Decorin is a small leucine-rich proteoglycan able to interact with several molecules of the subendothelial matrix, such as collagen and fibronectin. In this work, we investigated the ability of purified decorin to support adhesion of human platelets. We found that gel-filtered platelets were actually able to interact with immobilized decorin. Platelet adhesion to decorin was time dependent, required the presence of Mg2+ ions, and was totally mediated by the protein core of the proteoglycan. Platelet stimulation with either adenosine diphosphate (ADP) or a thrombin receptor–activating peptide significantly increased interaction of these cells with the proteoglycan. Upon adhesion to immobilized decorin a number of platelet proteins were found to become tyrosine-phosphorylated. By immunoprecipitation experiments with specific antibodies, the tyrosine phosphorylation of the tyrosine kinase Syk and the phospholipase Cγ2 (PLCγ2) isozyme was demonstrated in decorin-adherent platelets. Interaction of platelets with decorin was selectively prevented by 2 different antibodies against membrane integrin α2β1, but not by a number of antibodies against other membrane receptors. In addition, integrin α2β1, purified from platelet membranes, was able to specifically interact with immobilized decorin. Finally, purified decorin bound to Sepharose beads could precipitate integrin α2β1 from a platelet membrane glycoprotein preparation. Therefore, these results demonstrate that human platelets can bind to immobilized decorin through integrin α2β1, and that this interaction results in the tyrosine phosphorylation of intracellular proteins.
Introduction
Decorin is the prototype member of a growing family of structurally related extracellular matrix proteoglycans, known as small leucine-rich proteoglycans.1 It is composed by a 40-kDa protein core and one chondroitin/dermatan sulfate side chain linked to a serine residue at the N-terminal region of the protein. The protein core contains 10 leucine-rich repeats of 24 amino acids forming a typical α-helix/β-sheet structural motif, and is involved in protein-protein interaction. The N- and C-terminal regions of decorin are organized in globular structures stabilized by disulphide-bounded cysteines.
Decorin plays a key role in the regulation of extracellular matrix assembly by binding to several components such as collagen,2-5 thrombospondin,6 and fibronectin.7,8 Interaction of decorin with collagen has been shown to affect fibril formation by causing an initial delay in the lateral assembly and a reduction of the average fibril diameter.2,9 These effects may explain the phenotype of decorin-null mice characterized by abnormal skin fragility and loosely packed collagen fibers.10
In addition to its ability to modulate the assembly of the extracellular matrix, decorin also displays a number of biologic effects. For instance, this proteoglycan binds to growth factors, such as transforming growth factor β (TGFβ), and modulates TGFβ-dependent cell responses.11,12 Decorin is also involved in the control of cell growth: its synthesis is increased during growth arrest and is suppressed in several tumorigenic cell lines.13-15 The ability of decorin to suppress cell growth is mediated by binding to the epidermal growth factor (EGF) receptor.16 This interaction involves the protein core of the proteoglycan and causes the dimerization and autophosphorylation of the EGF receptor, leading to activation of mitogen-activated protein (MAP) kinases and induction of the growth suppressor p21.16-18 In addition to EGF receptor, another still-unidentified membrane receptor with a molecular mass of 51 kDa has been shown to bind decorin.19,20 This interaction leads to the endocytosis of the proteoglycan, which is necessary for intralysosomal degradation.19 20 Therefore, decorin is a regulator of both extracellular matrix assembly and cell function.
Upon a vessel wall injury, molecules of the subendothelial matrix are exposed to circulating blood cells. As an early event in the hemostatic processes, platelets rapidly adhere to several subendothelial components through specific membrane receptors. This event is followed by rapid platelet activation that leads to the formation of cell aggregates, representing a real hemostatic plug. Among the different components of the subendothelial matrix, several glycoproteins have been shown to mediate platelet adhesion and activation. For instance, platelet interaction with collagen and von Willebrand factor has been deeply investigated and certainly plays a major role in the recruitment of these cells at the site of vessel wall injury.21-23Similarly, adhesion to other glycoproteins of the subendothelial matrix, such as thrombospondin,24fibronectin,25 and laminin,26 has been reported. By contrast, no information is available on the possible interaction of human platelets with the proteoglycans, which are relevant components of the subendothelial matrix, and, in addition to adhesive glycoproteins, may play a role in primary hemostasis. In the light of the increasing interest arising from the dual role of the small proteoglycan decorin as regulator of matrix assembly and cell function, we investigated its possible involvement in the hemostatic processes by analyzing its ability to support platelet adhesion and activation. In this work we demonstrate that human platelets efficiently adhere to decorin under static conditions. This interaction is mediated by integrin α2β1 and leads to platelet activation through the stimulation of intracellular tyrosine kinases. These results suggest a novel role for the small leucine-rich proteoglycan decorin on the adhesion of human platelets to the subendothelial matrix.
