While studying patient plasma containing an unusual pattern of von Willebrand factor (VWF) multimers, we discovered a previously unreported phenomenon: heavy predominance of dimeric VWF. Genomic analysis revealed a new congenital mutation (Tyr87Ser) that altered the final stages of VWF biosynthesis. This mutation in the propeptide (VWFpp) resulted in synthesis of dimeric VWF with an almost complete loss of N-terminal multimerization. The multimer pattern in patient plasma appears to result from separate alleles' synthesizing wild-type or mutant (dimeric) VWF, with homodimers composing the predominant protomeric species. We have expressed VWF protein containing the Tyr87Ser mutation and analyzed the intracellular processing and resulting VWF biological functions. The expressed dimeric VWF displayed a loss of several specific functions: collagen binding, factor VIII binding, and ristocetin-induced platelet binding. However, granular storage of dimeric VWF was normal, demonstrating that the lack of multimerization does not preclude granular storage. Although the tertiary structure of the VWFpp remains unknown, the mutant amino acid is located in a region that is highly conserved across several species and may play a major role in the multimerization of VWF. Our data suggest that one function of the highly cysteine-rich VWFpp is to align the adjacent subunits of VWF into the correct configuration, serving as an intramolecular chaperone. The integrity of the VWFpp is essential to maintain the proper spacing and alignment of the multiple cysteines in the VWFpp and N-terminus of the mature VWF.
Introduction
Von Willebrand factor (VWF) is a large multimeric glycoprotein with a critical role in maintaining hemostasis. VWF serves as the carrier protein for the plasma coagulation protein factor VIII (FVIII) and promotes platelet adhesion and subsequent aggregation at the sites of vascular damage.1 The monomeric pro-VWF is composed of 4 repeated domains. The VWF propeptide (VWFpp) consists of D1-D2 domains, while the mature VWF protein consists of D′-D3-A1-A2-A3-D4-B-C1-C2 domains.2 VWF undergoes a series of intracellular modifications, including dimerization, glycosylation, sulfation, N-terminal multimerization, VWFpp cleavage, and FVIII binding.3,4 VWF is synthesized exclusively in endothelial cells and megakaryocytes. In these cells, VWF is packaged in releasable storage vesicles termed Weibel-Palade bodies in endothelium and α-granules in megakaryocytes and platelets.4 Several functions of VWF have been mapped to specific domains,1,5,6 such as FVIII binding (D′ and D3 domains); N-terminal multimerization (D3); glycoprotein Ibα (GPIbα) binding (A1); collagen binding (A1 and A3); and C-terminal dimerization (C2). Quantitative and qualitative abnormalities of VWF are caused by congenital mutations in the VWF gene and result in von Willebrand disease (VWD), a common inherited bleeding disorder.7Qualitative defects are grouped as VWD type 2 variants (2A, 2B, 2M, and 2N).7
While the extracellular function of the propeptide of VWF has yet to be fully defined, it has been demonstrated to be critical for multimerization and intracellular storage of the mature VWF protein.8-10 The 741–amino acid (aa) propeptide represents 26.3% of the primary translation pro-VWF product and consists of 2 homologous D domains that are cysteine-rich, with 32 cysteines in each D domain. The multimerization of pro-VWF dimers involves intermolecular disulfide bridges created between cysteine residues in the D3 domains of adjacent VWF molecules.5,11 The propeptides of several hormones and enzymes have been demonstrated to assist in the correct folding of the mature peptide and have been termed “intramolecular chaperones.”12 13 The VWFpp may function as an intramolecular chaperone to assist in VWF protein folding during multimerization and to promote vesicular segregation of VWF into storage granules.
This study represents a functional characterization of a naturally occurring mutation in the VWFpp. Although the patient in our study has mild VWD, the VWF multimer pattern has an unusual phenotype with a markedly increased dimer band among a relatively normal distribution of the remaining VWF multimers. In this report, we describe how a congenital defect located in the VWFpp results in a complete loss of VWF multimerization but maintenance of normal vesicular storage of VWF. The natural intramolecular chaperone function of the VWFpp has been altered by this defect. Heterozygosity in this patient presented a unique opportunity to study a VWF dimer mutant in vitro and determine the extent to which the VWFpp functions intracellularly during VWF multimer assembly in the secretory pathway.
Materials and methods
Isolation of total cellular RNA and genomic DNA
Reverse transcription–PCR and cDNA sequencing
Reverse transcription of purified platelet RNA was performed as previously described,14 15 with the use the VWF antisense primer a1144-1130 (CATTGCTGCAGATCC). DNA amplification of the cDNA was performed with VWF sense primer s(-14)-21:NcoI (ATTcCAtGGGAAGATGATTCCTGCCAGATTTGCC) and antisense primer a1115-1081:NsiI (CAAATGCAtGTGTTGCAGTCTCGAGAGAGGGAGGT). Lower-case letters indicate nucleotides altered for the purpose of introducing restriction sites. Second-round polymerase chain reactions (PCRs) were performed under the same conditions, except that the antisense primer was changed to a1059-1026:NsiI (GGAATGCAtGCAGGGACACTCGGTGCTCT CCACG). Second-round PCR fragments (1073 bp) were treated with Klenow fragment, purified after agarose gel electrophoresis by means of the Geneclean kit (BIO 101, La Jolla, CA), and inserted into the EcoRV site of pGEM-5Zf+ (Promega, Madison, WI). Sequence was determined on isolated clones of the patient's VWF insert with Sequenase 2.0 (United States Biochemical, Cleveland, OH) with the use of mini-prep DNA.
