but is instead caused by a cell-intrinsic defect in hematopoietic progenitor cells (as in W mutant mice). Table 7 illustrates the phenotypic similarities between the Slug mutant mice and specific Sl and W mice.
Other kit-expressing lineages, such as mast cells and most melanoblasts, show no obvious phenotypes in Slug mutant mice, suggesting that the cellular context is of great importance for the interpretation of the SCF/c-kit signal. Slug function in these cell types either is not required or can be compensated through synergy with other Snail family members. Another question concerns why the heterozygous loss-of-function of Slug leads to phenotypic abnormalities. This indicates that a loss-of-function mutation in one allele cannot be compensated for by the remaining wild-type allele of the same gene, defining Slug as a semidominant gene. This condition, known as haploinsufficiency, would generate Slug tissues that are mosaic with regard to the transcribed allele. Further experiments will be necessary to confirm that monoallelic expression may be the mechanism causing the haploinsufficiency of Slug genes.
Thus, mutations at Sl, W, or Slug impair the development of 3 stem cell populations: melanoblasts, hematopoietic progenitors cells, and germ cells. Accordingly, Slug is present in migratory c-kit+cells purified from control mice, but it is not present in c-kit+ cells derived from Sl/Sld mice or in bone marrow cells from W/Wv mice. SCF-induced migration was affected in primary c-kit+ cells purified from Slug−/− mice, providing evidence for a role of Slug in the acquisition of c-kit+ cells with the ability to migrate. The presence of Slug in spleen but not in BM c-kit+ cells from W/Wv mice could be explained by the fact that W/Wv mutants express c-kit, though with reduced surface expression and activity,22 indicating that intracellular signaling mediated by c-kit must exceed a critical threshold for Slug to become activated.
These findings are consistent with a model in which stem cells harboring the c-kit receptor would express Slug, promoting survival of the cell with dependence of the required external signal (SCF) and allowing cells to migrate outside their normal environment. If these are not achieved in a specific period of time, they would undergo apoptosis because they have been deprived of the required external signals to keep Slug expression. This would prevent migrating cells from entering territory inappropriate for their state of specification. These data indicate that signals regulating cell fate (or cell death) have an important role in maintaining patterns of cell specification and cell differentiation.
Thus, the results presented here indicate that Slug may be the factor that controls the migration and survival of c-kit+ cells. In this sense, it is known that p53 deficiency rescues the male fertility of W mice but has no effect on the survival of melanocytes and hematopoietic cells. Thus, male germ cell apoptosis in the absence of kit is p53 dependent.38 Our data show that Slug is involved in the development of Leydig, melanocyte, and hematopoietic cell lineages. Thus Slug, a protein that normally acts as a repressor,39 may down-regulate genes whose expression needs to be excluded from c-kit+ cells to migrate. Finally, the same molecules are used to trigger epithelial–mesenchymal transitions during embryonic development and in biologic functions mediated by the SCF/c-kit signaling pathway. These observations can pave the way to identify a common molecular mechanism conferring biologic specificity in the formation and migration of cells governed by other growth factors and their receptors through the Snail family of transcription factors.
Slug is a candidate gene for human piebaldism and hereditary anemias
Disorders of melanocyte development are characterized by heterogeneous distribution of pigmentation, so-called white spotting, typified by piebaldism and Waardenburg syndrome. It is now clear that these disorders of pigment cell development represent a subgroup of the neurocristopathies, involving defects of various neural crest cell lineages that include melanocytes.40 The present studies indicate that alterations in the Slug gene may be responsible for the piebald phenotype in some kindreds. Thus, in some patients, piebald trait may be prove to result from deletions in this gene rather than from mutations in the c-kit receptor gene.41
Another characteristic of Slug mutant mice is anemia. Human congenital anemias such as Diamond-Blackfan anemia, which are characterized by decreased erythroid progenitors in the marrow, resemble in some ways the anemia of Slug mutant mice. Thus, alterations in the Slug gene may be responsible for the anemia in some patients. However, it has been noted that humans with pathologic mutations of the KIT gene do not exhibit anemia.42 Now a direct test of this hypothesis is feasible.
SCF/c-kit Slug in transformation
The c-kit receptor is involved in leukemias and solid tumors. Mutations resulting in constitutive activation of c-kit have been described in acute myeloid leukemia,43,44 small cell lung cancer,45 gynecologic tumors,46 breast carcinoma,47 and colonic tumors derived from interstitial cells of Cajal (a cell type that is SCF dependent).48,49However, the oncogenic potential putatively conferred by alterations in c-kit activity in malignancy is uncertain. Our results show that Slug confers survival and migratory properties to c-kit+ cells. Thus, constitutive activation of c-kit could confer invasive properties to the tumor cells. In this regard, Slug may also represent a molecular event relevant to cell transformation by c-kit. Moreover, recent findings show that Slug is also expressed in t(17;19) leukemic cells,50 in rhabdomyosarcoma cells expressing the translocation PAX3-FKHR,51 and in cells expressing BCR-ABL (J.P.-L. et al, unpublished observations, May 2002). Thus, Slug may be a component of invasion in cancer biology by providing tumor cells with the ability to migrate. As such, Slug might constitute an attractive target for therapeutic modulation of invasiveness in the treatment of human cancer.
Potential uses for Slug
Progenitor/stem cell mobilization is important in clinical transplantation, gene therapy, and ex vivo expansion of hematopoietic stem cells. However, these and other applications of SCF have been limited by its mast cell–activating properties.2 The results presented here identify Slug as a molecule that contributes to the function of SCF/c-kit signaling pathway, suggesting that Slug could have clinical applications similar to those of SCF using current technology,52 with the advantage that Slug would not activate mast cells. Nevertheless, the effectiveness of this in hematopoietic stem cell mobilization remains to be determined.
We thank Dr D. Martı́n-Zanca for the PECE-kit expression vector and Dr T. Gridley for the Slug mutant mice and the BR1.4 plasmid. We thank Dr Pedro Soria for continuous and generous help with the mice irradiation and to J. C. Villoria-Terrón for excellent technical assistance with the mice. We also thank the members of laboratory 13 for helpful discussions, and we give special thanks to Prof R. González-Sarmiento for his unconditional help and support.
Supported by Direccion General Ciencia Y Tecnologia (DGCYT) (1FD97-0360, SAF2000-0148, BIO2000-0453-P4-02), Fundación Cientı́fica of the Asociacion Eespañola Contra el Cancer (AECC), Junta de Castilla y León (C.S.I. 3/01, CSI1/02), Fondo Investigacion Sanitaria (FIS) (99/0935, 01/0114), and the National Institutes of Health (1 R01 CA79955-01). A.R.-G. is a scholarship holder from Consejo Superior Investigaciones Cientificas (CSIC)-GLAXO.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Isidro Sánchez-Garcı́a, Instituto de Biologı́a Molecular y Celular del Cáncer, Centro de Investigación del Cáncer, CSIC/Universidad de Salamanca, Campus Unamuno, 37007 Salamanca, Spain; e-mail: isg@gugu.usal.es andjpl@usal.es.