Materials and methods
Materials
Sepharose CL-2B, protein G-sepharose, concanavalin A-Sepharose, streptavidin-Sepharose, DEAE-Sephacel, and PD10-columns were purchased from Amersham Pharmacia Biotech (Cologno Monzese, Italy). Chondroitinase ABC was from Seikagaku (Tokyo, Japan). Recombinant decorin was obtained from EMP Genetech (Denzlingen, Germany). Protein A-Sepharose, CNBr-activated Sepharose 4B, ADP, GRGDS, and apyrase were from Sigma (Milan, Italy). EZ-link sulfo-NHS-biotin and the bicinchoninic acid assay kit for protein determination were from Pierce (Pero, Italy). Microtiter plates were from Nunc International (Denmark), and polystyrene dishes were from Orange Scientific (Milan, Italy). Collagen type I was purchased from Collagen Corporation (Freemont, CA). The following antibodies were used in the present work: anti-integrin α2β1, P1E6 (Gibco, Milan, Italy), and 1998 (Chemicon, Rome, Italy); anti-integrin α2,1936 (Chemicon); anti-integrin αIIbβ3, P2 (Immunotech, Marseille, France); anti-GPIb, AK2 (provided by Dr P. Noris, IRCCS, Policlinico San Matteo, Pavia, Italy); anti-CD38, IB4 and anti-CD31, Moon-1 (a kind gift from Dr F. Malavasi, Laboratory of Cell Biology, University of Turin, Italy); anti-Syk and antiphospholipase Cγ2 (PLCγ2) (Santa Cruz Biotechnology, Heidelberg, Germany); anti–collagen type I (Sigma); antiphosphotyrosine (Upstate Biotechnology, Lake Placid, NY). Peroxidase-conjugated secondary antibodies were obtained from Bio-Rad (Milan, Italy).
Decorin purification
Decorin was purified as described elsewhere.27,28Briefly, proteoglycans were extracted from bovine tendon with 4 M guanidinium hydrochloride in 50 mM acetate buffer, 5 mM benzamidine, 0.1 M ε-aminocaproic acid, 10 mM EDTA (ethylenediaminetetraacetic acid), 1 mM phenylmethylsulfonyl fluoride (PMSF), pH 5.6, and purified by preparative ultracentrifugation (100 000g) in a CsCl gradient in the presence of buffered 4 M guanidinium hydrochloride. The fraction with density 1.5 g · mL−1was adsorbed on diethylaminoethanol (DEAE)–Sephacel and eluted with a linear 0-0.8 M NaCl gradient in the presence of 4 M urea. Decorin was desalted on PD-10 columns, freeze-dried, and stored at −80°C. The protein content of the decorin preparations was determined with the Bradford method. Decorin protein core was prepared by digestion of purified decorin with chondroitinase ABC (EC4.2.2.4) as reported by Yamagata et al.29
Platelet preparation
Blood was collected from healthy volunteers using citric acid–citrate-dextrose as anticoagulant and centrifuged at 120g for 10 minutes at room temperature to obtain the platelet-rich plasma (PRP). Platelets were then recovered by centrifugation of the PRP at 300g for 10 minutes and resuspended in a small volume (0.5-1 mL) of autologous plasma. Platelets were then isolated by gel-filtration on a 10-mL column of Sepharose CL-2B and eluted with HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (10 mM HEPES, 137 mM NaCl, 2.9 mM KCl, 12 mM NaHCO3, pH 7.4). Platelet count was adjusted to 1 × 109 cells/mL with the same buffer and then diluted for the adhesion assay.