Tyr87Ser mutant plasmid construction
The VWF expression vector pvW198 (a gift of Dennis Lynch, Dana Farber Cancer Center, Boston, MA) was digested with restriction enzymes XhoI and KpnI (Promega), and the resulting 525-bp fragment was cloned into pGEM7-Zf+ for use as mutagenic PCR template (Promega). Two separate first-round PCRs were performed with the use of the M13 forward universal primer (M13F) with aVWF271-249–mutant nucleotide (aVWF271-249-m) (ATTCCCCAAGAgACACGGAGAGG) producing a 590-bp product, and svWF249-271-m (CCTCTCCGTGTcTCTTGGGGAAT) with M13 reverse universal primer (M13R) producing a 174-bp product. A second round of PCR was performed on 2 μL of the first-round products and amplified with M13F and M13R. The resulting PCR fragment was then cloned into theXhoI/KpnI site of pG3vW-1 (an intermediate cloning vector constructed by cloning the 3302-bpSalI-KpnI fragment of pvW198 into theSalI-KpnI site of pGEM3-Zf+[Promega]). Finally, the vector expressing the Tyr87Ser-VWF defect (pvW198-Br) was constructed by a 3-fragment ligation with the use of the mutant 3302-bp SalI/KpnI fragment of pG3vW-1, and the 2415-bp KpnI/BamHI and the 7083-bpBamHI/SalI fragments of pvW198. Introduction of the patient's mutation into normal VWF sequence was verified by sequencing. A vector expressing only the propeptide containing the Tyr87Ser mutation was constructed by ligating a fragment containing the mutation into the previously described8 VWFpp expression vector.
Cell culture
Established cell culture lines were used in the course of this study: monkey kidney cells (COS-7; ATCC, Rockville, MD); mouse pituitary tumor cells (AtT-20/D16v-F2; ATCC); and human embryonic kidney cells (HEK 293T; provided by D. Ginsburg, University of Michigan, Ann Arbor, MI). AtT-20 cells are an adrenocorticotropic hormone (ACTH)–synthesizing model storage and secretory cell line16 that does not synthesize VWF, but will store VWF when transfected with VWF cDNA.8,10 COS-7 and HEK-293T cells are nonstorage cell lines used for high expression of VWF without VWF storage. All cell lines were cultured at 37°C in a 5% CO2 humidified incubator in either complete AtT-20 or HEK293T medium as previously described,8 or complete COS medium (RPMI 1640 [Life Technologies, Rockville, MD]; 10% fetal bovine serum [FBS]; and 2 mM L-glutamine].
Transient and stable expression of VWF
VWF expression vectors were transiently transfected into COS-7 cells and HEK-293T cells with the use of lipofectin (Life Technologies) and into AtT-20 cells with the use of lipofectamine (Life Technologies). Optimized transfection conditions were previously determined.3,17 Briefly, 24 hours prior to transfections, cultured cells were plated at 5 × 105 cells (COS-7) in 60-mm culture dishes or at 4 × 106 cells (AtT-20) in 100-mm dishes. Cells were incubated in OPTI-MEM I–reduced serum medium (Life Technologies) diluted with either 15 μg DNA plus 40 μg lipofectin or 8 μg DNA plus 192 μg lipofectamine for 5 hours at 37°C for the COS-7 and AtT-20 cell transfections, respectively. The DNA/lipid complexes were removed and replaced with complete media (with 10% FBS), and the plasmids were transiently expressed for 72 hours. Conditioned media were harvested from the cells, centrifuged to remove cellular debris, and analyzed by solid-phase capture enzyme-linked immunosorbent assays (ELISAs)18 and functional fluid-phase assays.18 19 The transfected cells were then lysed or fixed for immunofluorescent staining (see “Immunofluorescence staining”). AtT-20 cells were used to establish stable transfections of wild-type VWF (WT-VWF) (pvW198.1) or Tyr87Ser-VWF mutant (pvW198-Br) with the use of the selection agent G418 (Sigma, St Louis, MO) at 0.3 mg/mL. After several weeks of selection, positive colonies were selected by analysis of the secreted VWF in the conditioned media by VWF antigen-capture ELISA.
Antibodies used
Antibodies used in this study include AP-1, AVW-1, AVW-3, AVW-5, AVW-17, MBC 33.5, and polyclonal anti-VWF antibodies. The Hybridoma Core Laboratories at the Blood Center of Southeastern Wisconsin (Milwaukee) produced all above antibodies. AP-1 is a monoclonal antibody (mAb) against GPIbα that inhibits the platelet GPIbα binding of VWF. The monoclonal antibodies AVW-1, AVW-3, AVW-5, and AVW-17 recognize distinct epitopes in mature VWF. MBC 33.5 is an anti-VWFpp mAb.
Multimer analysis
Expressed VWF was immunoprecipitated from conditioned media with the use of mAb AVW-1 (anti-VWF) or MBC 33.5 (anti-VWFpp) covalently coupled to sepharose 4B agarose beads (Pharmacia Biotech, Piscataway, NJ), and eluted as previously described with the use of a buffer containing 8 M urea/6% sodium dodecyl sulfate (SDS)/13 mM Tris (tris(hydroxymethyl)aminomethane)/1 mM EDTA (ethylenediaminetetraacetic acid)/0.05% bromphenol blue (pH, 8.8).18 The eluted samples were electophoresed through 0.65% or 1.5% HGT(P) agarose (FMC Bioproducts, Rockland, ME) gels containing 1% SDS for 16 hours at 40 V. The protein was transblotted to nitrocellulose for 30 minutes at 35 V followed by 4 hours at 50 V. Membranes were blocked with 5% nonfat dry milk, incubated overnight with mAbs AVW-5 and AVW-17, and then incubated for 2 hours with horseradish peroxidase (HRP)–conjugated goat antimouse immunoglobulin G (IgG) (Pierce, Rockford, IL). Membranes were treated with electrogenerated chemiluminescent (ECL) substrate (Amersham Life Science, Arlington Heights, IL), and bands were detected by exposure to autoradiography film.
