Translocations involving immunoglobulin (Ig) loci and chromosome 13 monosomy (Δ13) are frequent cytogenetic findings in multiple myeloma (MM). Similar chromosomal aberrations have been identified in the monoclonal gammopathy of undetermined significance (MGUS), but their prevalence and significance remain uncertain. Bone marrow from 72 patients with MGUS (n = 62) and smoldering MM (n = 10) was evaluated for translocations between the Ig heavy chain (IgH) and chromosomes 4, 11, and 16, translocations involving Ig light chain–lambda (IgL-λ, and Δ13. Fluorescence in situ hybridization (FISH) analysis was done on clonal plasma cells (PCs) detected by immunofluorescence (cIg-FISH) of the cytoplasmic light chain. We also studied cells for cyclin D1 and FGFR3 up-regulation by immunohistochemistry and immunofluorescence, respectively. Twenty-seven (46%) of 59 patients had IgH translocations, and 4 (11%) of 37 had an IgL-λ translocation. A t(11;14)(q13;q32) was found in 15 (25%) of 59 patients, a t(4;14)(p16.3;q32) in 9% of patients, and a t(14;16)(q32;q23) in 5% of patients. All patients with t(4;14)(p16.3;q32) tested (n = 3) had intense cytoplasmic fluorescence with an anti-FGFR3 antibody. PC nuclear staining of cyclin D1 was only observed in patients with t(11;14)(q13;q32); Δ13 was detected in the clonal PCs in 50% of patients. The percentage of abnormal PCs varied with any given abnormality. No obvious clinical or biologic correlations were associated with these chromosome abnormalities. Similar translocations are found in both MGUS and MM, including t(4;14)(p16.3;q32) and t(14;16)(q32;q23). Moreover, Δ13 is common in MGUS and unlikely to play a predominant role in the evolution of MGUS to MM.
Introduction
Chromosomal translocations involving 14q32 are thought to be early events in the pathogenesis of many B-cell neoplasias,1,2 including multiple myeloma (MM). Nearly all MM cell lines harbor chromosome translocations involving the immunoglobulin H (IgH) locus, which are believed to originate from errors at the time of isotype-class switching.3-8 These translocations involve an array of nonrandom, recurrent, partner chromosome that includes, among others, loci 4p16.3, 11q13, and 16q23.3-8 These translocations result in dysregulation of putative oncogenes, including cyclin D1/myeov (11q13), c-maf (16q23), FGFR3/MMSET (4p16.3), and MUM-1 (6p25), among others.3-5,9 The estimated prevalence of IgH translocations for patients with MM is close to 60% when fluorescence in situ hybridization (FISH) is used to study interphase nuclei.10 11 The overexpression of these oncogenes (mostly proliferation genes) is thought to promote cell division and cause immortalization of neoplastic plasma cells (PCs).
Monoclonal gammopathy of undetermined significance (MGUS) is a precursor state to MM and thought to have similar cytogenetic abnormalities as clonal PCs of MM, including IgH translocations.11 Avet-Loiseau et al12reported that 46% of patients with MGUS (36 of 79) have translocations involving the IgH locus. They found that t(11;14)(q13;q32) is the most common translocation in MGUS and is present in about one sixth of patients. However, they rarely observed a t(4;14)(p16.3;q32). We have found t(11;14)(q13;q32) to be prevalent in the clonal PCs of patients with a related condition, light-chain amyloidosis (AL).13More recently, Malgeri and colleagues14 detected t(4;14)(p16.3;q32) in 1 of 16 patients with MGUS by reverse transcriptase–polymerase chain reaction for the IgH-multiple myeloma SET domain (MMSET) transcript. In addition, Perfetti et al15 used the same method to detect t(4;14)(p16.3;q32) transcripts in 14% of patients with AL. No systematic study of translocations involving IgL-λ in MGUS has been performed, although these translocations have been documented in human MM cell lines16 and patient samples.17,18 Recent reports also show the presence of Δ13 in the clonal PCs of some patients with MGUS, although the percentage of abnormal PCs may vary more extensively among patients with MGUS than in MM.19,20It has been suggested that Δ13 may be important for progression from MGUS to MM, but the incidence of Δ13 in MGUS is not dramatically different from that observed in MM.19,21 22
