DNA spotted microarrays were used to compare gene expression profiles from 2 functionally distinct human marrow stromal cell lines: HS-27a, which supports cobblestone area formation by early hematopoietic progenitors, and HS-5, which secretes multiple cytokines that support the proliferation of committed progenitors. One unexpected result was the high level of interleukin-7 receptor (IL-7R) gene expression in HS-27a stromal cells. Northern blot analysis confirmed the IL-7R RNA expression, and Western blots for the IL-7R protein detected both a full-length (90-kd) IL-7R and a smaller 30-kd fragment in both HS-27a cells and primary stromal cell cultures, whereas only the 90-kd receptor protein was detected in peripheral blood mononuclear cells. Biotinylated IL-7 was shown to bind to HS-27a cells under physiologic conditions, and this binding was inhibited by blocking anti–IL-7 antibodies. Tyrosine phosphorylation of several proteins (55 kd, 30 kd, and 24 kd) in HS-27a cells was rapidly increased after incubation with recombinant IL-7. One of the phosphorylated proteins proved to be the 30-kd IL-7R fragment. Exposure of HS-27a cells to IL-7 resulted in a 10-fold increase in secretion of IL-6 into culture supernatants but no increase in the cytokines stromal cell–derived factor 1, macrophage inflammatory protein 1α, or IL-1β. The up-regulation of IL-6 secretion is associated with a rapid but transient increase in detectable levels of IL-6 messenger RNA. These data suggest that IL-7 may function to regulate the milieu of the microenvironment by modulating IL-6 secretion by the IL-7R–expressing stromal elements.
Introduction
The bone marrow microenvironment represents a functional complex of cells and matrix that provides signals critical for the regulation of hematopoiesis. In an effort to dissect the microenvironment and define its functional components, we established a series of immortalized human stromal cell lines that differ in morphology and function. The cell line HS-27a was found to support cobblestone area formation by immature progenitors and to express a Notch ligand, Jagged-1, as well as other matrix molecules. In contrast, stromal cell line HS-5 does not support cobblestone area formation but does secrete many cytokines that support differentiation of committed progenitor cells.1 2
Differential gene expression in HS-27a and HS-5 cell lines was previously investigated on small-scale Affymetrix gene chips specifying 250 genes, most of which were cytokines and chemokines.3These original array data, although limited in scope, did confirm that the 2 cell lines were functionally distinct with HS-5 expressing more RNA for factors like granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF), whereas HS-27a expressed more message for stromal cell–derived factor 1 (SDF-1) and transforming growth factor-β (TGFβ). We have now used a large-scale DNA spotted microarray technology to provide a more comprehensive understanding of genes differentially expressed between HS-27a and HS-5. One unexpected result was high-level expression in HS-27a cells of the gene encoding the receptor for interleukin-7 (IL-7R). In this report we provide evidence that the IL-7R expressed by HS-27a is a functional receptor that transduces a signal, resulting in alterations in stromal cell function.
Materials and methods
Cell culture
Marrow stromal cell lines HS-27a and HS-5 were grown in RPMI 1640 medium (Gibco BRL, Carlsbad, CA) supplemented withl-glutamine (0.4 mg/mL), sodium pyruvate (1 nmol/L), and 10% fetal calf serum at 37°C under 95% air-5% CO2. After reaching confluency, cells were trypsinized and transferred at a cell density of 0.04 × 106 cells/cm2 to T-75 flasks (Costar, New York, NY) for DNA microarray analysis, or to multiwell plates for protein analysis, cell proliferation studies, and IL-7 binding assays. At this density, cultures were about 80% confluent and reached complete confluency at 3 days.
Primary long-term cultures (LTCs) from healthy donors were prepared under conditions previously described.4 Normal peripheral blood mononuclear cells (PBMCs) were isolated over Ficoll step gradients, hemolyzed, and washed 3 times in Hanks balanced salt solution (HBSS).
DNA microarray
Stromal cells were cultured in T-75 flasks for 3 days and total RNA was extracted using RNeasy spin columns (Qiagen, Valencia, CA). Four RNA preparations for each experimental group were prepared separately from continuously growing cultures over a period of 6 weeks. Human Universal RNA (Stratagene, La Jolla, CA) was used as a control RNA. RNA was annealed with oligo dT12-18 (Pharmacia, Piscataway, NJ), and reverse-transcribed using 0.5 mM deoxyguanosine triphosphate, 0.5 mM deoxycytidine triphosphate, 0.5 mM deoxyadenosine triphosphate, 0.3 mM deoxythymidine triphosphate, 0.2 mM aminoallyl deoxyuridine triphosphate (Sigma, St Louis, MO), and Superscript II reverse transcriptase (Gibco, Rockville, MD). After hydrolysis of RNA in 0.2 M NaOH, complementary DNA (cDNA) from stromal cells and the Universal control were coupled separately with Cy5 (Amersham, Piscataway, NJ) and Cy3, respectively, in 50 mM sodium bicarbonate, pH 9.0, followed by quenching with 2.7 M hydroxylamine. The Cy5- and Cy3-labeled cDNAs were combined and purified with a Qiaquick PCR purification kit (Qiagen), and suspended in 36 μL 3-fold concentrated saline sodium citrate (SSC) and 0.8 mg/mL poly-A (Roche, Indianapolis, IN). They were hybridized to the microarray containing over 17 000 known sequences. The hybridization was conducted in the DNA Array Facility at the Fred Hutchinson Cancer Research Center (FHCRC), and the 2-color microarray data were analyzed with GenePix Pro 3.0 software. Gene expression was shown as a normalized ratio of fluorescence intensity of Cy5-labeled stromal cell cDNA to Cy3-labeled cDNA from the Universal control RNA. The identity of Research Genetics clones and gene sequences of the microarray will be available through 2 Web sites: the NCBI Gene Expression Omnibus (GEO;www.ncbi.nlm.nih.gov/geo) and the FHCRC Web site (http://parma.fhcrc.org/MIwata) on publication.
Means and SDs of the ratios were measured, and gene sequences with statistically significant differences in gene expression were identified by unpaired Student t test (P < .05) as shown in Figure1.