Platelet adhesion assay
Platelet adhesion to decorin was studied in 60-mm polystyrene dishes coated for 16 hours at room temperature with 1 mL of 100 μg/mL decorin solution or decorin protein core in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA). Control plates were coated with 0.5% BSA in PBS for the same time. Dishes were then washed 3 times with 5 mL PBS and then blocked with 2 mL 5% BSA in PBS for 3 hours at room temperature. Decorin-coated and control dishes were finally washed again 3 times with 5 mL PBS. Gel-filtered platelets were diluted at a final concentration of 2 × 108 cells/mL with HEPES buffer containing 0.1% BSA, 5.5 mM glucose, and 2 mM MgCl2. Then, 0.5 mL platelet suspension (1 × 108 cells) were added to decorin-coated or control dishes and incubated for 90 minutes at room temperature. In some experiments, platelet samples were incubated with agonists or specific antibodies just before addition to decorin-coated or control dishes. Platelet stimulation was performed with 10 μM ADP or 10 μM thrombin receptor–activating peptide (TRAP). At the end of the incubation, nonadherent platelets were gently removed and either discharged or collected in separate tubes for further analysis. Dishes were washed 3 times with 5 mL PBS, and the adherent platelets were lysed and scraped into 0.1 mL 2% sodium dodecyl sulfate (SDS) in HEPES buffer at 90°C. Platelet adhesion was quantified using a colorimetric assay based on the consideration that the number of adherent cells is proportional to the amount of cell-derived proteins as previously described.30 31 Lysed, adherent cells were transferred to a test tube, and the protein content was determined by the bicinchoninic acid assay. To correlate the amount of measured proteins with the number of adherent cells, parallel samples containing 1 × 108 platelets from the same cell suspension used for the adhesion experiments (corresponding to the number of cells added to each dish) were prepared, lysed with 2% SDS, and subjected to protein determination. Results are generally expressed as percentage of adherent cells referred to the total added platelets.
Immunoprecipitation
For immunoprecipitation experiments, adherent platelets were lysed with 0.25 mL ice-cold immunoprecipitation buffer (10 mM Tris/HCl pH 7.4, 158 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 5 mM EGTA (ethyleneglycoltetraacetic acid), 1 mM PMSF, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 1 mM Na3VO4). Platelet lysates were placed on ice for 15 minutes. Nonadherent platelets, collected after the adhesion assay, were lysed by adding an equal volume of ice-cold immunoprecipitation buffer 2X and placed on ice. Similarly, an aliquot of total gel-filtered platelets in HEPES buffer was also lysed with immunoprecipitation buffer. Upon incubation on ice, the samples were centrifuged at 13 000 rpm for 10 minutes at 4°C to remove insoluble materials, and the protein concentration in the supernatant was determined by the bicinchoninic acid assay. Aliquots of each sample, containing equal amount of proteins, were precleared for 1 hour at 4°C with 100 μL protein A-Sepharose (50 mg/mL stock solution). The cleared lysates were incubated with 1 μg anti-Syk or anti-PLCγ2 antisera for 2 hours at 4°C. Immunocomplexes were then recovered by incubation with 100 μL protein A-Sepharose for 45 minutes. After centrifugation, immunoprecipitates were washed 3 times with immunoprecipitation buffer and finally resuspended with 25 μL SDS-sample buffer (25 mM Tris, 192 mM glycine, 2% SDS, 0.5% DTT, 10% glycerol, 0.01% bromophenol blue, pH 8.3) and heated at 95°C for 3 minutes.
Immunoblotting
Analysis of protein tyrosine phosphorylation was performed on both immunoprecipitates and whole cell lysates. In the latter case, aliquots containing the same amount of proteins from total platelets, nonadherent or adherent platelets to decorin of BSA-coated dishes, were added to an equal volume of SDS-sample buffer and heated at 95°C for 3 minutes. Immunoprecipitates, as well as samples of whole platelet lysates, were subjected to SDS-PAGE on 7.5% acrylamide gels. Proteins were transferred to nitrocellulose and tested with antibodies against phosphotyrosine, Syk, or PLCγ2, as previously described.32
Purification of integrin α2β1
Integrin α2β1 was purified from platelet membranes by affinity chromatography on collagen-Sepharose 4B, essentially as described by Kern et al.33 Platelet membranes were prepared from transfusion units obtained from the local blood bank, as previously described,34 and solubilized in extraction buffer (50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 1 mM MnCl2, 2 mM MgCl2, 100 mM n-octyl β-D-glucopyranoside, 10 μg/mL leupeptin, 10 μg/mL aprotinin). Type I collagen was coupled to cyanogen bromide–activated Sepharose 4B, according to the manufacturer's instructions. The efficiency of the coupling was evaluated by hydroxyproline assay. A 5 mL column of collagen-Sepharose 4B was packed and equilibrated with extraction buffer at a flow rate of 30 mL per hour. Solubilized membrane proteins were loaded at a flow rate of 10 mL per hour. After extensive washes of the column, bound proteins were eluted with Tris/HCl 50 mM, pH 7.4, 150 mM NaCl, 25 mM n-octyl β-D-glucopyranoside, 25 mM EDTA, and monitored continuously by spectrophotometry at 280 nm. Positive fractions were pooled and extensively dialysed against Tris/HCl 50 mM, pH 7.4, 150 mM NaCl, 25 mM n-octyl β-D-glucopyranoside, 2 mM MgCl2, 1 mM MnCl2. The amount of proteins was determined by the bicinchoninic acid assay, and the purity of integrin α2β1 was confirmed by electrophoretical analysis followed by silver staining of the gels.