PCs are, by definition, rare in bone marrow (BM) samples of patients with MGUS.23 To improve the sensitivity of the interphase FISH method, we used simultaneous immunofluorescent detection of the cytoplasmic light chains and cytomorphology (cIg-FISH)24 to analyze clonal PCs. The purpose of this study of MGUS patients was to determine the presence and frequency of translocations involving IgH and IgL-λ, including t(4;14)(p16.3;q32) and t(14;16)(q32;q23). We also studied oncogene up-regulation and other biologic and clinical association with these translocations.4 5
Patients, materials, and methods
Patients and BM samples
BM samples of patients with MGUS (n = 62) and smoldering MM (SMM) (n = 10) were obtained at the time of routine clinical procurement and under informed consent. Approval was obtained from the Mayo Clinic institutional review board, and the experiments were performed according to the guidelines for research with human subjects. Patients were classified as having MGUS according to standard criteria and were required to have no evidence of target organ damage indicative of MM.23,25 Patients with 10% or fewer clonal PCs in the BM and a monoclonal serum protein concentration of 3 g/L or less were categorized as MGUS. Patients with more than 10% clonal PCs in the BM or a monoclonal serum protein concentration of more than 3 g/L were categorized as SMM. This study was limited to patients with either IgG or IgA MGUS because patients with IgM MGUS have different biology.26
Slide preparation and interphase FISH
BM samples were enriched for mononuclear cells using the Ficoll-gradient centrifugation method. Cytospin slides were made with these cells and subjected to immunofluorescent detection of the cytoplasmic light chain with clone-specific, 7-amino-4-methylcoumarin-3-acetic acid–labeled antibodies (anti-κ or anti-λ).24 The signal was amplified with a second 7-amino-4-methylcoumarin-3-acetic acid–labeled anti-IgG antibody. Subsequently the cells were fixed with 2% paraformaldehyde and permeabilized with proteinase-K (10 μg/mL) at room temperature. Nick-translated probes were codenatured under a coverslip using a hybridization mixture containing 50% formamide and 10% dextran sulfate. The cells were hybridized overnight and washed with 0.4 × saline sodium citrate (SSC) for 5 minutes at 72°C, and antifade was added. For 34 of 72 patients, we enriched BM samples by performing CD138 bead selection to augment the number of PCs available for analysis by cIg-FISH.
Probes
Break-apart strategy (segregation).
The probes used in these experiments were directly labeled via nick translation with either SpectrumGreen or SpectrumRed (Vysis, Downers Grove, IL) (Figure 1). For the IgH segregation (“break-apart”) strategy we used both a bacterial artificial chromosome (BAC) clone for the constant region of the IgH locus (CH, labeled with SpectrumGreen) and a cosmid clone for the IgH variable region (VH, labeled with SpectrumRed) (Figure 1).13,27 For the IgL-λ segregation strategy we used 2 BAC clones for the Vλ and Cλ region of IgL-λ locus: These probes were kindly provided by W. M. Kuehl (Center for Cancer Research, Bethesda, MD) and described by Shou et al.16
Pictures with both the normal and abnormal patterns for the break-apart and fusion strategies.
In all cases the clonal PCs are identified by the intense blue fluorescence of the cytoplasm as detected by the cIg-FISH procedure. (A) A PC with the normal configuration for the VH (red signal) and CH probes (green signal). Arrows point to the closely associated pairs of signals indicative of a normal pattern. (B) Segregation (break-apart) of the VH (green) and CH (red) probes consistent with a 14q32 translocation. (C) A cell with fusion signals (arrows) resulting from comigration of the pools of probes at 14q32 (green) and 4p16.3 (red) consistent with a t(4;14)(p16.3;q32). (D) The fusion signal (arrows) resulting from comigration of the pools of probes at 14q32 (green) and 16q23 (red) consistent with a t(14;16)(q32;q23). (× 100 magnification, Leica DMRXA microscope [Wetzlar, Germany]).
Pictures with both the normal and abnormal patterns for the break-apart and fusion strategies.