Platelet-binding assays
Binding of expressed VWF to platelets was determined by means of a modification of the platelet-binding assay previously described by our laboratory.19 In these assays, conditioned media samples from transiently transfected cells were diluted to equal amounts of VWF (determined by ELISA) and mixed with125I-labeled AVW-1 (anti-VWF mAb). After 60 minutes, formalin-fixed human platelets were added to this mixture in the presence or absence of either ristocetin (1.2 mg/mL) or botrocetin (1.0 μg/mL) under nonstirring conditions. After 30 minutes, reactions were centrifuged at 10 000g for 10 minutes. Both platelet pellets and supernatants were counted to quantitate the amount of VWF bound to the platelets as determined by measuring the 125I–AVW-1. All data were averaged from 6 independent assays of separate transient transfections and normalized to WT-VWF, which has been established as 100% binding. Controls included media from nontransfected cells and diluted normal pool plasma.
Collagen binding
Binding of expressed VWF to collagen was determined in a microtiter plate assay. Immulon I 96-well plates (Dynatech, Chantilly, VA) were coated with 300 ng per well of collagen Type III from human placenta (Sigma). Fixed amounts of expressed recombinant VWF (rVWF) (100 ng/mL) were added to the collagen plate and incubated for 60 minutes. Samples were incubated with polyclonal anti-VWF antiserum for 30 minutes and then incubated with AP-conjugated goat antirabbit IgG (Pierce) for 30 minutes. Color was developed with ImmunoSelect substrate (Life Technologies) and read at 492 nm in the ThermoMax microplate reader (Molecular Devices, Menlo Park, CA). The relative amount of VWF bound (VWF:CB) was calculated by means of SOFTmax, version 2.34 (Molecular Devices), on the basis of a standard curve generated by plating serially diluted pooled normal human plasma.
FVIII binding
Binding of FVIII to VWF was measured in a microtiter plate assay essentially as described by Nishino et al20 and modified by Kroner et al.17 18 Samples were diluted to a final VWF concentration of 10 mU/mL. Equal volumes were added to an AVW-1–coated microtiter plate (Immulon I) and incubated for 2 hours. Plates were washed and incubated for 1 hour at 37°C with 50 μL recombinant human FVIII (Baxter Healthcare, Glendale, CA) ranging in concentration from 0 to 4 U/mL. Plates were washed, and the VWF-bound FVIII was determined by chromogenic assay by means of the Chromogenix Coatest FVIII:C/4 kit (diaPharma, West Chester, OH). Plates were incubated at 37°C for the enzymatic reactions, and the resulting chromogenic substrate conversion was read at 405 nm in the ThermoMax reader. The amount of FVIII bound was calculated by means of SOFTmax software, on the basis of a standard curve generated by the addition of serially diluted r-hFVIII to microtiter plates wells prior to the chromogenic FVIII assay.
Immunofluorescence staining
Cultured cells transfected with VWF plasmids were analyzed for the subcellular localization of VWF with immunofluorescent antibody labeling of the expressed antigens and confocal laser scanning microscopy, as previously described.3,21Transfected AtT-20 cells were grown on 35-mm culture plates (Fisher Scientific, Itasca, IL) in Dulbecco modified Eagle medium/G418 media for 72 hours. Negative controls (nontransfected and mock-transfected cells) were processed in parallel with each immunofluorescence labeling experiment. The cells were processed and immunofluorescently labeled by the sequential antibody staining method.10 22 Purified anti-VWF monoclonal and polyclonal antibodies, and anti-VWFpp monoclonal antibodies, were used as primary antibodies. Fluorescein isothiocyanate (FITC)– and Texas Red (TXR)–conjugated donkey IgG (H + L) (F(ab′)2fragments) (Jackson ImmunoLabs, West Grove, PA) were the secondary antibodies (diluted at 1:200 and 1:1000, respectively).
Submarine gel analysis
To examine the formation of homodimers and heterodimers of VWF, 2 types of VWF were expressed. Wild-type VWF was coexpressed with hereditary persistence of pro-VWF (HPP-VWF) that contains a disrupted furin-cleavage site. The HPP-VWF mutation was identified in a patient and results in an alternative splice (Δbp2269-2270 and Δbp2282-2288) that changes the amino acid sequence from756Pro-Leu-Ser-His-Arg-Ser-Lys-Arg-Ser-Leu-Serto 756Pro-Val-Ser-Ser-Glu-Ser-Leu-Ser(underlined sequence is mature VWF sequence).23 The HPP-VWF expresses a pro-VWF that is 741 amino acids larger than wild-type VWF, contains uncleaved propeptide, multimerizes, and is stored in granules in AtT-20 cells.23 This HPP-VWF was used to create a larger protein that could be distinguished from wild-type VWF by SDS-agarose electrophoresis. The 2 VWF constructs were expressed independently and coexpressed in HEK293T cells with the use of the transfection conditions as described above. A DNA ratio of 40:1 (HPP-VWF to WT-VWF) was used and had been optimized to produce similar concentrations of the 2 types of expressed VWF. Culture supernatants were harvested and quantitated by ELISA. Samples were analyzed on a 3% MetaPhor Agarose gel (FMC BioProducts) dissolved in 90 mM Tris, 90 mM boric acid, pH 8.5; and run in the same buffer in the presence of 0.1% SDS by means of a BioRad Submarine Gel System at 7.5 mA (40 V) for 16 hours at 4°C. Western blotting was performed as described above.