Solid-phase binding assay
Wells of a microtiter plate were coated with 50 μL decorin (400 μg/mL in PBS) for 16 hours at 4°C. Plates were washed 3 times with 100 μL washing solution (150 mM NaCl and 0.1% Tween 20) to remove unbound proteins. Additional binding sites were saturated for 2 hours with 100 μL 1% BSA in PBS. Increasing amounts of purified integrin α2β1 (0.1, 0.5, 1, and 2 μg) in a final volume of 50 μL Tris/HCl 50 mM, pH 7.4, 150 mM NaCl, 25 mM n-octyl β-D-glucopyranoside, 2 mM MgCl2, 1 mM MnCl2 were added, and incubation was performed for 2 hours at room temperature. Wells were then washed 4 times with washing solution containing 2 mM MgCl2 and incubated with 50 μL of the anti-integrin α2 antibody 1936 (1:1000 dilution) in PBS containing 1% BSA, 0.05% Tween 20, and 2 mM MgCl2for 2 hours at room temperature, followed by incubation with a peroxidase-conjugated secondary antibody for an additional hour. Bound proteins were detected by a colorimetric reaction using o-phenylenediamine dihydrochloride as substrate. Control wells were prepared by omitting either coating with decorin or incubation with purified integrin α2β1.
Integrin α2β1 pull-down assay
This was performed by a modification of the method described by Navdaev et al.35 Briefly, purified decorin was biotinylated by incubation with EZ-link sulfo-NHS-biotin at a molar ratio of 1:20 for 2 hours at room temperature. Biotin-linked decorin was separated from free biotin by extensive dialysis. Solubilized platelet membrane proteins were applied on a column of concanavalin A-Sepharose, and bound glycoproteins were eluted with 100 mM methyl-α-mannopyranoside in Tris/HCl 50 mM, pH 7.4, 135 mM NaCl, 1 mM CaCl2, 2 mM MgCl2, 1 mM MnCl2, 10 mM n-octyl β-D-glucopyranoside. Fractions containing eluted glycoproteins were pooled, and 1 mL samples were pretreated with 200 μL streptavidin-Sepharose (20% slurry) for 1 hour at 4°C. The cleared samples were incubated either with 100 μg biotinylated decorin or an equal volume of buffer for 2 hours at room temperature, and then streptavidin-Sepharose was added. After further incubation for 1 hour, the beads were collected by centrifugation and washed 3 times, and the associated proteins solubilized with 25 μL SDS-sample buffer. The presence of integrin α2β1 among the glycoproteins bound by biotinylated decorin was analyzed by immunoblotting with the anti-integrin α2 antibody 1936.
Results
Characterization of purified decorin
The decorin preparations used in this study were first characterized by electrophoretic analysis under denaturating conditions, before and after digestion with chondroitinase ABC: a single band of about 100 kDa and 40 kDa, respectively, was observed. Purified decorin was then subjected to N-terminal amino acid sequencing. In all the preparations, a unique and correct sequence was determined, confirming the absence of any contaminating proteins. The structural conformation of purified decorin was analyzed by circular dichroism spectroscopy: spectra at 20°C were very similar to those reported in the literature for recombinant decorin.
In addition, we specifically tested the decorin preparations for contaminating collagen. Amino acid analysis of hydrolyzed samples did not reveal the presence of hydroxyproline, and the amount of proline was consistent with that expected for purified decorin. Moreover, the presence of collagen was also tested by dot blot experiments using a specific antiserum against collagen type I. Even when 10 μg purified decorin was tested, no reactivity to the anticollagen antibody was observed. By spotting on nitrocellulose filters increasing amounts of purified collagen, we found that this immunological system was able to detect amounts of collagen as low as 10 ng, indicating that if undetectable traces of collagen were present in the decorin preparations, they accounted for < 0.1% (data not shown). Therefore, we conclude that decorin used in this study was highly purified and essentially free of contaminating collagen.
