We describe a woman with severe neutropenia and dependency on red blood cell transfusions who had previously undergone Billroth II surgery and whose bone marrow (BM) showed morphologic characteristics typical of myelodysplastic syndrome (MDS) with ringed sideroblasts. She had transient reversal of anemia and severe neutropenia after therapy with erythropoietin and granulocyte colony-stimulating factor. Because of relapse while receiving growth factors, the patient was referred for allogeneic BM transplantation. A pretransplantation nutritional evaluation revealed severe copper deficiency, and her hematologic abnormalities resolved fully with copper therapy. This case shows that copper deficiency should be an integral part of the differential diagnosis of sideroblastic MDS, even in patients not requiring parenteral nutrition.
Study design
A 44-year-old woman with macrocytic anemia and leukopenia had undergone a gastric resection with Billroth I anastomosis for peptic ulcer disease 9 years earlier. Four years after the operation, recurrent gastric ulceration and gastric outlet obstruction developed and the Billroth I anastomosis was converted to a Billroth II. Dumping symptoms with chronic diarrhea developed, but the patient's weight remained stable. The patient described a history of chronic anemia treated with oral and parenteral iron, B12, and folate, without response. One year before referral to us, the patient's hematocrit was 0.23 and her white blood cell (WBC) count was 2.3 × 109/L. She required red blood cell (RBC) transfusions approximately every 2 months.
On presentation, the patient had the following laboratory results: WBC count, 1.5 × 109/L, with 0.19 neutrophils; hemoglobin (Hb) level, 64 g/L; mean corpuscular volume, (MCV) 102 fL; and platelet count, 192 × 109/L. Occasional oval macrocytes were observed on blood films. The patient was thin and appeared chronically ill, but a physical examination revealed otherwise unremarkable results. There was no splenomegaly. Serum levels of B12, RBC folate, and ferritin were elevated; the albumin level was 34 g/L (normal, 39-48 g/L). A bone marrow (BM) assessment showed dyserythropoiesis, dysmyelopoiesis, ringed sideroblasts (RSs), and prominent hemosiderin in plasma cells (Figure 1 and Table1). The results of cytogenetic studies were normal.
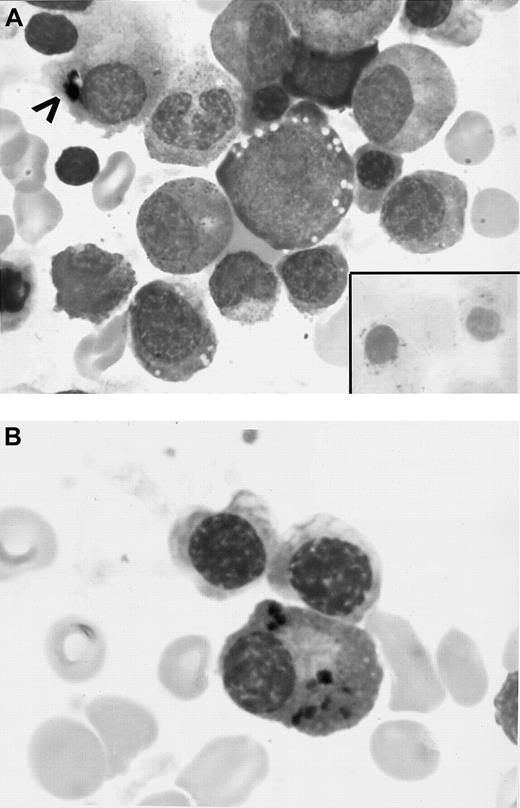
BM aspirate.
(A) Initial BM smear showing vacuoles in the erythroid precursors, dyspoietic changes, several RSs (inset), and iron granules in plasma cell cytoplasm (arrowhead) (original magnifications, × 890). (B) BM aspirate smears (initial BM aspirate) showing several plasma cells containing blue-black particulate material in the cytoplasm that stained positive with Prussian blue reaction (hemosiderin deposits) (original magnification, × 970).
BM aspirate.
(A) Initial BM smear showing vacuoles in the erythroid precursors, dyspoietic changes, several RSs (inset), and iron granules in plasma cell cytoplasm (arrowhead) (original magnifications, × 890). (B) BM aspirate smears (initial BM aspirate) showing several plasma cells containing blue-black particulate material in the cytoplasm that stained positive with Prussian blue reaction (hemosiderin deposits) (original magnification, × 970).
Because of the BM morphologic findings, the patient was presumed to have myelodysplastic syndrome (MDS), French-American-British subtype refractory anemia with RSs (RARS). There was no response to pyridoxine therapy. The patient was then given granulocyte colony-stimulating factor (G-CSF; 75 μg/day) and erythropoietin (EPO; 5000 U/day). Peripheral blood counts became normal (Table 1) 3 weeks after therapy began, and no further RBC transfusions were given.
The Hb response was not sustained, however, and 3 months after initiation of therapy, the patient again required frequent RBC transfusions. On the other hand, even though the G-CSF dose was tapered to every other day, WBC counts remained in the normal range (Table 1). Another BM examination showed hypercellular BM with the same morphologic abnormalities shown in Figure 1 and listed on Table 1. During this interval, the patient's diarrhea resolved spontaneously. An esophagogastroduodenoscopy with small-bowel biopsy was performed, and the results were normal. The patient was also found to have a severe progressive peripheral neuropathy and an optic neuritis.