Results
Identification and expression of mutant VWF
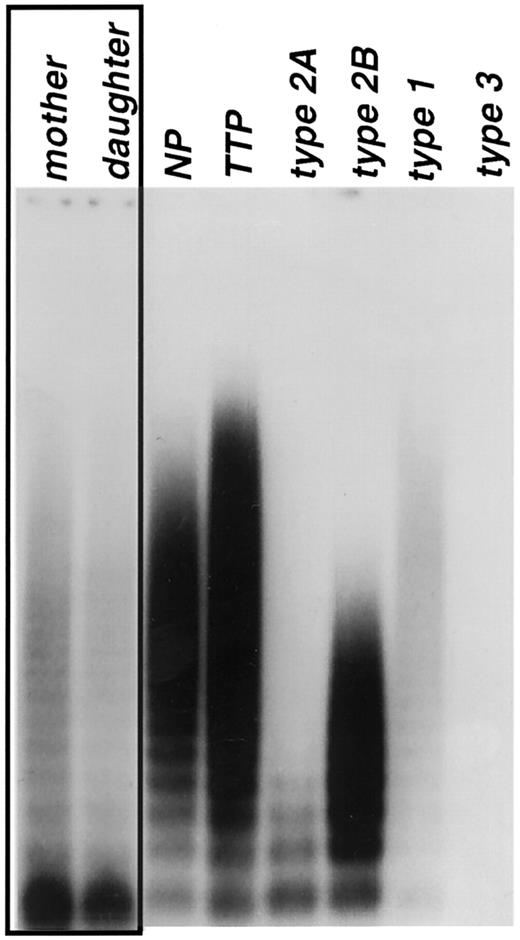
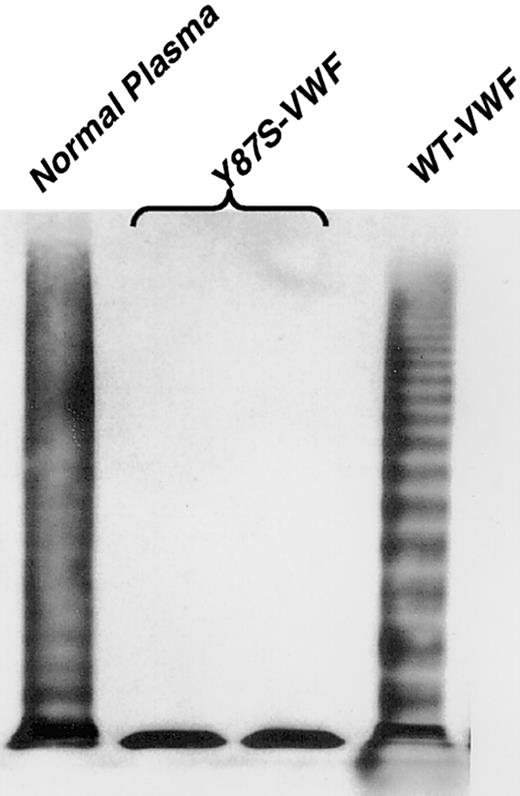
In the process of studying a family with clinical symptoms of type 1 VWD, we discovered that the mother and the daughter had a previously unreported multimer pattern. A heavy predominance of VWF dimers was observed in both plasmas (Figure 1, lanes 1, 2), equaling approximately 50% of the total VWF antigen, while the remainder of the VWF appeared to multimerize normally. A single missense mutation (260A>C) was identified in exon 4 (D1 domain), converting Tyr87 to a serine (Tyr87Ser) (Figure2). When a VWF expression vector containing this mutation was transfected into COS-7 cells, the expressed VWF consisted predominantly of dimers, with a nearly complete loss of multimerization beyond the dimer band (Figure3, lanes 2, 3).
Unique multimeric structure of patient's plasma VWF.
Plasma samples were obtained from the patient and a family member (mother and daughter, respectively) and 5 VWD controls, analyzed by means of agarose gel electrophoresis (1% agarose), and detected by Western blot with AVW-5 and AVW-17. Control plasma samples included normal pool plasma (NP, lane 3); thrombotic thrombocytopenic purpura (TTP, lane 4); and type 2A (lane 5), type 2B (lane 6), type 1 (lane 7), and type 3 (lane 8) for typing comparisons. Plasma samples obtained from the mother and daughter are shown in lanes 1 and 2. The VWF multimeric patterns of both patients (lanes 1 and 2) do not resemble any of the known type 2 VWD multimeric patterns.
Unique multimeric structure of patient's plasma VWF.
Plasma samples were obtained from the patient and a family member (mother and daughter, respectively) and 5 VWD controls, analyzed by means of agarose gel electrophoresis (1% agarose), and detected by Western blot with AVW-5 and AVW-17. Control plasma samples included normal pool plasma (NP, lane 3); thrombotic thrombocytopenic purpura (TTP, lane 4); and type 2A (lane 5), type 2B (lane 6), type 1 (lane 7), and type 3 (lane 8) for typing comparisons. Plasma samples obtained from the mother and daughter are shown in lanes 1 and 2. The VWF multimeric patterns of both patients (lanes 1 and 2) do not resemble any of the known type 2 VWD multimeric patterns.
VWF structure and binding sites in relationship to the VWFpp mutation.
(A) The protein schematic of the pre-pro-VWF molecule. The propeptide (VWFpp) is 741 aa's with a 22-aa signal peptide, and is cleaved intracellularly at residue 763. Mature VWF is 2050 aa's in length. (B) Structure and function of VWF domains. The modular array of the repeating domains of VWFpp and VWF is shown. Several known functions of VWF have been mapped specifically to domains, listed below the drawing. The s-s sites shown for the D1 and D2 domains represent the vicinal cystines. The —S— depicts the intermolecular cystines, which bridge the VWF monomers into dimers (C2) and multimers (D3). The functional A1 and A3 loops are also depicted, above the domains. (C) The cDNA structure of the VWF. The different VWD mutations, types IIC, 2N, 2B, 2M, and 2A, are listed below along with the site of the Tyr87Ser defect.
VWF structure and binding sites in relationship to the VWFpp mutation.
(A) The protein schematic of the pre-pro-VWF molecule. The propeptide (VWFpp) is 741 aa's with a 22-aa signal peptide, and is cleaved intracellularly at residue 763. Mature VWF is 2050 aa's in length. (B) Structure and function of VWF domains. The modular array of the repeating domains of VWFpp and VWF is shown. Several known functions of VWF have been mapped specifically to domains, listed below the drawing. The s-s sites shown for the D1 and D2 domains represent the vicinal cystines. The —S— depicts the intermolecular cystines, which bridge the VWF monomers into dimers (C2) and multimers (D3). The functional A1 and A3 loops are also depicted, above the domains. (C) The cDNA structure of the VWF. The different VWD mutations, types IIC, 2N, 2B, 2M, and 2A, are listed below along with the site of the Tyr87Ser defect.