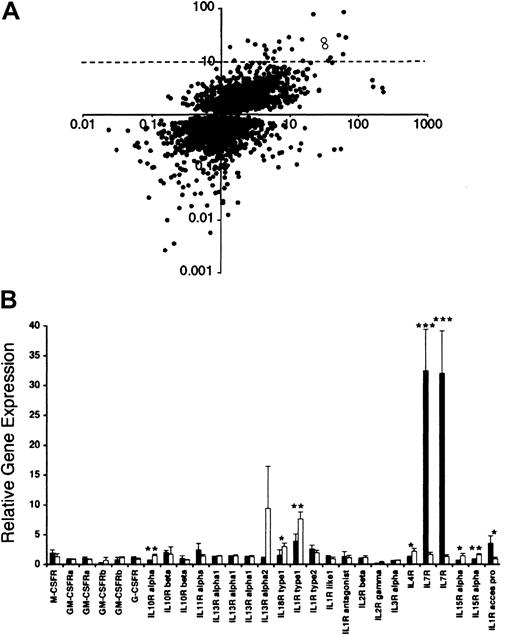
Microarray data.
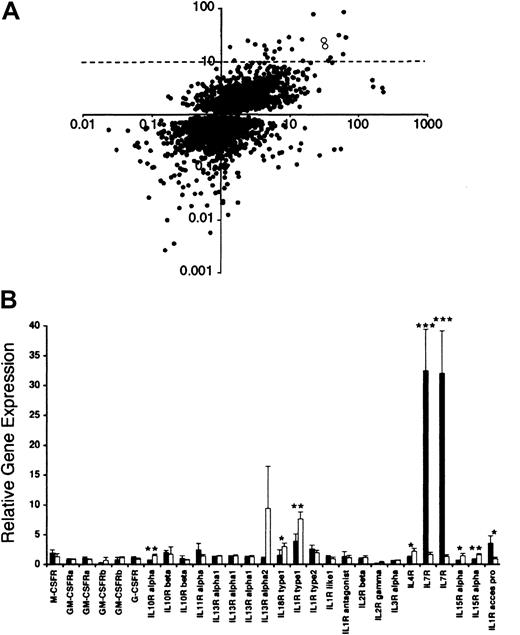
(A) Comparative analysis of microarray data of HS-27a and HS-5 cell lines. Gene sequences that were determined to have significantly different (P < .05) levels of expression in HS-27a cells compared to HS-5 cells are represented by a dot. Gene expression levels in HS-27a cells are shown on the x-axis. The y-axis indicates gene expression of HS-27a divided by HS-5. Therefore, genes expressed highly in HS-27a compared to the Universal control are at the far right in both the upper and lower quadrants. Genes that are also expressed to a greater degree in HS-27a compared to HS-5 are in the upper right quadrant. Two open circles in the upper right quadrant designate IL-7R. The dotted line indicates 10-fold higher gene expression for HS-27a than HS-5. (B) Microarray data on cytokine receptors in HS-27a and HS-5. Gene expression of 22 cytokine receptors from HS-27a (solid bars) and HS-5 (open bars) is shown as the ratio of hybridization signal from each stromal cell line to the hybridization signal from the Universal control RNA. Each bar represents the average ± SD for 4 samples. Stars indicate cytokine receptors whose gene expression is statistically different between HS-27a and HS-5 (*P < .05, **P < .005, and ***P < .0001).
Microarray data.
(A) Comparative analysis of microarray data of HS-27a and HS-5 cell lines. Gene sequences that were determined to have significantly different (P < .05) levels of expression in HS-27a cells compared to HS-5 cells are represented by a dot. Gene expression levels in HS-27a cells are shown on the x-axis. The y-axis indicates gene expression of HS-27a divided by HS-5. Therefore, genes expressed highly in HS-27a compared to the Universal control are at the far right in both the upper and lower quadrants. Genes that are also expressed to a greater degree in HS-27a compared to HS-5 are in the upper right quadrant. Two open circles in the upper right quadrant designate IL-7R. The dotted line indicates 10-fold higher gene expression for HS-27a than HS-5. (B) Microarray data on cytokine receptors in HS-27a and HS-5. Gene expression of 22 cytokine receptors from HS-27a (solid bars) and HS-5 (open bars) is shown as the ratio of hybridization signal from each stromal cell line to the hybridization signal from the Universal control RNA. Each bar represents the average ± SD for 4 samples. Stars indicate cytokine receptors whose gene expression is statistically different between HS-27a and HS-5 (*P < .05, **P < .005, and ***P < .0001).
Northern blot
Northern blot analysis was performed using a P32-labeled DNA probe of IL-7R (DNA clone no. 1096966, American Type Culture Collection, Manassas, VA), which has the same DNA sequence as one of 2 DNA spots for IL-7R in the spotted microarray (Research Genetics clone ID 840460). A plasmid containing the IL-6 sequence was a gift from Genetic Institute (Cambridge, MA). Ten or 20 μg total RNA was prepared from stromal cells and PBMCs after culturing in the presence or absence of IL-7 (100 ng/mL). Formaldehyde RNA agarose gels and capillary blotting were conducted as previously described.5 The blot was exposed to a phosphor screen that was quantitatively scanned by a Typhoon scanner (Molecular Dynamics, Santa Cruz, CA), and analysis was done using ImageQuant software.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotting
The HS-27a or primary LTC cells were grown in T-75 flasks or 6-well plates, washed with phosphate-buffered saline (PBS) 3 times, and removed using a cell scraper. PBMCs were centrifuged at 500gfor 5 to 10 minutes and washed twice with PBS. Supernatant was completely removed, and 2 volumes of boiling Laemmli sample buffer (Bio-Rad, Hercules, CA) containing 5% β-mercaptoethanol was added to the cell pellet. Proteins were extracted by heat denaturation at 98°C for 5 minutes and sonicated to reduce viscosity. They were then centrifuged at 20 000g for 5 minutes. The supernatant was saved, and its protein concentration was measured by a BCA protein assay kit (Pierce, Rockford, IL) after 8% trichloroacetic acid precipitation of proteins.
The sodium dodecyl sulfate–polyacrylamide electrophoresis (SDS-PAGE) gels (Invitrogen, Carlsbad, CA) with a 4% to 12% linear gradient of acrylamide were used. Immunoblots of these gels were stained with Ponceau S (Sigma, St Louis, MO) for proteins, blocked in Blotto, and probed sequentially with rabbit antibodies against a 20mer peptide mapping at the carboxy terminus of IL-7R (anti–IL7R-IC, Santa Cruz Biotechnology, Santa Cruz, CA) and horseradish peroxidase (HRP)–conjugated secondary antibodies (Amersham). Bound HRP activity was detected by an enhanced chemiluminescent method (Roche). Prestained molecular weight marker proteins (Gibco) were used to calculate molecular weight of proteins.