In all cases the clonal PCs are identified by the intense blue fluorescence of the cytoplasm as detected by the cIg-FISH procedure. (A) A PC with the normal configuration for the VH (red signal) and CH probes (green signal). Arrows point to the closely associated pairs of signals indicative of a normal pattern. (B) Segregation (break-apart) of the VH (green) and CH (red) probes consistent with a 14q32 translocation. (C) A cell with fusion signals (arrows) resulting from comigration of the pools of probes at 14q32 (green) and 4p16.3 (red) consistent with a t(4;14)(p16.3;q32). (D) The fusion signal (arrows) resulting from comigration of the pools of probes at 14q32 (green) and 16q23 (red) consistent with a t(14;16)(q32;q23). (× 100 magnification, Leica DMRXA microscope [Wetzlar, Germany]).
Fusion strategy (colocalization).
For detection of translocation fusion signals, we used probes for 4p16.3, 11q13, and 16q23 (labeled with SpectrumRed); that bracket all reported breakpoints in the human MM cell lines, along with the VH and CH probes (labeled with SpectrumGreen). For the 11q13 region, we used a contig of probes spanning approximately 100 kilobases (kb) telomeric and 600 kb centromeric to the cyclin D1 gene (all directly labeled in SpectrumRed; Vysis).28-30 This pool contained the cosmids cCL11-505, cCL11-44, and cCL11-356 (provided by K. Hashimoto, Japanese Collection of Research Bioresources, Tokyo, Japan), cos3.62 and cos3.91 (provided by Ed Schuuring at Leiden University, The Netherlands), and the P1 clones ICRF 700 B1587 and ICRF 700 J077 (provided by R. Kochan, Resource Center and Primary Database [RZDP], Berlin, Germany). For the 4p16.3 region, we used the cosmid probes described in detail by Chesi et al4 and spanning a total of approximately 200 kb and encompassing all reported 4p16.3 breakpoints in human MM cell lines. For the detection of translocations involving 16q23, we used 2 clones obtained from a BAC library (clones 356D21 and 484H2; Research Genetics, Huntsville, AL) and the BAC clones 10205 and 10206, as described by Chesi et al.5 The clone 356D21 (120 kb) is located centromeric to 484H2 (95 kb), and both are separated approximately 500 kb apart (both are centromeric to c-maf).
Ploidy and Δ13 probes.
In 15 cases we determined the percentage of PCs with aneuploidy by using cIg-FISH and centromeric probes for chromosomes 7, 9, 11, 15, 18, and X (Vysis).24 We used the highest proportion of aneuploid PCs for comparison of the proportion of clonal PCs that had any given chromosomal translocation. To calculate, we used the following formula: We subtracted from 100% the percentage of cells with a normal FISH pattern. Therefore, this percentage includes all cells with a given abnormality for a set of probes used simultaneously. We also compared percentage of abnormal PCs by translocation assay versus percentage abnormal PCs by the Δ13 assay. The Δ13 assay was performed simultaneously using FISH probes D13S319 and LSI13Rb from Vysis, which we previously validated for the study of PCs.31
Normal and abnormal signal patterns
Probe validation and normal range
Before doing this investigation, we studied normal metaphases to establish that the probes hybridized to the correct chromosomal loci, and we found no cross-hybridization to inappropriate loci. We also did separate hybridization experiments on specimens known to have either t(11;14)(q13;q32) (clinical samples), t(4;14)(p16.3;q32) (cell line KMS11), or t(14;16)(q32;q23) (cell lines OCI-MY5, JJN3, and MM.1) to confirm that these probes could accurately detect these specific translocations. We tested these probes on normal cells to establish the incidence of nuclei with false-positive signal patterns: The upper limit of normal plus 3 SD proved to be 9%. We attempted to count as many PCs as possible (> 20 in all samples). However, to further improve the stringency of our criteria and because of the uncertain biologic significance of patients with low percentages of abnormal PCs, we used the following cutoff values: Less than 10% of cells with an abnormal pattern indicated no translocation, 10% to 24% abnormal cells was an equivocal result, and 25% or more abnormal cells indicated a translocation. For the Δ13 assay we used our previously determined upper limit of normal (≥ 10% is indicative of Δ13) to discern samples with and without the abnormality.31,32 We previously reported the normal range for cells that are aneuploid by the cIg-FISH strategy.24
Scoring
The scoring process included a cytomorphologic assessment to exclude non-PCs with cIg positivity, including lymphocytes and monocytes, and we attempted to score only the cIg-positive PCs. A decision to score a cell was made a priori using the 4,6-diamidino-2-phenylindole (DAPI) filter to assess cell morphology. If at least one signal of each probe was observed, the cell was scored.