Platelet adhesion to immobilized decorin
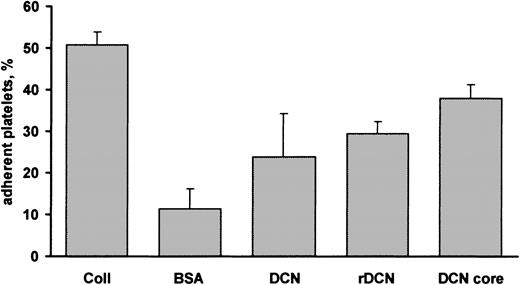
To investigate the ability of human platelets to interact with decorin, polystyrene dishes were coated with either purified decorin or BSA as a control. Gel-filtered platelets were incubated with immobilized ligands for 90 minutes at room temperature in the presence of 2 mM MgCl2, and the extent of adhesion was estimated by a colorimetric assay as described in “Material and methods.” Figure1 shows that under these conditions about 24% of added platelets were able to adhere to decorin-coated dishes, while the nonspecific binding to immobilized BSA was only about 10%. Under the same conditions, platelet adhesion to collagen-coated dishes was about 50%, indicating that interaction of platelets with decorin, although significant, was less efficient. When polystyrene dishes were coated with recombinant decorin, the percentage of platelet adhesion was very similar to that observed using dishes coated with the proteoglycan purified in our laboratory (Figure 1). To investigate whether decorin interaction with platelets was mediated by the proteoglycan protein core or by the glycosaminoglycan side chain, purified decorin was digested with chondroitinase ABC, and the isolated protein core was used to coat polystyrene dishes. As shown in Figure 1, a significant platelet adhesion to the protein core was observed. The level of adhesion to the decorin protein core was about 37%, and, therefore, subsequent experiments were performed using purified decorin protein core.
Platelet adhesion to decorin.
Polystyrene dishes were coated with 100 μg collagen (Coll), purified decorin (DCN), recombinant decorin (rDCN), or decorin protein core (DCN core). Control dishes were coated with BSA. Gel-filtered platelets (1 × 108 platelets) were added to the dishes and incubated for 90 minutes at room temperature. Nonadherent cells were removed, and the percentage of adherent platelets was determined by a colorimetric assay as described in “Materials and methods.” Results are the means ± SD of 3 to 8 experiments.
Platelet adhesion to decorin.
Polystyrene dishes were coated with 100 μg collagen (Coll), purified decorin (DCN), recombinant decorin (rDCN), or decorin protein core (DCN core). Control dishes were coated with BSA. Gel-filtered platelets (1 × 108 platelets) were added to the dishes and incubated for 90 minutes at room temperature. Nonadherent cells were removed, and the percentage of adherent platelets was determined by a colorimetric assay as described in “Materials and methods.” Results are the means ± SD of 3 to 8 experiments.
In time course experiments, platelet interaction with decorin appeared to be quite rapid. After 30 minutes of incubation the percentage of cell adhesion was already about 28%, and then further increased to reach a maximum after 90 minutes (data not shown). The morphology of decorin-adherent platelets was analyzed by fluorescence microscopy upon staining with trypan blue. Platelets adhered to immobilized decorin as single cells, and no aggregates were detected. Adherent platelets appeared as round cells with several thin and long pseudopods, indicating that some degree of activation occurred (data not shown).
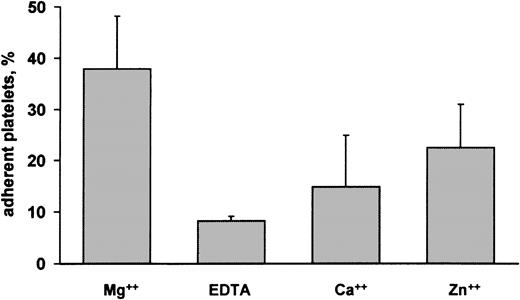
Platelet adhesion to decorin was investigated in the presence of 2 mM MgCl2. To analyze the role of this ion on platelet-decorin interaction, adhesion assays were performed in the absence of MgCl2 or in the presence of different divalent cations such as Ca++ and Zn2+. Figure2 shows that platelet adhesion to decorin was actually Mg2+ dependent. In the presence of EDTA, adhesion dramatically dropped to a percentage comparable to that observed in BSA-coated dishes. Figure 2 also shows that Mg2+ could not be substituted with Ca++, while only a slight increase of platelet adhesion to decorin was observed in the presence of Zn2+. Another divalent ion, Mn2+, could not be tested in our experimental system because it was found to strongly interfere with the colorimetric assay used to quantify platelet adhesion.
Role of divalent ions on platelet adhesion to decorin.
Adhesion of gel-filtered platelets to decorin-coated dishes was analyzed in the presence of 2 mM MgCl2, (Mg++), 2 mM EDTA (EDTA), 2 mM CaCl2 (Ca++), or 2 mM ZnCl2 (Zn++). Results are expressed as percentage of adherent platelets and represent the mean ± SD of 3 separate experiments.
Role of divalent ions on platelet adhesion to decorin.
Adhesion of gel-filtered platelets to decorin-coated dishes was analyzed in the presence of 2 mM MgCl2, (Mg++), 2 mM EDTA (EDTA), 2 mM CaCl2 (Ca++), or 2 mM ZnCl2 (Zn++). Results are expressed as percentage of adherent platelets and represent the mean ± SD of 3 separate experiments.