For detecting phosphotyrosine, SDS-polyacrylamide gels (Invitrogen) with 14% acrylamide were used. Immunoblots of these gels were probed with a monoclonal antibody against phosphotyrosine (PY20, BD Biosciences, San Diego, CA) and HRP-conjugated secondary antibodies according to the manufacturer's protocol.
Subcellular fractionation
Proteins from HS-27a cells were sequentially separated into cytosol, membrane, and insoluble fractions by the following procedure. Cells (6 × 106 cells in 2 T-75 flasks) were cultured for 3 days, washed with PBS 3 times, removed by a rubber scraper, and centrifuged at 500g for 10 minutes. The pelleted cells were suspended in 3 to 5 volumes of 50 mM Hepes, pH 7.4, 10 mM EDTA, and a protease inhibitor cocktail (1 tablet of complete protease inhibitor [Roche], 5 μL diisopropylfluorophosphate, and 10 μg iodoacetamide/10 mL buffer), and homogenized by sonication on ice. The homogenate was ultracentrifuged at 100 000g for 1 hour at 4°C using a Sorval RC M120 ultracentrifuge (Dupont, Newton, CT). The supernatant was saved as a cytosol fraction. The pellet was re-extracted under the same conditions as the first homogenization with a buffer containing 0.1% Triton X-100, 0.1 M NaCl, 10 mM EDTA, 50 mM Hepes, pH 7.4, and the protease inhibitor cocktail. The resulting second supernatant was saved as a membrane fraction. The pellet was homogenized under the same conditions with 8 M urea, 0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 0.1M NaCl, 10 mM EDTA, 50 mM Hepes, pH 7.4, and the protease inhibitor cocktail. The homogenate was vigorously shaken for 1 hour at room temperature and ultracentrifuged as described above at 15°C. The resulting supernatant was saved as an insoluble fraction.
Protein concentration in the subcellular fractions was measured by a BCA assay and adjusted to 1.3 mg/mL in each buffer. Two volumes of Laemmli sample buffer containing 5% β-mercaptoethanol was added, and the proteins were heat-denatured for electrophoresis.
Immunofluorescence
HS-27a cells were cultured in 8-chamber coverglass slides (Nalge Nunc, Naperville, IL) coated with 25 ng/mL human fibronectin at 40 000 cells/chamber for 2 days. They were washed with PBS twice, fixed in methanol for 5 minutes at room temperature, and washed with PBS 3 times. The slides were incubated with a blocking solution (10% goat serum in culture media) for over 30 minutes at 37°C to prevent nonspecific antibody binding. They were then incubated at 37°C for 2 hours with rabbit anti–IL7R-IC antibodies or control rabbit immunoglobulins (Zymed, San Francisco, CA) at 10 μg/mL in fresh blocking solution. After washing with PBS, the slides were incubated with Cy5-conjugated goat antirabbit IgG (Jackson, West Grove, PA) at 13 μg/mL in the blocking media for 30 minutes. They were washed with PBS 4 times and stained with 4′6-diamidino-2-phenylindole (DAPI) at 1 μg/mL in PBS for 5 minutes. After a further wash with PBS, the staining of Cy5 and DAPI was observed using a wide-field deconvolution fluorescence microscope (Applied Precision, Issaquah, WA).
Detection of IL-7 binding to HS-27a
Binding of biotinylated IL-7 to HS-27a cells was detected using a Fluorokine kit (R & D Systems, Minneapolis, MN). Cells were grown in T-75 flasks and removed from the flasks with PBS containing 0.5 mM EDTA. The cells were centrifuged at 500g for 5 minutes, washed twice with PBS, and suspended at 4 × 106 cells/mL in PBS. The cells in suspension were incubated at 4°C with biotinylated IL-7 (BTN–IL-7) or biotinylated soybean trypsin inhibitor (BTN-Ctrl) as a negative control according to the manufacturer's protocol. Biotinylated proteins bound to the cells were detected using fluorescein isothiocyanate (FITC)–conjugated streptavidin (FITC-avidin), and analyzed by flow cytometry (FACScan, Becton Dickinson, San Jose, CA).
Under adherent conditions, cells were cultured in 96-well plates at 0.8 × 104 cells/well for 1 day. They were washed twice in HBSS and incubated with 0.2 mg/mL goat IgG for 15 minutes at room temperature to block Fc-mediated interactions. The cells were then incubated for 1 hour at 4°C with 35 μL/well of BTN–IL-7 (0.71 μg/mL), BTN-Ctrl (1.42 μg/mL), or the mixture of BTN–IL-7 and blocking antibodies to test the specificity of the assay. For the antibody mixture, 20 μL anti–IL-7 blocking antibodies (2 mg/mL, R & D Systems) was mixed with 10 μL BTN–IL-7 (2.5 μg/mL) and 5 μL goat IgG (1 mg/mL), and incubated for 15 minutes at room temperature prior to addition to the cells. FITC-avidin (10 μL/well) was added to the cells, gently mixed by pipetting, and incubated for 30 minutes at 4°C in the dark. The cells were washed with RDF1 washing buffer provided by the manufacturer, and fluorescence intensity of FITC-avidin bound to the cells was measured using a fluorescence plate reader (CytoFluor II, PerSeptive Biosystems, Framingham, MA) with 485 nm excitation and 530 nm emission filters.
In some experiments, cells were cultured in 8-well chambered coverslides, followed by incubation with BTN–IL-7 or the mixture of BTN–IL-7 and blocking antibodies as described above. They were stained with FITC-avidin, and nuclei were counterstained with 5 μg/mL Hoechst in RDF1 washing buffer. The staining of FITC and Hoechst was observed using the wide-field deconvolution fluorescence microscope.
Detection of tyrosine phosphorylation
HS-27a cells were grown in 6-well plates at 0.4 × 106 cells/well for 1 day. IL-7 at 10 to 100 ng/mL or IL-1β at 2 ng/mL was added, and the cells were cultured for 0 to 70 minutes at 37°C. The cells were washed with cold PBS containing 1 mM sodium ortho-vanadate and 10 mM NaF 3 times. After complete aspiration of the washing solution, 0.2 mL/well of boiling Laemmli sample buffer containing 5% β-mercaptoethanol was added. The cells were scraped from the wells, transferred to a tube, and heat denatured for 5 minutes. The extracts were sonicated briefly to reduce viscosity, and 15 μL of the extracts was applied to SDS-PAGE followed by immunoblot with the PY-20 antiphosphotyrosine antibody as described above.