Immunohistochemistry and immunofluorescence
Immunohistochemical detection of cyclin D1up-regulation was done on paraffin-embedded BM core biopsy samples as previously described by Hoyer et al.33 A sample was considered positive if the PCs had nuclear positivity (± cytoplasmic stain). Immunofluorescent detection of fibroblast growth factor–receptor 3 (FGFR3) up-regulation was performed on cytospin slides as reported by Chesi et al.34 A sample was considered positive if it displayed strong cytoplasmic staining.
Results
Patient demographics
Seventy-two patients were studied. The medical history of each patient met the criteria for the diagnosis of MGUS or SMM.23 All patients had a monoclonal protein in the serum detected by protein electrophoresis and/or immunofixation (κ = 42, λ = 30). Patients were not eligible for this study if they had evidence of MM complications. All MGUS patients had discrete clonal proliferations of PCs (< 10%) in their BM as detected by PC labeling index (PCLI) or aspirate. The median value of the monoclonal protein on the serum protein electrophoresis was 13.5 g/L [1.35 g/dL] (range immunofixation only to 35 g/L [3.5 g/dL]). Nine patients had a monoclonal protein that could only be detected by immunofixation. The isotypes were as follows: 64 patients had an IgG protein, 4 had IgA, and 4 had light-chain–only disease (idiopathic Bence-Jones proteinemia). The distribution of isotypes is similar to what has been described for other MGUS series, with only a slight lower proportion of cases with a monoclonal IgA protein.23 This can have some, albeit minor, influence on the prevalence of the specific chromosomal abnormalities observed because they are thought to correlate with heavy-chain isotype usage.
IgH and IgL-λ translocations
A translocation involving either an IgH or IgL-λ locus was observed in 30 (49%) of 61 patients (Tables1,2). A translocation specifically involving the IgH locus was seen in 27 (46%) of 59 patients. The median percentage of abnormal PCs was 57% (range, 27%-100%). A total of 14 patients had equivocal results for a translocation involving the IgH locus and no other definitive translocation (Table3). Translocations involving the IgL-λ locus were seen in 4 (11%) of 37 patients. The median percentage of abnormal PCs was 63% (range, 25%-90%). Another 3 patients had equivocal results for a translocation involving the IgL-λ locus. Altogether, 47 (77%) of 61 patients had evidence of a definitive or equivocal result for an IgH translocation. There was no significant difference in the prevalence of the translocation between patients with samples that were enriched versus not (P > .2) and between patients in whom the abnormality was detected by immunofixation only versus those that had a measurable monoclonal protein (P > .2).
Specific partners and disease progression
Fifteen (25%) of 59 patients had evidence of a t(11;14)(q13;q32) (21 [36%] of 59 if equivocals were included) (Table 1). The median percentage of abnormal PCs was 64% (range, 25%-100%). None of these patients progressed since the time of translocation detection (range, 0-52 months) or diagnosis (range, 0-55 months). Five (9%) of 56 patients had a t(4;14)(p16.3;q32) detected (7 [13%] of 56 if equivocals included) (Figure 1). Four of these 5 patients had a predominant pattern of only 1 fusion signal. These 5 patients were followed for 6.5 to more than 14 months since the time of translocation detection, 21 to 58 months since the time MGUS was diagnosed, and had no evidence of disease progression. The median percentage of abnormal PCs was 79% (range, 37%-100%). Three (5%) of 58 patients had the t(14;16)(q32;q23) detected in 25%, 50%, and 100% of PCs (Figure 1). Two of these patients had a predominant pattern of only 1 fusion signal. These patients have been followed for 7 to 25 months since the time of translocation detection, for 24 to 66 months since the time MGUS was diagnosed, and had no evidence of disease progression.