Multimeric composition of expressed mutant VWF.
The Tyr87Ser-VWF and WT-VWF plasmid constructs were transiently expressed in COS-7 cells, and the expressed proteins analyzed on 1.5% agarose-SDS gels (see “Materials and methods”). The type of VWF plasmid transfected is listed above the lanes, with normal plasma run for comparison purposes (lane 1). Two separate transfections of Tyr87Ser-VWF plasmids in COS-7 cells have been analyzed on this Western blot (lanes 2 and 3). Compared with the WT-VWF (lane 4), there is clearly a lack of multimerization by the Tyr87Ser (lanes 2 and 3) VWF defect, and only dimers are visualized.
Multimeric composition of expressed mutant VWF.
The Tyr87Ser-VWF and WT-VWF plasmid constructs were transiently expressed in COS-7 cells, and the expressed proteins analyzed on 1.5% agarose-SDS gels (see “Materials and methods”). The type of VWF plasmid transfected is listed above the lanes, with normal plasma run for comparison purposes (lane 1). Two separate transfections of Tyr87Ser-VWF plasmids in COS-7 cells have been analyzed on this Western blot (lanes 2 and 3). Compared with the WT-VWF (lane 4), there is clearly a lack of multimerization by the Tyr87Ser (lanes 2 and 3) VWF defect, and only dimers are visualized.
Effect of dimeric VWF on platelet binding
To determine whether the VWFpp mutation had an effect on the platelet-binding function of VWF, we analyzed the binding capability of expressed VWF in platelet-binding assays performed with ristocetin and botrocetin as agonists (Table 1). The antibiotic ristocetin was used to induce the interaction between VWF and the platelet GPIbα receptor.6 Botrocetin binds directly to the A1 loop of VWF, which contains the GPIb-binding domain, and induces the binding of VWF to platelet GPIb.1 The expressed WT-VWF bound as expected to platelets when induced by either ristocetin or botrocetin, similarly to pooled normal human plasma (NP) controls run for each assay (data not shown). While the Tyr87Ser mutant rVWF showed normal botrocetin-induced platelet binding (97.2% of control WT), there was a marked loss of ristocetin-induced platelet binding (16.8% of control WT).
Further functional characterization of the dimeric VWF species
We questioned whether the other functions of VWF might be similarly affected. We explored the potential effects on the critical VWF functions of collagen and FVIII binding by the dimeric VWF species. Since VWF interactions with subendothelial matrix proteins such as collagen are necessary to promote platelet adhesion and wound repair, we analyzed the ability of the recombinant VWF dimers to bind collagen. Compared with the VWF expressed by WT-VWF–transfected cells, the rVWF dimers expressed by Tyr87Ser-VWF–transfected cells displayed only 23% of the collagen-binding potential (Table 1).
In a solid-phase assay for VWF-to-FVIIIB activity, mAb-captured expressed rVWF was incubated with rFVIII in varying amounts (see “Materials and methods”). Table 1 compares the binding of FVIII results with rVWF samples from transient transfections of WT, Arg854Gln, Tyr87Ser, and mock plasmids. The Arg854Gln is a type 2N mutant construct shown to bind only 20% to 25% of rFVIII as compared with wild-type VWF. Binding of FVIII to the dimeric (Tyr87Ser mutant) rVWF species was detected, albeit at reduced levels (Tyr87Ser at 36.6% of WT), similar to that seen with the Arg854Gln VWF (26.1% of WT). Additionally, when an assay was performed with the use of plasma from this family in place of the cultured media samples (data not shown), the ratio of FVIII bound to VWF captured was between 53% and 62% (normal plasma controls were 91%). In this assay, a heterozygote 2N VWD control displayed a binding ratio of 53%, indicating that the patient's plasma possesses a degree of FVIII binding similar to that of the control 2N VWF.
Intracellular storage of Tyr87Ser-VWF
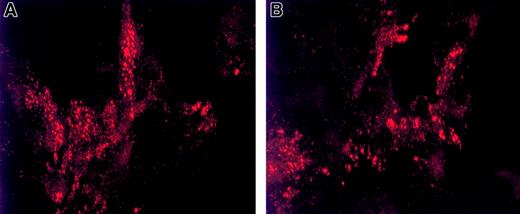
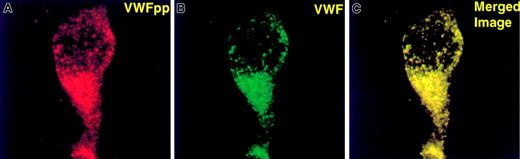
Since multimerization occurs prior to VWF regulated storage,8,22 we next addressed whether this VWFpp mutation would affect VWF intracellular storage. When expressed in AtT-20 cells, the Tyr87Ser dimeric VWF and WT-VWF were secreted at similar levels (303 ± 18 versus 264 ± 27 ng VWF per 1 × 106cells, respectively). To determine whether a dimeric VWF species could be stored in secretory granules, AtT-20 cells expressing mutant and wild-type VWF were stained with anti-VWF mAbs to assess intracellular localization of VWF. The punctate granular pattern detected by indirect immunofluorescent microscopy of cells expressing Tyr87Ser-VWF (Figure4B) appears as small and medium-sized granules similar to those observed in WT-VWF expressing AtT-20 cells (Figure 4A). Additional experiments focused on the capability of the mutant propeptide to direct noncontiguous VWF protein to storage. When the Tyr87Ser-VWFpp and mature VWF (Δpro)8 plasmids were cotransfected in AtT-20 cells as 2 separate gene products(in trans), both the mutant propeptide (Figure5, top panel) and VWF (Figure 5, center panel) were stored colocalized in granules as shown in the lower panel of Figure 5.
Intracellular storage of dimeric VWF in granules.