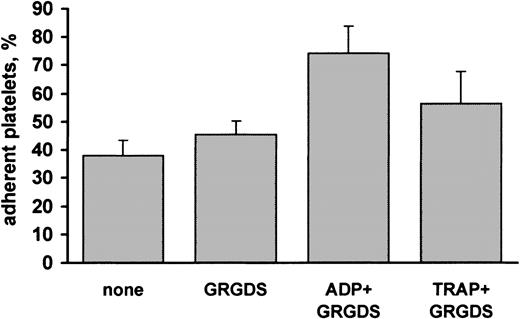
It is known that the ability of platelets to interact with several adhesive proteins is increased upon stimulation of these cells.23 Therefore, we investigated the effect of platelet stimulation on interaction with decorin. Platelets were allowed to adhere to decorin-coated dishes in the presence of 10 μM ADP or 10 μM TRAP, a selective thrombin receptor activating peptide. Stimulation with either ADP or TRAP increased the ability of platelets to interact with immobilized decorin (Figure3). In particular, in ADP-stimulated platelets the percentage of adhesion was approximately 70%. Because, even in the absence of stirring, platelet activation may result in the formation of small cell aggregates that can cause an overestimation of the percentage of adherent cells, assays with stimulated platelets were performed in the presence of 1 mM Gly-Arg-Gly-Asp-Ser (GRGDS) peptide, a potent antagonist of fibrinogen binding to integrin αIIbβ3, and, consequently, an inhibitor of platelet aggregation. Figure 3 shows that the GRGDS peptide alone did not significantly affect platelet adhesion to decorin. These results indicate that platelet binding to decorin is potentiated upon stimulation.
Effect of platelet stimulation on adhesion to decorin.
Samples of gel-filtered platelets were mixed with buffer (none), 1 mM peptide GRGDS, 10 μM ADP, or 10 μM thrombin receptor activating peptide (TRAP) in the presence of 1 mM GRGDS and immediately added to decorin-coated plates. After incubation for 90 minutes, the percentage of adherent platelets was determined by a colorimetric assay. Results are the mean ± SD of 3 to 4 separate experiments.
Effect of platelet stimulation on adhesion to decorin.
Samples of gel-filtered platelets were mixed with buffer (none), 1 mM peptide GRGDS, 10 μM ADP, or 10 μM thrombin receptor activating peptide (TRAP) in the presence of 1 mM GRGDS and immediately added to decorin-coated plates. After incubation for 90 minutes, the percentage of adherent platelets was determined by a colorimetric assay. Results are the mean ± SD of 3 to 4 separate experiments.
Platelet adhesion to decorin activates tyrosine kinases
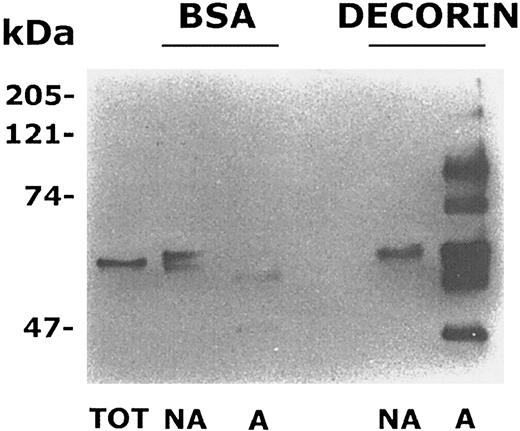
It is known that platelet interaction with some components of the subendothelial matrix such as collagen, von Willebrand factor, or fibrinogen leads to cell activation associated with stimulation of tyrosine kinases and tyrosine phosphorylation of several intracellular proteins.32,36,37 Therefore, we analyzed the level of protein tyrosine phosphorylation in decorin-adherent platelets. Gel-filtered platelets were incubated with BSA- or decorin-coated dishes for 90 minutes. Adherent as well as nonadherent platelets were lysed, and an equal amount of proteins was separated on a 7.5% acrylamide gel, transferred to nitrocellulose, and probed with antiphosphotyrosine antibodies. In samples from nonadherent platelets, a single tyrosine phosphorylated protein with an apparent molecular mass of about 60 kDa (that in some samples appeared as a doublet by unknown reasons) was evident (Figure 4). This band was also present in lysates from untreated resting platelets and probably represented the tyrosine kinase pp60src, which is highly expressed and constitutively tyrosine phosphorylated in these cells.38 By contrast, a number of additional tyrosine phosphorylated proteins were detected in platelets adherent to decorin- but not to BSA-coated dishes (Figure 4). The main bands appeared to have molecular masses of about 38, 50, 60, 70, 120, and 150 kDa. These results indicate that adhesion of platelets to decorin specifically induces the tyrosine phosphorylation of multiple substrates.