Immunoprecipitation
HS-27a cells were seeded in T-25 flasks at 3 × 106 cells/flask, and cultured for 3 days in the presence or absence of 100 ng/mL IL-7. The cells were washed with PBS containing 1 mM sodium ortho-vanadate and 10 mM NaF 3 times. The cells were lysed in a boiling 1% SDS, 10 mM Tris-HCl, pH 7.4, and 1 mM sodium ortho-vanadate, transferred to a tube, and heat denatured for 5 minutes. They were centrifuged at 14 000g for 5 minutes and supernatants were saved as denatured total cell extracts. Immunoprecipitation of tyrosine phosphorylated proteins in the extracts (80 μg) was conducted by using a biotinylated recombinant antiphosphotyrosine (RC20; BD Biosciences) and streptavidin-conjugated agarose beads (Sigma) according to the manufacturer's protocol. The immunoprecipitated proteins were denatured in the Laemmli sample buffer containing 5% β-mercaptoethanol, and applied to SDS-PAGE and immunoblotted using the anti–IL7R-IC antibodies as described above.
Cell proliferation assay
To study the effect of IL-7 on cell proliferation, HS-27a or LTC cells were seeded in 48-well plates at 1.5 × 104 or 0.5 × 104 cells/well, respectively. They were cultured in the presence or absence of 100 ng/mL IL-7 for various times, and cell numbers were estimated with the green fluorescent dye CyQuant GR (Molecular Probes, Eugene, OR), as described elsewhere.6
Cytokine and endotoxin analysis
Culture supernatants were harvested from cell cultures and frozen at −20°C until analyzed. Cytokine enzyme-linked immunosorbent assays (ELISAs) were performed by the FHCRC Shared Resources Facility.
Endotoxin levels of media, cytokine, and reagents were tested using a Limulus Amebocyte Lysate test kit (Charles River Laboratories, Wilmington MA) by kinetic-turbidmetric method performed by the Biologics Production Facility at FHCRC.
Results
Microarray comparison of gene expression in HS-27a and HS-5 cells
Gene expression in HS-27a and HS-5 was compared by DNA spotted microarrays specifying over 17 000 known human sequences. The expression level was determined relative to a Universal control RNA, which is a mixture of RNA from 10 control cell lines. This allows comparison of multiple samples and culture conditions and provides an internal control for array-to-array differences in spotting and overall hybridization efficiency. As shown in Figure 1A, gene expression of 4933 gene sequences was significantly different between HS-27a and HS-5 stromal cells. Among them, 25 sequences were expressed at a more than 10-fold higher level in HS-27a than HS-5. Fifty-two sequences were expressed at a more than 10-fold lower level in HS-27a than HS-5 (ratio HS-27a/HS −5, < 0.1). Identities of these genes with 10-fold and significant differences in expression between the 2 cell lines are presented by Graf et al34 (see accompanying Letter to the editor, page 1509). The present large-scale microarray data cover a whole spectrum of gene sequences of proteins including cytokines and chemokines and their receptors, signal transduction molecules, components of the cytoskeleton, cell cycle regulators, transcription factors, and metabolic enzymes. The complete data set and gene identification will be available through http://parma.fhcrc.org/MIwataand the NCBI Gene Expression Omnibus Web site (www.ncbi.nlm.nih.gov/geo) on publication.
We compared the present large-scale microarray data with our previously published data from small-scale gene chips specifying 250 genes of immunologic interest.3 Relative gene expression levels between HS-5 and HS-27a were consistent between the 2 data sets. Importantly, ratios of gene expression for several known stromal factors were also consistently reflected in levels of secreted proteins. HS-5 expressed approximately 100-fold more message for IL-1 and IL-6 compared to HS-27a. This was reflected in more than 100-fold more protein detected by ELISA (98.4 ± 18.2 pg/mL IL-1β in HS-5 compared to none detectable in HS27a; 1031.8 ± 379.9 ng/mL IL-6 compared to 0.4 ± 0.1 ng/mL, respectively). HS-5 expressed about 10-fold more message for monocyte chemoattractant protein 1 (MCP-1) and this too was reflected in ELISA data (HS-5 secreted 104.4 ± 54.8 ng/mL MCP-1, whereas HS-27a secreted 11.0 ± 1.4 ng/mL). SDF-1 message was expressed more than 10-fold more in HS-27a; this was consistent with ELISA data (9.1 ± 0.1 ng/mL in HS-27a compared to nondetectable in HS-5).
We were interested in the much higher expression of IL-7R in HS-27a than HS-5 (Figure 1A, open dots), because IL-7R has been well documented in hematopoietic cells but not in bone marrow stromal cells. Higher expression of IL-7R in HS-27a was observed for both sequences specifying IL-7R. One Research Genetics clone specifying IL-7R (ID 840460, first pair of the bars for IL-7R in Figure 1B) had a ratio of 32.4 ± 7.0 in HS-27a and 1.7 ± 0.4 in HS-5 (n = 4;P = .0001). The second Research Genetics clone specifying IL-7R (ID 841238) showed similar ratios and P value. We obtained a plasmid containing the gene sequence of clone ID 840460, and verified that its DNA sequence is identical to the 3′ end of the IL-7R coding sequence. Gene sequences of 22 cytokine receptors in 28 different spots are included in the microarrays, and their expression levels in HS-27a and HS-5 are also shown in Figure 1B.