Intracellular storage of dimeric VWF in granules is similar to that of wild-type VWF. Indirect immunofluorescence detection of VWF storage granules in stably transfected AtT-20 cells demonstrates the vesicular storage of dimeric VWF. AtT-20 cells were stably transfected with WT-VWF (panel A) or Tyr87Ser-VWF (panel B). The fixed and permeabilized cells were immunolabeled as described in “Materials and methods.” All cells shown in this Figure have been labeled with anti-VWF polyclonal antibodies (detected by Texas Red) to visualize the intracellular localization of VWF. Shown are 3-D projections of confocal images taken by confocal laser scanning microscopy (Bio-Rad C600, × 1500 magnification). Dimeric VWF resulting from the Tyr87Ser mutation is stored in Weibel-Palade body–like granules similar to those of WT-VWF storage.
Intracellular storage of dimeric VWF in granules.
Intracellular storage of dimeric VWF in granules is similar to that of wild-type VWF. Indirect immunofluorescence detection of VWF storage granules in stably transfected AtT-20 cells demonstrates the vesicular storage of dimeric VWF. AtT-20 cells were stably transfected with WT-VWF (panel A) or Tyr87Ser-VWF (panel B). The fixed and permeabilized cells were immunolabeled as described in “Materials and methods.” All cells shown in this Figure have been labeled with anti-VWF polyclonal antibodies (detected by Texas Red) to visualize the intracellular localization of VWF. Shown are 3-D projections of confocal images taken by confocal laser scanning microscopy (Bio-Rad C600, × 1500 magnification). Dimeric VWF resulting from the Tyr87Ser mutation is stored in Weibel-Palade body–like granules similar to those of WT-VWF storage.
Mutant Tyr87Ser-VWFpp facilitation of intracellular storage of VWF.
The mutant Tyr87Ser-VWFpp functions in trans to facilitate intracellular storage of VWF. The Tyr87Ser-VWFpp was coexpressed in trans with Δpro (mature VWF) as 2 separate plasmids. The fixed and permeabilized cells were immunolabeled as described in “Materials and methods.” Transfected cells were labeled with anti-VWF polyclonal antibodies (detected by FITC) and anti-VWFpp monoclonal antibodies (detected by TXR) to visualize the intracellular localization of VWF and VWFpp. Panel A shows anti-VWFpp staining (red); panel C shows anti-VWF staining (green); and panel B represents the merge of VWFpp and VWF (colocalization is shown in yellow). Tyr87Ser-VWFpp directs mature VWF into storage granules.
Mutant Tyr87Ser-VWFpp facilitation of intracellular storage of VWF.
The mutant Tyr87Ser-VWFpp functions in trans to facilitate intracellular storage of VWF. The Tyr87Ser-VWFpp was coexpressed in trans with Δpro (mature VWF) as 2 separate plasmids. The fixed and permeabilized cells were immunolabeled as described in “Materials and methods.” Transfected cells were labeled with anti-VWF polyclonal antibodies (detected by FITC) and anti-VWFpp monoclonal antibodies (detected by TXR) to visualize the intracellular localization of VWF and VWFpp. Panel A shows anti-VWFpp staining (red); panel C shows anti-VWF staining (green); and panel B represents the merge of VWFpp and VWF (colocalization is shown in yellow). Tyr87Ser-VWFpp directs mature VWF into storage granules.
Formation of VWF dimers
Patient plasma exhibited what appeared to be dimers from the abnormal allele and normal multimers from the normal allele as displayed in Figure 1. Recently, Bodo et al24reported heterodimerization of a mutant (Cys1149Arg) VWF and normal subunits as the cause of intracellular retention and degradation resulting in a quantitative VWF deficiency. To investigate the origin of the patient multimer patterns, we examined whether the predominant protomeric species was a homodimer derived from message from the same allele. In addition to wild-type VWF, a second construct (HPP-VWF) expressing pro-VWF with a disrupted furin-cleavage site was used to create a larger protein that could be distinguished from wild type by SDS-agarose gel electrophoresis. This HPP-VWF protein has been shown to multimerize normally.23 The 2 VWF constructs were expressed independently and coexpressed in HEK293T cells. The resulting conditioned media were analyzed on submarine MetaPhor-agarose gels that better define dimeric VWF molecules (Figure6). As shown in Figure 6, our results demonstrate that there was not significant formation of heterodimers (less than 10%, lane 1, middle band). Instead, the predominant species appears to be mainly homodimers (39% HPP-VWF, lane 1, upper band, and 52% WT-VWF, lane 1, lower band). These experiments were repeated with the use of a VWF deletion mutant (A3 domain deleted) with the HPP-VWF. Similar results were obtained: 8% heterodimer, 46% HPP-VWF homodimer, and 46% ΔA3-VWF homodimer (data not shown).
Facilitation of multimerization.
The propeptide functions in cis and trans to facilitate multimerization. The question of homodimer versus heterodimer formation in VWF biosynthesis was addressed by the coexpression of HPP-VWF (wild-type VWF containing a disrupted furin-cleavage site) with wild-type VWF. When these 2 VWF proteins were coexpressed in HEK293T cells, the resulting protein expression showed nearly exclusive formation of homodimers (lane 1). As a size reference, each single plasmid transfection was included in the gel analysis (WT-VWF, lane 2, and HPP-VWF, lane 3). The immunoblot shown was scanned and the band densities were calculated. Each peak is listed with the scanned area and percentage of total area per gel lane. Only 8.6% of the total VWF detected in lane 1 is heterodimeric. Dimer formation appears to be primarily homodimeric.
Facilitation of multimerization.
The propeptide functions in cis and trans to facilitate multimerization. The question of homodimer versus heterodimer formation in VWF biosynthesis was addressed by the coexpression of HPP-VWF (wild-type VWF containing a disrupted furin-cleavage site) with wild-type VWF. When these 2 VWF proteins were coexpressed in HEK293T cells, the resulting protein expression showed nearly exclusive formation of homodimers (lane 1). As a size reference, each single plasmid transfection was included in the gel analysis (WT-VWF, lane 2, and HPP-VWF, lane 3). The immunoblot shown was scanned and the band densities were calculated. Each peak is listed with the scanned area and percentage of total area per gel lane. Only 8.6% of the total VWF detected in lane 1 is heterodimeric. Dimer formation appears to be primarily homodimeric.