Two patients had clear evidence of 2 IgH translocations (Table1). One patient had 91% of the cells with a t(4;14)(p16.3;q32) fusion abnormality pattern, while there were also 30% of cells with a t(11;14)(q13;q32). This patient had 2 subclones detected in the IgH break-apart strategy; 70% of the cells had 1 red, 1 green, and 1 fusion pattern, while 30% of the cells had a 2 reds, 1 green, and 1 fusion pattern. Presumptively. the t(11;14)(q13;q32) represents a secondary rearrangement in a clone already established with a t(4;14)(p16.3;q32). Another patient had 100% of the cells with t(4;14)(p16.3;q32) and 100% of cells with a t(14;16)(q32;q23). There was one predominant pattern in the IgH break-apart strategy: 1 red, 1 green, and 1 fusion.
Chromosome 13 abnormalities
The Δ13 was detected in 24 (50%) of 48 patients using the standard cutoff criterion. If we used the same and more stringent criterion for IgH translocations (≥ 25% abnormal cells), we still found the abnormality at high prevalence (19 [40%] of 48 cases). In all cases both signals were simultaneously deleted, likely indicating a large deletion or monosomy, as we and others have reported for MM.31,35 The median percentage of abnormal PCs was 91% (range, 10%-100%). Patients with t(11;14)(q13;q32) (7 of 12 patients,P > .2) or IgL-λ (1 of 3, P > .2) were equally likely to have Δ13 but not those with t(4;14)(p16.3;q32), where Δ13 appeared to be more prevalent (3 of 4 patients).36 No meaningful information was available for patients with the t(14;16)(q32;q23).
Biologic and prognostic variables
Each of the chromosomal abnormalities was correlated with laboratory biologic and prognostic variables of MM (serum monoclonal protein concentration, hemoglobin, creatinine, β2-microglobulin, BM PC%, and PCLI). Given the small number of patients, no specific clinical and biologic features could be established, although there was a suggestion that patients with Δ13 had higher concentrations of serum monoclonal protein (WilcoxonP = .07).
Aneuploidy correlation
We compared the percentage of abnormal cells with Δ13 versus those with aneuploidy (Table 4). Heterogeneity was common for both abnormalities, although in general Δ13 had a greater tendency to involve a large fraction of the clonal cells. Among all patients with translocations, a significant proportion of them did have the abnormality detected in less than 50% of clonal PCs: 9 (38%) of 24 with an VH/CHtranslocation, 3 (20%) of 15 with t(11;14)(q13;q32), 1 of 5 with t(4;14)(p16.3;q32), and 1 of 3 with t(14;16)(q32;q23). In 15 patients, we attempted to correlate the proportion of PCs with aneuploidy with the percentage of PCs with an Ig translocation (IgH, specific partners, or IgL-λ). In several patients the percentage of abnormal PCs with aneuploidy was higher than the abnormal PCs with a translocation or with Δ13.
Cyclin D1 and FGFR3 detection
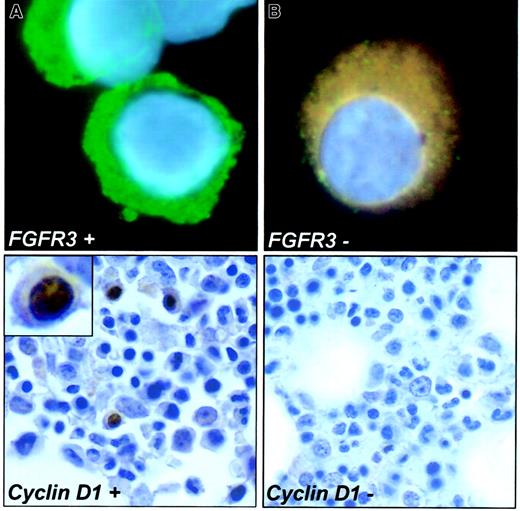
We tested for FGFR3 up-regulation by immunofluorescence in 3 patients using leftover slides and found a 1:1 correlation with t(4;14)(p16.3;q32) (Figure2). We also found that cyclin D1–positive nuclear staining could only be demonstrated in patients with t(11;14)(q13;q32). Eight (57%) of 14 patients with t(11;14) (q13;q32) had cyclin D1–positive staining in the cell nucleus. In contrast, none of the 6 patients without t(11;14)(q13;q32) had cyclin D1–positive staining.
Genetic up-regulation of FGFR3 and cyclin D1.