Confirmation of IL-7R expression difference
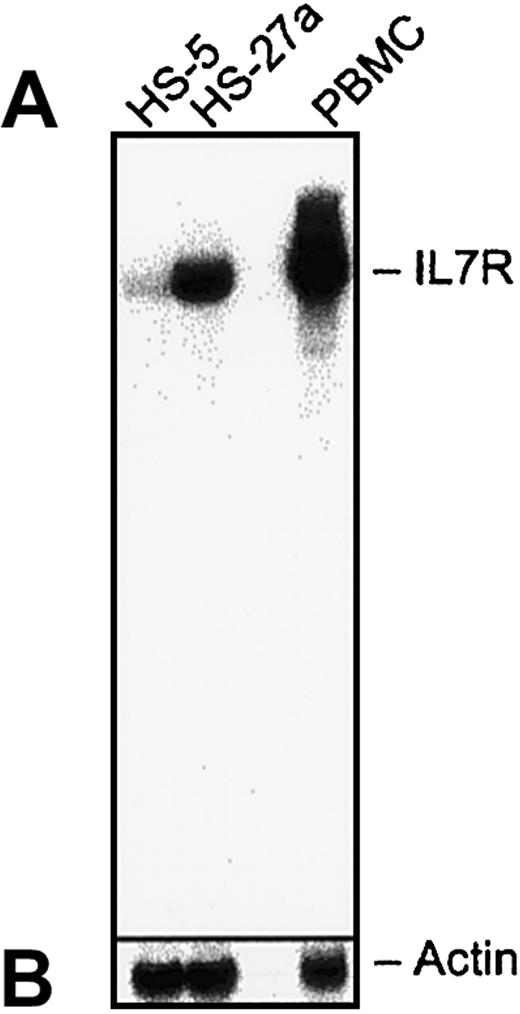
The difference in IL-7R RNA expression between the 2 stromal cell lines was confirmed by Northern blot analysis, as shown in Figure2. Also shown as a positive control is the expected band from peripheral blood lymphocytes. Quantitation by Phosphor Image analysis showed a more than 6-fold difference in hybridization of the IL-7R probe between HS-27 and HS-5 cells, whereas the actin signal in the 2 samples was equal. These data verify the microarray data of IL-7R.
Northern blot analysis of IL-7R gene expression.
Total RNA (20 μg/lane) from HS-27a, HS-5, or PBMCs was separated on an RNA agarose gel and blotted onto a nylon membrane. The membrane was probed with a P32-labeled IL-7R probe (A). The DNA sequence of the probe covers 1098 base pairs from the 3′ end and was confirmed to hybridize to human IL-7R. The bound probe was stripped off, and the membrane was reprobed with a P32-labeled actin probe (B). The data indicate a strong signal for IL-7R sequences in RNA isolated from HS-27a and PBMCs but not HS-5 cells.
Northern blot analysis of IL-7R gene expression.
Total RNA (20 μg/lane) from HS-27a, HS-5, or PBMCs was separated on an RNA agarose gel and blotted onto a nylon membrane. The membrane was probed with a P32-labeled IL-7R probe (A). The DNA sequence of the probe covers 1098 base pairs from the 3′ end and was confirmed to hybridize to human IL-7R. The bound probe was stripped off, and the membrane was reprobed with a P32-labeled actin probe (B). The data indicate a strong signal for IL-7R sequences in RNA isolated from HS-27a and PBMCs but not HS-5 cells.
IL-7R protein expression correlates with IL-7R gene expression
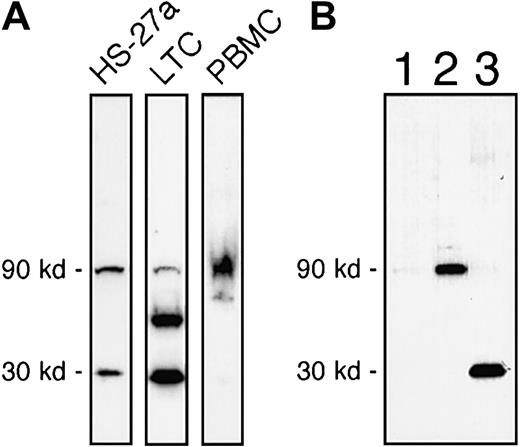
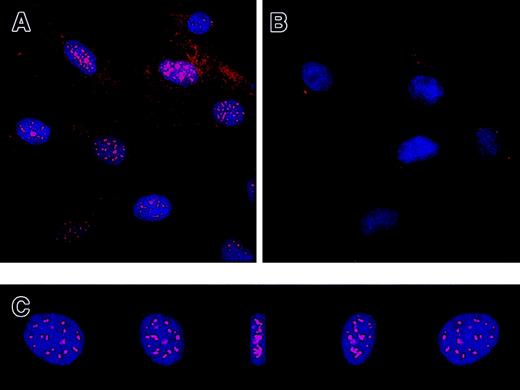
Protein expression of IL-7R in HS-27a and HS-5 by Western blot using commercially available antibodies against a synthetic peptide (20mer) mapping at the carboxyl terminus of the intracellular domain of the receptor (anti–IL7R-IC) is shown in Figure3. The intracellular domain of human IL-7R does not share significant homology with other proteins.7 Full-length human IL-7R has an expected molecular weight of 90 kd in reducing SDS-PAGE.8 A 90-kd band of IL-7R was detected in HS-27a, primary LTCs of bone marrow, and PBMCs (Figure 3A). However, immunoblotting for IL-7R protein in whole cell extracts of HS-27a occasionally revealed a smaller 30-kd band. The antibodies are specific for the IL-7R because the antibody binding to both 90-kd and 30-kd proteins was blocked when the antibodies were mixed with the 20mer peptide prior to incubation with the blot (data not shown). This 30-kd form was also consistently observed in primary LTCs, as shown in Figure 3A (middle lane). In contrast, the 30-kd form was not detected in PBMCs (Figure 3A, right lane). Three additional hematopoietic cell lines (HEL, KG-1a, and K562) were tested and found not to have a 30-kd band. Further characterization of the 30-kd form was undertaken by subcellular fractionation of HS-27a and Western blotting, as shown in Figure 3B. Cells were sequentially extracted into cytosol, membrane, and insoluble fractions. The full-length IL-7R was present in the membrane fraction (Figure 3B, lane 2), but the 30-kd form was exclusively found in the insoluble fraction (Figure 3B, lane 3). This suggested that the 30-kd form is tightly associated with cytoskeleton, nuclear matrix, or extracellular matrix of HS-27a. Punctate fluorescent staining was detected in the nucleus by immunofluorescence microscopy using anti–IL7R-IC antibodies, indicating that the 30-kd form might be tightly associated with the nuclear matrix (Figure 4A). Control rabbit immunoglobulins did not show nuclear staining (Figure 4B). The staining appeared to be inside the nucleus, as shown by reconstructing and rotating a 3-dimensional image (Figure 4C).
Immunoblot analysis of IL-7R in HS-27a, LTCs, and PBMCs.