Discussion
Our laboratory has been investigating the role of the VWFpp in the processing of the mature VWF molecule. A key congenital mutation was discovered in the VWFpp resulting in an unusual multimerization pattern in which there is a marked increase of the protomeric species of VWF, C-terminal dimers. The multimeric pattern displayed in Figure 1 is different from those published for subtype IIC VWD,25which also contain an overabundance of the dimeric species but with a selective loss of the high–molecular weight VWF multimers when studied with low-percentage agarose electrophoresis. The abnormality (Tyr87Ser) in this study is one that affects the later stages of VWF biosynthesis: the generation of VWF multimers. When this mutation (Tyr87Ser) was introduced into a VWF expression vector, the secreted VWF was nearly exclusively the dimeric subunit (Figure 3). Previous experiments indicated that multimerization is a process that is separate from dimerization.5,9,26 Our studies confirm these findings; the Tyr87Ser VWFpp defect directly affects the multimerization process of VWF. Furthermore, lack of multimerization did not preclude storage in AtT-20 cells (Figures 4 and 5), confirming that multimerization and granular storage are 2 independent processes.8
We theorize that the multimer pattern presented in Figure 1 is the result of multimers derived from separate alleles synthesizing wild-type (full-length multimers) and mutant VWF (dimer molecules) with little evidence for production of heterodimers. Recently, Bodo et al24 reported that a Cys1141Arg mutation in VWF had a dominant negative effect, causing endoplasmic reticulum retention of the normal subunit as a result of heterodimer formation with the mutant subunit. In this report, we did not find a random formation of heterodimer. In our study, we observed that less than 10% of the dimer population consisted of heterodimers (Figure 6). This result was obtained with the use of essentially wild-type VWF proteins or A3 domain–deleted VWF. We have found the propensity for dimer formation to be primarily homodimeric, indicating that assembly of dimers (C-terminal) may occur cotranslationally. The drive for homodimer formation could be the result of C-terminal interaction with the nearest neighboring VWF monomer, which most likely would be a monomer being produced by the same polysome and therefore the same mRNA molecule.
Although the mutation affects the propeptide, the inhibitory effect of this defect manifests itself in a dramatic loss of several critical functions for the processed VWF protein. While the ability of the dimeric rVWF to participate in botrocetin-induced platelet binding was unaffected, the dimeric rVWF displayed diminished ristocetin-induced platelet binding, collagen binding, and FVIII binding (Table 1). The decrease in platelet binding to these dimers in the presence of ristocetin is not surprising, since it is known that high–molecular weight multimers have a greater predilection for platelets in the presence of ristocetin.6 In contrast, botrocetin is not affected by the multimeric size of VWF.6 Even though the binding sites for collagen in the A1 and A3 domains are not mutated, there was a marked decrease in rVWF dimer binding to type III collagen (Table 1). This could be attributed to the loss of HMW multimers since it has been shown that the highest MW multimers bind collagen with the greatest avidity.1 6 The dimeric rVWF also binds FVIII, but at reduced levels, similar to type 2N VWD controls. This suggests a dependency of FVIII binding on VWF multimer size, and rVWF binding of FVIII may depend on the degree of cooperation between neighboring FVIII-binding sites in more fully multimerized VWF molecules.
Defects and deletions in the propeptide of VWF have demonstrated the obligatory nature of VWFpp in the multimerization process. Experiments involving deletion of parts of VWFpp (ΔD1 and ΔD2) or the entire VWFpp (Δpro-VWF) demonstrated that deletion of these regions resulted in the expression of only the dimeric form of VWF and prevented the synthesis of VWF multimers.9,27 When transfected in trans, the separate and noncontiguous VWFpp retains the ability to correctly orientate the mature-only subunits of Δpro-VWF to create multimers in vivo27 and in vitro.28 In patients with a heterozygous abnormality, the functional wild-type VWFpp is involved in multimerization of the wild-type VWF molecules and is unavailable to function in trans. Naturally occurring mutations in the VWFpp (Tyr87Ser [this study], Arg273Trp,29 Asn528Ser,30Gly550Arg,31 Cys623Trp,25 and 625insGly25) all demonstrate altered multimerization, presumably resulting from their VWFpp defects, yet none are located in close proximity to any functional areas. An intact VWFpp appears to be prerequisite for the N-terminal multimerization of VWF in the trans Golgi network (TGN).
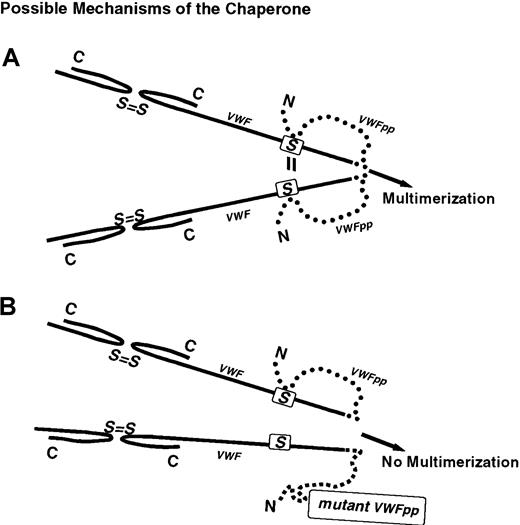
Propeptides of peptide hormones have been reported to act upon their mature subunits as intramolecular chaperones, including subtilisin, preprosomatostatin, lactase-phlorizin hydrolase, and prouroguanylin.32-35 The uncleaved, contiguous propeptide functions at only one point in the folding pathway: assisting in protein folding and stabilization prior to protein maturation.32 These chaperones remain attached to the mature sequence until the maturation process is complete, and then are proteolytically removed by autoprocessing or another endopeptidase.32 Analogous to other intramolecular chaperones, the lengthy propeptide (741 aa) of VWF appears to stabilize the intramolecular disulfide interactions between cysteines within individual D1, D2, and D3 domains. It is our hypothesis that the propeptide of VWF functions during the maturation process as an intramolecular chaperone. In our model (Figure7), the propeptide serves as a molecular chaperone during the final stages of protein folding, aligning the dimeric pro-VWF subunits in the proper spacial arrangement necessary to create disulfide bridges between the free sulfhydryls in N-termini (D3 domain) of adjacent pro-VWF molecules.