The left upper panel shows a PC with the intense cytoplasmic fluorescence for the FGFR3 (FITC) and is counterstained with DAPI. This patient had a t(4;14)(p16.3;q32) detected by cIg-FISH. The PC in the upper right panel shows no reactivity to the FGFR3antibody (no t(4;14)(p16.3;q32) detected by cIg-FISH). The lower left panel depicts a PC with intense nuclear positivity for cyclin D1 by immunohistochemistry (light H-E counterstain). This patient also had the t(11;14)(q13;q32) detected. Inset shows nuclear localization in enlarged cell. The lower right panel shows a PC of a patient negative for cyclin D1 and for the t(11;14)(q13;q32) by FISH (100 × magnification, Leica DMRXA microscope).
Genetic up-regulation of FGFR3 and cyclin D1.
The left upper panel shows a PC with the intense cytoplasmic fluorescence for the FGFR3 (FITC) and is counterstained with DAPI. This patient had a t(4;14)(p16.3;q32) detected by cIg-FISH. The PC in the upper right panel shows no reactivity to the FGFR3antibody (no t(4;14)(p16.3;q32) detected by cIg-FISH). The lower left panel depicts a PC with intense nuclear positivity for cyclin D1 by immunohistochemistry (light H-E counterstain). This patient also had the t(11;14)(q13;q32) detected. Inset shows nuclear localization in enlarged cell. The lower right panel shows a PC of a patient negative for cyclin D1 and for the t(11;14)(q13;q32) by FISH (100 × magnification, Leica DMRXA microscope).
Discussion
All translocations observed in MM are also seen in MGUS
We have been able to identify the chromosomal partner regions for IgH translocations in 21 (77%) of 27 patients, leaving only about one fourth of patients with suspected various partners. Our results now also show that translocations involving the IgL-λ locus also occur in 11% of patients with MGUS. However, and in contrast to the results of Avet-Loiseau and colleagues,12 we observed t(4;14)(p16.3;q32) and t(14;16)(q32;q23) in 9% and 5% of patients with MGUS, respectively, which is a similar prevalence we have found for these translocations in patients with MM (R.F., unpublished observations, June 2002, and Fonseca et al36).
Our ability to detect t(4;14)(p16.3;q32) or t(14;16)(q32;q23) in MGUS may be related to the sets of probes, which bracket all reported human MM cell line breakpoints in these partner chromosomal regions, we used in this study. For instance, we used a pool of probes instead of a single clone because we have observed deletions surrounding the translocation breakpoints in 16q23 translocations in human MM cell lines with t(14;16)(q32;q23) (R.F., unpublished data, May 2002) and, thus, probe signals may be lost as a consequence of translocation events resulting in false-negative results. In many cases we have found a pattern consistent with loss of a derivative chromosome when studying t(4;14)(p16.3;q32) in MM samples resulting in only 1 fusion signal. In our MGUS series we found a high concordance between the percentage of PCs with abnormal signal patterns by both the fusion and IgH break-apart strategy in individual patients (Table 1). Alternatively, there may be differences in the referral patterns or populations that are being studied that account for these variations.
Progression to MM
The relative short follow-up time of patients in our study prevents us from estimating the predictive capacity of evolution to MM of specific Ig translocations in MGUS. The long period of stability after diagnosis (and translocation detection) in patients with t(4;14)(p16.3;q32) or t(14;16)(q32;q23) suggests that, much like the t(11;14)(q13;q32), these abnormalities alone, at least in some cases, are not sufficient for progression from MGUS to MM and are likely primary events for disease pathogenesis. It is, however, possible that time of evolution of these patients may be shorter than for patients without the abnormality. Compared with other studies,37 we observed a higher prevalence of t(11;14)(q13;q32) in MGUS than in MM38,39 and more recently have also found a high incidence of this same abnormality in the clonal PCs of AL patients.13 If these data are correct, it may suggest t(11;14)(q13;q32) is negatively selected for progression from an early-state PC disorder to MM. This hypothesis can be tested through longitudinal studies of MGUS patients.