(A) Total protein extracts of HS-27a, LTCs, and PBMCs were analyzed for IL-7R by Western blot. The 30-kd form, which bound to the anti–IL-7R-IC antibodies, was detected in both HS-27a and primary stromal cell extracts (LTCs) but was almost absent in the PBMC extract. Comparable results were obtained from 4 different cell preparations. (B) Subcellular fractions (26 μg/lane) of HS-27a cells were analyzed by Western blot. Lane 1 represents the cytosol fraction; lane 2, the membrane fraction; and lane 3, the insoluble fraction. The full-length form of IL-7R (90 kd) was found in the membrane fraction, and the 30-kd form was exclusively found in the insoluble fraction of HS-27a cells. This is in keeping with the immunocytochemistry data that show staining localized to the nucleus.
Immunoblot analysis of IL-7R in HS-27a, LTCs, and PBMCs.
(A) Total protein extracts of HS-27a, LTCs, and PBMCs were analyzed for IL-7R by Western blot. The 30-kd form, which bound to the anti–IL-7R-IC antibodies, was detected in both HS-27a and primary stromal cell extracts (LTCs) but was almost absent in the PBMC extract. Comparable results were obtained from 4 different cell preparations. (B) Subcellular fractions (26 μg/lane) of HS-27a cells were analyzed by Western blot. Lane 1 represents the cytosol fraction; lane 2, the membrane fraction; and lane 3, the insoluble fraction. The full-length form of IL-7R (90 kd) was found in the membrane fraction, and the 30-kd form was exclusively found in the insoluble fraction of HS-27a cells. This is in keeping with the immunocytochemistry data that show staining localized to the nucleus.
Immunofluorescence localization of intracellular IL-7R.
HS-27a cells were fixed with methanol and incubated with the anti–IL7R-IC antibodies (A) or control immunoglobulins (B). The bound antibodies were detected with Cy5-conjugated secondary antibodies (red). Nuclei of the cells were counterstained with DAPI (blue), and 2-color fluorescence images were captured with a deconvolution fluorescence microscope. The images of a nucleus stained with the anti–IL7R-IC antibodies are reconstructed into a 3-dimensional image and rotated by 45° 4 times as shown in panel C. This clearly shows localization of the intracellular receptor in the nucleus. Original magnification × 400.
Immunofluorescence localization of intracellular IL-7R.
HS-27a cells were fixed with methanol and incubated with the anti–IL7R-IC antibodies (A) or control immunoglobulins (B). The bound antibodies were detected with Cy5-conjugated secondary antibodies (red). Nuclei of the cells were counterstained with DAPI (blue), and 2-color fluorescence images were captured with a deconvolution fluorescence microscope. The images of a nucleus stained with the anti–IL7R-IC antibodies are reconstructed into a 3-dimensional image and rotated by 45° 4 times as shown in panel C. This clearly shows localization of the intracellular receptor in the nucleus. Original magnification × 400.
HS-27a cells can bind IL-7
The binding of IL-7 to HS-27a cells was tested using a commercially available kit with BTN–IL-7 (R & D Systems). Biotinylated soybean trypsin inhibitor (BTN-Ctrl) was used as a negative control. Binding was detected by addition of streptavidin-FITC and flow cytometry after gating for viable cells (Figure5A-B). The binding of BTN–IL-7 to the cells was reduced to the background level by blocking anti–IL-7 antibodies as shown in Figure 5C (P < .0001). Cell surface binding of BTN–IL-7 was visualized by fluorescence microscopy (Figure 5D-E). IL-7R was also detected on HS-27a by a phycoerythrin (PE)–conjugated antibody against the extracellular domain of IL-7R. The immunofluorescent staining colocalized with biotinylated IL-7 (data not shown). These data suggest that BTN–IL-7 binds to the IL-7R on HS-27a under physiologic conditions.
IL-7 binds to HS-27a cells.
Cells in suspension were incubated in PBS containing BTN–IL-7, BTN-Ctrl, or no protein (FITC only), followed by incubation with FITC-avidin. Live cells were gated, as shown in panel A, and analyzed by flow cytometry (B). Neg indicates the position of unstained control cells. (C) Cells were incubated under adherent conditions with BTN–IL-7, the mixture of BTN–IL-7 plus blocking anti–IL-7 antibodies (BTN–IL-7+Ab), or BTN-Ctrl. The cells were incubated with FITC-avidin and washed, and the fluorescence was measured by a fluorescence plate reader. Each bar represents the average ± SD for 4 samples. Nuclei of the cells incubated with BTN–IL-7 or the mixture of BTN–IL-7 plus blocking anti–IL-7 were counterstained with Hoechst dye, and staining of FITC (green) and Hoechst (blue) was visualized by fluorescence microscopy in panel D or E, respectively. Original magnification × 600.
IL-7 binds to HS-27a cells.
Cells in suspension were incubated in PBS containing BTN–IL-7, BTN-Ctrl, or no protein (FITC only), followed by incubation with FITC-avidin. Live cells were gated, as shown in panel A, and analyzed by flow cytometry (B). Neg indicates the position of unstained control cells. (C) Cells were incubated under adherent conditions with BTN–IL-7, the mixture of BTN–IL-7 plus blocking anti–IL-7 antibodies (BTN–IL-7+Ab), or BTN-Ctrl. The cells were incubated with FITC-avidin and washed, and the fluorescence was measured by a fluorescence plate reader. Each bar represents the average ± SD for 4 samples. Nuclei of the cells incubated with BTN–IL-7 or the mixture of BTN–IL-7 plus blocking anti–IL-7 were counterstained with Hoechst dye, and staining of FITC (green) and Hoechst (blue) was visualized by fluorescence microscopy in panel D or E, respectively. Original magnification × 600.
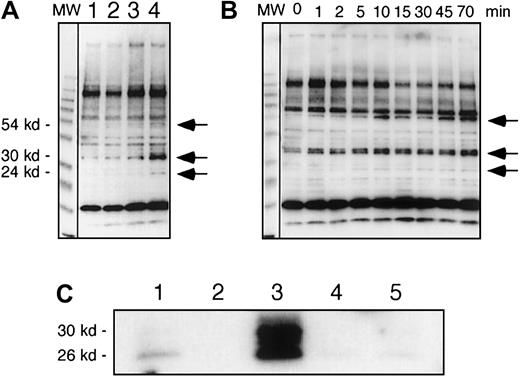
Recombinant IL-7 enhanced tyrosine phosphorylation of several proteins in HS-27a, demonstrating that the receptor is functionally active
Tyrosine phosphorylation of cellular proteins was analyzed after cells were incubated with recombinant IL-7, as seen in Figure6A. Tyrosine phosphorylation of 54-kd, 30-kd, and 24-kd proteins was increased after 5 minutes of IL-7 stimulation. Another cytokine, IL-1β, which has been previously shown to up-regulate gene expression of several cytokines and chemokines in HS-27a,3 had no effect on tyrosine phosphorylation within 5 minutes. A time course showed that increased tyrosine phosphorylation of the 30-kd protein could be detected as soon as 1 minute after addition of IL-7 (Figure 6B).