The patient was referred for evaluation for BM transplantation to treat MDS. As part of the pretransplantation evaluation, a nutrition consultation was obtained, and the patient's copper level was found to be undetectable (< 1.57 μmol/L [< 10 μ/dL]; normal, 11-24.3 μmol/L [70-155 μ/dL]). She was treated with intravenous copper chloride (2.5 mg daily for 14 doses; total dose, 35 mg) and EPO and G-CSF were discontinued. The patient's Hb concentration became normal within 6 weeks. She was given oral copper supplementation (3 mg copper sulfate 3 times daily). Three months after initiation of copper therapy, the patient's copper level was normal (14.4 μmol/L [92 μ/dL]), as were her ceruloplasmin, complete blood count, and MCV values. BM aspiration and biopsy showed reversal of previous abnormalities (Table 1). Hemosiderin was observed only in macrophages, and plasma cells did not contain histologically identifiable iron. The patient's general well-being improved, but the peripheral and optic neuropathies persisted and her weight remained stable.
Results and discussion
Deficiency of copper has been reported to result in anemia, neutropenia,1 and less commonly, thrombocytopenia.2 BM morphologic findings vary, but vacuolated erythroid precursors are often found. Other abnormalities include megaloblastic changes and RSs. Although textbooks describe anemia of copper deficiency as microcytic,3 a review of the literature revealed that macrocytic, microcytic, and normocytic anemia occur.1 4-6 Our patient had macrocytic anemia, with occasional oval macrocytes, a finding further supporting the suspected diagnosis of MDS. The importance of the plasma cell iron in the patient was unclear; however, it was likely due to copper deficiency, since it resolved after copper therapy.
Most cases of copper deficiency in adults occur in patients receiving total parenteral hyperalimentation,3 although there have been cases resulting from enteral feeding that did not include copper.7 To our knowledge, there have been only 2 previous reports4,5 of copper deficiency due to intestinal malabsorption after partial gastrectomy in patients who, like our patient, were not receiving parenteral or enteral nutrition. One of these patients5 also had neurologic abnormalities.
The mechanism by which copper deficiency induces anemia and other cytopenias is unknown. Copper is an essential cofactor for various redox enzymes, and decreased activity of copper-dependent enzymes, such as ceruloplasmin ferroxidase and cytochrome oxidase, has been hypothesized to be a potential cause. Mitochondria isolated from copper-deficient animals were deficient in cytochrome oxidase activity and failed to synthesize heme from ferric iron and protoporphyrin at the normal rate, perhaps leading to mitochondrial iron accumulation, that is, RSs.8 The association of copper and iron metabolism is of increasing interest. The molecular basis for the anemia due to a defect in intestinal iron transport in sex-linked anemic (sla) mice was identified as a multicopper ferroxidase, hephaestin.9 The Wilson disease protein product was localized to mitochondria,10 the copper-containing mitochondrial transporter frataxin was identified in yeast,11 and its human analogue was identified subsequently.12 Interestingly, acquired somatic mutations of mitochondrial cytochrome c oxidase (a copper-containing enzyme) were found in 2 patients with acquired sideroblastic anemia (MDS); one of them had macrocytic anemia and the other had microcytic anemia.6 An acquired somatic mutation is not a satisfactory explanation for our patient's condition, given that her hematologic abnormalities reversed rapidly with copper repletion. The molecular defect in sideroblastic anemia of copper deficiency remains to be elucidated.
The cause of neutropenia in copper deficiency remains obscure, but such neutropenia is observed consistently.8 Similarly, we do not know whether the severe peripheral and optic neuropathies in our patient, which did not respond to copper replacement, were related to the copper deficiency. However, copper deficiency associated with myelopathy in a patient who also had microcytic anemia and neutropenia (no mention of RSs) was reported previously,5and myelopathy has also been observed in copper-deficient sheep.13
Our patient's condition responded to hematopoietic growth factors with normalization of Hb levels and neutrophil counts, although the Hb response was transient. To our knowledge, this response has not previously been reported. However, this observation shows that therapeutic responses to these cytokines may occur regardless of the causative event.
Copper deficiency is not often considered as a cause of cytopenias in adults. Most current hematology textbooks do not list copper deficiency in the differential diagnosis of RARS, indicating a limited awareness of this correctable cause of sideroblastic anemia that can sometimes, as in our patient, be particularly severe. The diagnosis may not be suspected in patients not receiving nutritional support, since one recent hematology textbook's discussion of hematologic complications of copper deficiency contains the statement that copper deficiency occurs “only in malnourished premature infants or patients receiving long-term parenteral nutrition.”14 Our case illustrates the nonspecificity of the morphologic abnormalities and shows that copper deficiency should be considered a possible cause of RARS, even in patients not receiving parenteral nutrition. It also demonstrates that RARS may respond transiently to cytokine therapy.
Prepublished online as Blood First Edition Paper, May 17, 2002; DOI 10.1182/blood-2002-01-0256.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Josef T. Prchal, Baylor College of Medicine, One Baylor Plaza, 802E, Houston, TX 77030; e-mail: jprchal@bcm.tmc.edu.