Chromosome 13 abnormalities
The prevalence of Δ13 among the patients in the present study was approximately 50% and is similar to that reported in MM.19,21,31 Other investigators have reported Δ13 in 15% to 50% of patients with MGUS.19,20 Most studies indicate that when a chromosome abnormality is detected in MM, it occurs in most clonal PCs,31,35 and this is consistent with our findings in MGUS, although other reports show greater heterogeneity in MGUS.35 These observations suggest that for many patients the acquisition of Δ13 is not critical for evolution from MGUS to MM. Patients with t(11;14)(q13;q32) and MGUS appear to be approximately equally likely to have Δ13 (about 50%) as patients with MM and t(11;14)(q13;q32) (54%).38 In contrast, we recently observed a strong association between Δ13 and t(4;14)(p16.3;q32) since the early stages of PC disorders.36 We have found Δ13 in more than 85% of patients with MM and the t(4;14)(p16.3;q32) and have also found it in 3 of 4 patients with MGUS,36 but this observation needs to be confirmed in a larger cohort of patients. Our sample size is too small to infer much about the association of t(14;16)(q32;q23) and Δ13. The results presented here are in disagreement with several of the published series and would suggest that Δ13 is not necessarily important in the progression from MGUS to MM, but this issue is clearly in need of further study.
Percentage of abnormal cells and relation to prevalence
This study shows that in most MGUS patients, and like has been reported by Avet-Loiseau et al,12 chromosome abnormalities are seen in most of the clonal cells. Clonal cells were defined in their study by virtue of aneuploidy, while we estimated the actual number of clonal cells by virtue of the light-chain cytoplasmic stain (κ or λ) restriction.12 In most cases tested we found a chromosomal abnormality, including translocations, ploidy, or Δ13, in most of the light-chain–restricted cells. However, and like in Avet-Loiseau's study,12 we also found patients where translocations involved a smaller percentage of light-chain–restricted clonal cells. Although it is possible that not all light-chain–restricted PCs form part of the clone (some polyclonal PCs with the same light chain usage “contaminate” the scoring), their contribution is likely minimal. It is also possible that translocations do not involve most clonal PCs, because they may occur early in the process of disease pathogenesis (MGUS) but may not be the first events. Because of this, and the observation that several more patients had a low proportion of PCs with abnormal fusions (equivocal results), we may be underestimating the actual incidence of Ig translocations in MGUS.
Aneuploidy versus structural chromosomal abnormalities
It is unknown in the PC disorders which genetic lesion occurs first in the clonal PCs of patients with MGUS: aneuploidy or translocations. Because of the ubiquitous nature of aneuploidy, the high prevalence of IgH translocations in MGUS, and the lack of a clearly defined precursor to MGUS, this question remains unsolved. The results of our study make us speculate that in some cases aneuploidy may occur before IgH translocations. This would be consistent with a model where genomic instability occurs first, results in aneuploidy, and is the permissive event for structural and numeric chromosomal abnormalities to occur.12,40 41 In MGUS, and in contrast to MM, IgH and IgL-λ translocations frequently occur in less than 75% of PCs. In this study we encountered several patients (Table 4) where the percentage of aneuploid PCs (by centromeric probes or Δ13 assay) was greater than the percentage of PCs with any given translocations. In many patients the differences between the percentage of abnormal cells by a ploidy assay and translocation percentage abnormal cells were modest (perhaps technical), but in some the absolute percentage difference exceeded 35 points (likely real). Even when larger numbers of patients need to be studied, our results would suggest that aneuploidy arises at the time or just before IgH translocations occur.
Comparison with other series of patients
While our series shows similarities with that published by Avet-Loiseau and colleagues,12 several differences are notorious and will need to be further clarified in future studies. Some of these differences are of modest percentage difference but may have important implications for the interpretation of our results. The major differences between both series of patients include that we can detect the 3 main partner chromosomes to IgH translocations (11q13, 4p16.3, and 16q23) in 77% of cases while they have been able to identify these same chromosomal partners in only 37% of IgH translocations. In many cases we have found that clonal chromosomal abnormalities, but particularly IgH translocations, may involve only a fraction of the light-chain–restricted cells. We also found a significantly higher incidence of Δ13 (about 50%), and lastly we found that when the abnormality is detected it is present in most clonal PCs.