Proposed intramolecular chaperone function of the VWFpp.
The propeptide of VWF functions as an intramolecular chaperone. The contiguous molecule folds VWF into the correct orientation necessary for proper intermolecular bridging by the cysteines in the adjacent dimers (panel A). The VWFpp role may be to tether the molecules together, allowing the cysteines to interact. The Tyr87Ser VWF defect and possibly other type IIC VWD defects cause the region to assume an incorrect configuration (panel B), and thus prevent the proper contact between adjacent dimers that results in a loss of N-terminal multimerization.
Proposed intramolecular chaperone function of the VWFpp.
The propeptide of VWF functions as an intramolecular chaperone. The contiguous molecule folds VWF into the correct orientation necessary for proper intermolecular bridging by the cysteines in the adjacent dimers (panel A). The VWFpp role may be to tether the molecules together, allowing the cysteines to interact. The Tyr87Ser VWF defect and possibly other type IIC VWD defects cause the region to assume an incorrect configuration (panel B), and thus prevent the proper contact between adjacent dimers that results in a loss of N-terminal multimerization.
The tertiary and quaternary structures have yet to be deciphered for VWFpp. Hence, it is not known how the Tyr87Ser mutation would disrupt the structure or function of the VWFpp. In order for a defect to inhibit the multimerization of VWF, it must disrupt the alignment of the VWFpp and the mature VWF protein. We have discovered that our novel mutation is situated in a highly conserved region of the VWFpp. Alignment of the human VWFpp with the VWFpp's of 4 divergent mammals (canine,8 porcine [unpublished, accession no. AY004876], bovine,36 and murine [unpublished sequence obtained in our laboratory]) shows an expanse of 12 aa's that are perfectly conserved around the mutation (Figure 8). This defect must disrupt the conformation of VWFpp in such a way that its ability to function as an intramolecular chaperone is impaired.
High degree of homology in the D1 domain across species.
The VWFpp of human, porcine, bovine, canine, and murine VWF have been aligned. Shown in this Figure is a 60-aa span of the D1 domain for 5 species and the Tyr87Ser mutation in human sequence. Murine VWF has been reported for only a small portion of the D1 domain, shown here from residues 74 through 120. Only differences are shown, with · depicting a perfect homology match. Between the 5 species' VWFpp's, there is a very strong homology throughout this region in the D1 domain of perfectly conserved and well-conserved amino acids (86% to 95%), indicating that the Tyr87Ser mutation may alter the configuration of the region.
High degree of homology in the D1 domain across species.
The VWFpp of human, porcine, bovine, canine, and murine VWF have been aligned. Shown in this Figure is a 60-aa span of the D1 domain for 5 species and the Tyr87Ser mutation in human sequence. Murine VWF has been reported for only a small portion of the D1 domain, shown here from residues 74 through 120. Only differences are shown, with · depicting a perfect homology match. Between the 5 species' VWFpp's, there is a very strong homology throughout this region in the D1 domain of perfectly conserved and well-conserved amino acids (86% to 95%), indicating that the Tyr87Ser mutation may alter the configuration of the region.
A second potential function ascribed to the VWFpp is to direct VWF into vesicular storage via an intrinsic TGN-sorting signal.8,26When the dimer-generating VWF mutant was expressed in AtT-20 cells, granular storage of transfected VWF was observed (Figure 4). Furthermore, as the double transfection (in trans) with Tyr87Ser-VWFpp and Δpro-VWF plasmids of AtT-20 cells demonstrated, this mutant propeptide could still interact with and direct the mature VWF into storage granules (Figure 5). Hence, even though the mutation in the propeptide rendered the region incapable of its key chaperone function, it does not preclude interactions necessary for granular storage. While other studies have demonstrated multimerization of VWF to be necessary for granular storage,37 38 our results demonstrate that VWF multimerization is not a prerequisite for storage. Rather, our model of VWF storage requires intact propeptide and structurally competent VWF that can associate noncovalently with VWFpp to cotraffick to storage granules. Deletion of one or more domains from the mature VWF protein may result in structurally incompetent VWF that cannot associate with VWFpp, leading to loss of VWF granular storage.
The correct folding of VWF into C-terminal dimers does occur without the presence of an intact VWFpp. However, if the VWFpp is defective, the final stages of VWF biosynthesis (N-terminal multimerization) are aberrant with no multimerization observed. It is evident that there are multiple domains or motifs present in the propeptide of VWF that together in the correct tertiary configuration confer this function. The entire propolypeptide appears to function in this chaperone capacity; thereby, missense mutations (eg, Tyr87Ser) abolish this vital VWF biosynthesis function. The propeptide functions as an obligatory intramolecular chaperone necessary for the correct alignment of the C-terminal dimers into the tail-to-tail position during VWF N-terminal multimerization.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2002-03-0789.
Supported by grants from the National Hemophilia Foundation—Judith Graham Pool Fellowship (J.B.R.), National Blood Foundation (J.B.R.), the American Heart Association (BGIA 0060437Z to J.B.R.), National Institutes of Health (grants HL-44612 and HL-33721 to R.R.M.), National Institutes of Health Clinical Research Center Program Grant MO1-RR00058 (M.J. Dunn, Medical College of Wisconsin), and the Malcolm Hewitt Wiener Foundation, Greenwich, CT (R.R.M.).
J.B.R. and S.L.H. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert R. Montgomery, Department of Pediatrics, The Medical College of Wisconsin, 8701 W Watertown Plank Rd, Milwaukee, WI 53226; e-mail: bob@bcsew.edu.