Recombinant IL-7 enhanced tyrosine phosphorylation of proteins in HS-27a.
In panels A and B, tyrosine phosphorylated proteins were analyzed by immunoblots using an antiphosphotyrosine antibody. (A) HS-27a cells were cultured for 5 minutes in standard media (lane 1), or media containing 2 ng/mL IL-1β (lane 2), 10 ng/mL IL-7 (lane 3), or 100 ng/mL IL-7 (lane 4). (B) Cells were cultured in the presence of 100 ng/mL IL-7 for various times as shown. Arrows indicate positions of the tyrosine phosphorylated proteins, which increased after exposure to recombinant IL-7. MW shows a lane of molecular weight marker proteins. (C) Tyrosine-phosphorylated proteins were immunoprecipitated with the biotinylated recombinant RC20 antiphosphotyrosine antibody and immunoblotted with the anti–IL7R-IC antibodies. Cells were cultured in the presence (lanes 3 and 4) or absence (lanes 1 and 2) of 100 ng/mL IL-7 for 1 hour. Total proteins of the cells were extracted under denaturing conditions and immunoprecipitated with (lanes 1 and 3) or without (lanes 2 and4) the RC20 antibody. Mock immunoprecipitation was conducted with the RC20 antibody but without cellular extracts (lane 5).
Recombinant IL-7 enhanced tyrosine phosphorylation of proteins in HS-27a.
In panels A and B, tyrosine phosphorylated proteins were analyzed by immunoblots using an antiphosphotyrosine antibody. (A) HS-27a cells were cultured for 5 minutes in standard media (lane 1), or media containing 2 ng/mL IL-1β (lane 2), 10 ng/mL IL-7 (lane 3), or 100 ng/mL IL-7 (lane 4). (B) Cells were cultured in the presence of 100 ng/mL IL-7 for various times as shown. Arrows indicate positions of the tyrosine phosphorylated proteins, which increased after exposure to recombinant IL-7. MW shows a lane of molecular weight marker proteins. (C) Tyrosine-phosphorylated proteins were immunoprecipitated with the biotinylated recombinant RC20 antiphosphotyrosine antibody and immunoblotted with the anti–IL7R-IC antibodies. Cells were cultured in the presence (lanes 3 and 4) or absence (lanes 1 and 2) of 100 ng/mL IL-7 for 1 hour. Total proteins of the cells were extracted under denaturing conditions and immunoprecipitated with (lanes 1 and 3) or without (lanes 2 and4) the RC20 antibody. Mock immunoprecipitation was conducted with the RC20 antibody but without cellular extracts (lane 5).
The phosphorylated 30-kd protein was shown by immunoprecipitation and Western blot to be the 30-kd form of IL-7R (Figure 6C). Total proteins in HS-27a cells were extracted after 0 or 1 hour of stimulation with IL-7, and tyrosine phosphorylated proteins were immunoprecipitated using biotinylated recombinant IgG variable regions of the antiphosphotyrosine antibody RC20 (BD Biosciences). The immunoprecipitated phosphorylated proteins were analyzed by Western blot using anti–IL-7R antibodies. The phosphorylated 30-kd form of IL-7R was detected in both control and IL-7–stimulated cells, but an increase of the phosphorylated 30-kd form was observed after exposure to IL-7 (compare lanes 1 and 3 in Figure 6C). Smaller proteins (26 kd and 28 kd) were detected in the immunoblot after the immunoprecipitation. Whether these are the result of multiple truncations after IL-7 stimulation or an artifact of protease digestion during the immunoprecipitation procedure despite the presence of the protease inhibitors is not clear.
IL-7 does not affect proliferation of HS-27a and LTC cells
It is reported that IL-7 promotes the growth of human adult T cells.9 Therefore we asked if recombinant IL-7 might increase cell growth of HS-27a and primary stromal cells. For this purpose, cells were cultured in the presence or absence of recombinant IL-7 (100 ng/mL) for 6 days. The cells were harvested every day, and cell growth was monitored by measuring DNA content using a CyQuant cell proliferation assay kit. Because HS-27a cells are not subject to contact inhibition, a linear growth curve was obtained during 6 days of culture. Results indicated that IL-7 did not influence cell proliferation of either HS-27a or primary stromal cultures over a 6-day period (data not shown).
Increased secretion of IL-6 protein by IL-7–stimulated HS-27a cells
The ELISA-based analyses of culture supernatants of HS-27a indicated a 10-fold increase in IL-6 protein levels after IL-7 stimulation (Figure 7A). The other cytokines tested were not similarly affected. An increase of IL-6 protein secretion of 2-fold was also observed in IL-7–stimulated primary stromal cultures (data not shown). The more modest increase realized in primary cultures is most likely due to already high constitutive levels of IL-6 detected in primary stroma. All media and cytokines used in these experiments were tested for endotoxin and found to be negative, thereby excluding the possibility of endotoxin-induced IL-6 secretion.
Cytokine production of IL-7–stimulated HS-27a cells.
(A) Concentrations of the cytokines IL-6, SDF-1α, IL-1β, and macrophage inhibitory protein 1-α (MIP-1α) in conditioned media were determined after 6 days of culture without (open bars) or with 100 ng/mL IL-7 (hatched bars) by ELISA. Each bar represents the average ± SD for 4 samples. (B) Northern blot analysis to measure the IL-6 (upper gel) and actin (lower gel) mRNA levels in IL-7 stimulated (+) or control (−) HS-27a cells at different time points. Each lane contained total RNA (10 μg/lane) blotted onto a nylon membrane. The membrane was probed with a P32-labeled IL-6 probe (upper gel). The bound probe was stripped off, and the membrane was reprobed with a P32-labeled actin probe (lower gel). (C) The blots were quantitatively scanned, and the ratio of IL-6 RNA to actin RNA was calculated. The IL-7–stimulated and control cells are represented by solid and dashed lines, respectively.
Cytokine production of IL-7–stimulated HS-27a cells.