Platelet adhesion to decorin stimulates protein tyrosine phosphorylation.
Samples of whole cell lysates from adherent (A) and nonadherent (NA) platelets to decorin or BSA-coated dishes containing the same amount of proteins (10 μg) together with a sample of resting untreated platelets (TOT) were separated by SDS-PAGE on a 7.5% acrylamide gel. Proteins were transferred to nitrocellulose and probed with antiphosphotyrosine antibody. The positions of standard molecular weight markers are reported on the left.
Platelet adhesion to decorin stimulates protein tyrosine phosphorylation.
Samples of whole cell lysates from adherent (A) and nonadherent (NA) platelets to decorin or BSA-coated dishes containing the same amount of proteins (10 μg) together with a sample of resting untreated platelets (TOT) were separated by SDS-PAGE on a 7.5% acrylamide gel. Proteins were transferred to nitrocellulose and probed with antiphosphotyrosine antibody. The positions of standard molecular weight markers are reported on the left.
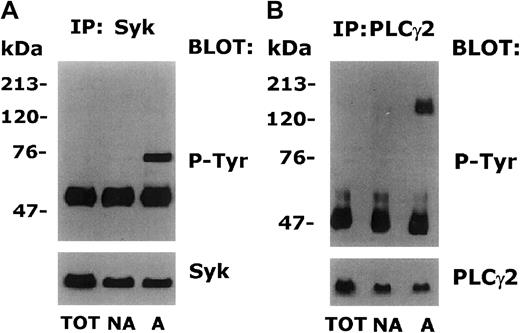
We next tried to identify some of the proteins that became tyrosine phosphorylated upon platelet adhesion to decorin. It is known that platelet interaction with collagen or von Willebrand factor induces the tyrosine phosphorylation of the kinase Syk, which in turn mediates the phosphorylation of PLCγ2, an enzyme involved in the generation of the intracellular second messengers IP3 and DAG.37,39,40 To investigate whether Syk and PLCγ2 were tyrosine phosphorylated also upon platelet adhesion to decorin, aliquots of adherent and nonadherent platelets containing the same amount of proteins were immunoprecipitated with specific antibodies against Syk and PLCγ2. Immunoprecipitates were analyzed by immunoblotting with antiphosphotyrosine antibodies, followed by a second staining with the same antibody used for the immunoprecipitation. As shown in Figures5A and 5B, both Syk and PLCγ2 immunoprecipitated from decorin-adherent platelets were found to be tyrosine phosphorylated. By contrast, the same proteins in samples of untreated or nonadherent platelets were not reactive to antiphosphotyrosine antibodies. Therefore, we conclude that platelet adhesion to decorin triggers tyrosine phosphorylation of Syk and PLCγ2. Because previous works have shown that release of ADP from dense granules during platelet adhesion may by responsible for the observed tyrosine phosphorylation of some proteins,36 41adhesion assays to decorin-coated dishes were performed in the presence of 1 U/mL ADP scavenger apyrase. We found that the level of Syk and PLCγ2 tyrosine phosphorylation was not affected by the presence of apyrase (data not shown), indicating that these events are directly triggered by platelet interaction with decorin and are not mediated by the release of intracellular ADP.
Tyrosine phosphorylation of Syk and PLCγ2 in platelets adherent to decorin.
Samples of total platelets (TOT) and platelets nonadherent (NA) or adherent (A) to decorin were lysed with immunoprecipitation buffer. Aliquots of each sample containing the same amount of proteins (100 μg) were immunoprecipitated with 1 μg of anti-Syk (A) or anti-PLCγ2 (B). Immunoprecipitated proteins were separated by SDS-PAGE on a 7.5% acrylamide gradient gel and transferred to nitrocellulose. Filters were then probed with antiphosphotyrosine antibody (P-Tyr). Upon inhibition of the peroxidase-conjugated secondary antibody with NaN3, the nitrocellulose filters were reprobed with the same antibody used for immunoprecipitation (Syk or PLCγ2). The positions of standard molecular weight markers are reported on the left.
Tyrosine phosphorylation of Syk and PLCγ2 in platelets adherent to decorin.