Oncogene up-regulation
Because of the oncogenic potential ofFGFR334 and cyclin D1,42we examined clonal PCs in MGUS (a low proliferative index clonal state) for their genetic up-regulation. Using the technique published by Chesi et al,34 we confirmed up-regulation of FGFR3 by the t(4;14)(p16.3;q32) in MGUS, which further supports our finding of these abnormalities in the early stages of the PC disorders.34,43 We also used immunohistochemical detection of cyclin D1 by a method recently described by us.33 While nuclear positivity was seen only in cases with the t(11;14)(q13;q32), the test was positive in only 8 (57%) of 14 patients, and in other patients the staining was difficult to interpret due to the low percentage of clonal PCs present in the BM biopsy.44
MGUS/SMM clonal cells can have more than one translocation
In MGUS and SMM the clonal cells can have 2 coexistent Ig translocations, each with its putative dysregulation of oncogenes. In this series of patients we detected 3 patients with 2 translocations. While it is possible that separate clonal populations have different translocations, in 2 of 3 patients the clonal PCs shared 2 translocations in the same PC; the aggregate percentage exceeded 100 (199% and 121%). This has been previously detected in other studies, although these appear to be rare.17,18 However, we recently finished a large cIg-FISH study of IgH translocations in MM36,38 but could not find 2 coexistent translocations in the clonal PCs of MM, and others have made similar observations.10,39 This issue may be better addressed through a detailed metaphase-FISH16 or spectral karyotype (SKY)17 18 study.
Importance
Preliminary observations suggest a biologic uniqueness to groups of MM defined by the specific cytogenetic markers, as detected by cIg-FISH. We recently reported that patients with the t(11;14)(q13;q32), as detected by FISH, appear to have specific biologic features and better survival than patients without the abnormality.38 These observations further validate that Ig translocations are likely important in the pathogenesis of the PC disorders and may be seen as candidates for therapeutic interventions aimed at the consequences of oncogene up-regulation.
Supported by the Multiple Myeloma Research Foundation and the Mayo Foundation. R.F. and S.V.R. are Leukemia and Lymphoma Society Translational Research Awardees. R.F. is supported by the CI-5 Cancer Research Fund–Lilly Clinical Investigator Award of the Damon Runyon–Walter Winchell Foundation. This work is also supported in part by Public Health Service grant R01 CA83724-01 from the National Cancer Institute (R.F.) and grant P01 CA62242 (R.A.K., J.A.L., G.J.A., P.R.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rafael Fonseca, Division of Hematology and Internal Medicine, Stabile 6-28, Rochester, MN 55905; e-mail:fonseca.rafael@mayo.edu.

![Fig. 1. Pictures with both the normal and abnormal patterns for the break-apart and fusion strategies. / In all cases the clonal PCs are identified by the intense blue fluorescence of the cytoplasm as detected by the cIg-FISH procedure. (A) A PC with the normal configuration for the VH (red signal) and CH probes (green signal). Arrows point to the closely associated pairs of signals indicative of a normal pattern. (B) Segregation (break-apart) of the VH (green) and CH (red) probes consistent with a 14q32 translocation. (C) A cell with fusion signals (arrows) resulting from comigration of the pools of probes at 14q32 (green) and 4p16.3 (red) consistent with a t(4;14)(p16.3;q32). (D) The fusion signal (arrows) resulting from comigration of the pools of probes at 14q32 (green) and 16q23 (red) consistent with a t(14;16)(q32;q23). (× 100 magnification, Leica DMRXA microscope [Wetzlar, Germany]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/4/10.1182_blood.v100.4.1417.h81602001417_1417_1424/6/m_h81622989001.jpeg?Expires=1764501488&Signature=izUhUi8z2nZhB6ZfKWRZL-Q~u9an-SWG5rRjl-cPrckThgra4XqOqHvxwQkOfS8onii0CdJoSVgo2bHJIQ3UpBe1a6ayMhG~Zl35R0MJsLZeZZk3toRUlRABaf9n4IcixxuIisrTdwe1NW3EniMeZa3NK0iJFikgGfnel5FiG2FUIxwd-g6ElQFfxDlGDPVm9YpuJjAtrtWDjmv4LFVJJohLhQrFRws8s46yBU0mx0ojHVBpI~EOuhT~6RRb0dtotH4CoBbuZcbZMPIixb3Mh7H84bRbreFmP18Cyk1Wn6esIAMBHq23FJo7EmJNlvA2gQFnGWUTH710VrAEdZGHfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)