(A) Concentrations of the cytokines IL-6, SDF-1α, IL-1β, and macrophage inhibitory protein 1-α (MIP-1α) in conditioned media were determined after 6 days of culture without (open bars) or with 100 ng/mL IL-7 (hatched bars) by ELISA. Each bar represents the average ± SD for 4 samples. (B) Northern blot analysis to measure the IL-6 (upper gel) and actin (lower gel) mRNA levels in IL-7 stimulated (+) or control (−) HS-27a cells at different time points. Each lane contained total RNA (10 μg/lane) blotted onto a nylon membrane. The membrane was probed with a P32-labeled IL-6 probe (upper gel). The bound probe was stripped off, and the membrane was reprobed with a P32-labeled actin probe (lower gel). (C) The blots were quantitatively scanned, and the ratio of IL-6 RNA to actin RNA was calculated. The IL-7–stimulated and control cells are represented by solid and dashed lines, respectively.
Levels of IL-6 messenger RNA (mRNA) were analyzed by Northern blots (Figure 7B-C). After 2 to 8 hours of IL-7 stimulation, a rapid but transient increase in IL-6 mRNA was seen in HS-27a. The mRNA levels returned to near the normal level 15 hours after IL-7 addition.
Discussion
In the present study, we used DNA spotted microarray technology capable of providing information on more than 17 000 known human gene sequences. Our goal was to identify differentially expressed gene sequences that may define functional differences between HS-5 and HS-27a cells. In so doing we found a 20-fold higher expression of IL-7R in HS-27a compared to HS-5 cells. Given that IL-7R is not reported to be expressed by marrow stroma, we felt additional studies were warranted to determine the validity of the microarray data.
The DNA spotted array data of IL-7R gene expression in HS-27a was confirmed by Northern and Western blots. Further, biotinylated IL-7 was found to bind to HS-27a cells under physiologic conditions. Binding of recombinant IL-7 induced tyrosine phosphorylation of several proteins, including what appears to be a 30-kd form of the IL-7R that is specific to stromal cells. By immunofluorescence staining and biochemical analysis, we found the 30-kd form of the receptor to be tightly associated with the nucleus.
These data suggest a model in which IL-7 may induce phosphorylation and truncation of IL-7R, whereby the truncated IL-7R translocates into the nucleus. Several transmembrane receptors, including those for IL-1 and epidermal growth factor, have also been detected in the nucleus.10-12 It is suggested that once in the nucleus, these receptors bind to chromatin or to specific DNA sequences to activate gene expression.13 Nuclear localization might therefore be a general feature of some cytokine receptors, although truncation of the receptors has not yet been reported. Clearly a more detailed analysis of this stroma-specific IL-7–mediated signaling pathway is warranted.
Interleukin 7 is produced by thymic, splenic, or bone marrow stroma14-17 and shows diverse and potent effects on progenitor cells of the lymphoid lineage. IL-7 increases proliferation of both immature and mature T cells and pro-B cells, but not mature B cells, in both humans and mice.9,18,19 Although it was originally cloned as a murine pre-B cell growth factor, IL-7 does not affect pre-B cell proliferation in humans.9,14 IL-7 is required for early T-cell development primarily to inhibit programmed cell death by regulating bcl-2 expression.20-22 Recently, IL-7 was found to down-regulate p27kip1, a cyclin-dependent kinase inhibitor and to promote cell cycle progression in T-cell acute lymphoblastic leukemia.23 In both IL-7– and IL-7R–deficient mice the number of B lymphocytes is markedly reduced.24,25 On the other hand, IL-7– overexpressing transgenic mice develop a lymphoproliferative disorder that results in B- and T-cell lymphomas.26 27
Interleukin 7 has been shown to bind to IL-7R with high affinity atKd of 0.25 nM to IL-7R alone andKd of 0.04 nM to a receptor complex of an IL-7R and the common γ chains.28 IL-7 also binds to heparin and heparan sulfate with a much lower affinity (Kd = 25 nM), as do many cytokines and growth factors, through heparin-binding consensus sequences.29-31These molecules may serve as coreceptors that facilitate interaction of growth factors with their receptors or provide a weakly associated docking site for the cytokines on the cell surface and extracellular matrix. HS-27a produces both IL-7R and heparan sulfate proteoglycans (present study and Graf et al34). It is reasonable to speculate that heparan sulfate proteoglycans from HS-27a cells may influence molecular interactions between IL-7 and IL-7R.
In the current study, IL-7 did not promote proliferation of IL-7R–expressing stromal cells. These data suggest that not only the proximal signaling pathway, but also the consequences of signaling, are different between lymphopoietic and stromal cells. In keeping with this, we found that exposure of HS-27a cells to IL-7 caused a 10-fold increase in the amount IL-6 secreted over a 6-day time course. This increased secretion of IL-6 could be explained in part by the rapid but transient increase in IL-6 mRNA seen between 2 and 12 hours of IL-7 stimulation. The same consequence of signaling, that is, increased IL-6 secretion, was also observed in primary cultures of human stromal cells.
Many cell types in addition to marrow stroma make IL-6. These include macrophages, lymphocytes, keratinocytes, endothelial cells, and some tumors.32 IL-6 in turn has wide-ranging proinflammatory effects. In addition to its stimulation of acute-phase protein biosynthesis in liver, IL-6 supports the proliferation of many cell types including early hematopoietic progenitors, B and T lymphocytes, and plasma cells.33 IL-7 in contrast is reported to have a more restricted function, limited primarily to the survival and expansion of lymphoid precursors. Here we report that IL-7 also stimulates marrow stroma to secrete IL-6. Hypothetically, this IL-7–induced change in the milieu of the marrow microenvironment could serve to promote the local expansion of immature progenitors, lymphocytes or plasma cells, or alternatively contribute to a systemic proinflammatory response. The actual physiologic role that IL-7 and IL-7–responsive stroma may play in regulating IL-6 production and the potential effect of this regulation on hematopoiesis or lymphopoiesis require further study.
The authors thank Ludmilla Golubev for maintaining the HS-27a and HS-5 cell lines, and Helen Crawford and Bonnie Larson for preparing the manuscript.
Prepublished online as Blood First Edition Paper, May 24, 2002; DOI 10.1182/blood-2002-01-0062.
Supported in part by grants HL62923, CA15704, and DK56465 from the National Institutes of Health, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mineo Iwata, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Mail-stop D1-100, PO Box 19024, Seattle, WA 98109; e-mail: miwata@fhcrc.org.