Samples of total platelets (TOT) and platelets nonadherent (NA) or adherent (A) to decorin were lysed with immunoprecipitation buffer. Aliquots of each sample containing the same amount of proteins (100 μg) were immunoprecipitated with 1 μg of anti-Syk (A) or anti-PLCγ2 (B). Immunoprecipitated proteins were separated by SDS-PAGE on a 7.5% acrylamide gradient gel and transferred to nitrocellulose. Filters were then probed with antiphosphotyrosine antibody (P-Tyr). Upon inhibition of the peroxidase-conjugated secondary antibody with NaN3, the nitrocellulose filters were reprobed with the same antibody used for immunoprecipitation (Syk or PLCγ2). The positions of standard molecular weight markers are reported on the left.
Platelet adhesion to decorin is mediated by integrin α2β1
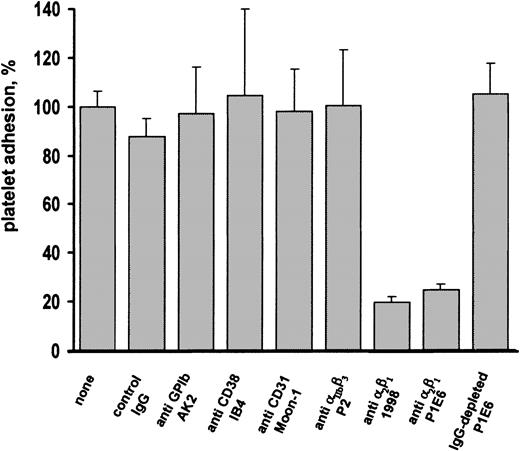
We next tried to identify the membrane receptor responsible for platelet interaction with decorin. Previous studies have shown that the EGF receptor can actually bind decorin and leads to the activation of intracellular kinases.17 18 By immunoprecipitation and immunoblotting experiments with specific antibodies, we failed to detect the presence of the EGF receptor in human platelets (data not shown). We therefore analyzed the effects of a panel of monoclonal antibodies against the main known platelet surface receptors involved in cell adhesion on the interaction of platelets with immobilized decorin. Preincubation of platelets with unrelated IgG or with antibodies against GP Ib-V-IX complex, integrin αIIbβ3, CD38, or CD31 did not affect platelet adhesion to immobilized decorin (Figure6). By contrast, 2 distinct antibodies against integrin α2β1 (1998 and P1E6) almost completely inhibited the adhesion of human platelets to decorin. Because the P1E6 antibody was used as ascite, to confirm that its ability to block platelet interaction with decorin was actually due to the antibody specificity, aliquots of P1E6 were pretreated with protein G-Sepharose. As shown in Figure 6, antibody depletion by protein G-Sepharose specifically abolished the ability of P1E6 to inhibit platelet adhesion to decorin.
Effect of the preincubation of platelets with antibodies against different membrane receptors on adhesion to decorin.
Samples of gel-filtered platelets (1 × 108 platelets, 0.5 mL) were incubated with buffer (none), 1 μg unrelated mouse IgG (control IgG), 1 μL anti-GPIb ascitic fluid AK2, 1 μg anti-CD38 monoclonal antibody IB4, 1 μg anti-CD31 monoclonal antibody Moon-1, 1 μg anti-integrin αIIbβ3 monoclonal antibody P2, 1 μg anti-integrin α2β1monoclonal antibody 1998, 1 μL anti-integrin α2β1 ascitic fluid P1E6, or 1 μL anti-integrin α2β1 ascitic fluid P1E6 pretreated with protein G-Sepharose for IgG depletion (IgG-depleted P1E6). After 5 minutes of incubation, platelet samples were added to decorin-coated dishes and allowed to adhere for 90 minutes. Results are the mean ± SD of 3 to 4 separate experiments and are expressed as percentage of platelet adhesion, considering 100% the adhesion measured in the absence of added antibodies.
Effect of the preincubation of platelets with antibodies against different membrane receptors on adhesion to decorin.
Samples of gel-filtered platelets (1 × 108 platelets, 0.5 mL) were incubated with buffer (none), 1 μg unrelated mouse IgG (control IgG), 1 μL anti-GPIb ascitic fluid AK2, 1 μg anti-CD38 monoclonal antibody IB4, 1 μg anti-CD31 monoclonal antibody Moon-1, 1 μg anti-integrin αIIbβ3 monoclonal antibody P2, 1 μg anti-integrin α2β1monoclonal antibody 1998, 1 μL anti-integrin α2β1 ascitic fluid P1E6, or 1 μL anti-integrin α2β1 ascitic fluid P1E6 pretreated with protein G-Sepharose for IgG depletion (IgG-depleted P1E6). After 5 minutes of incubation, platelet samples were added to decorin-coated dishes and allowed to adhere for 90 minutes. Results are the mean ± SD of 3 to 4 separate experiments and are expressed as percentage of platelet adhesion, considering 100% the adhesion measured in the absence of added antibodies.
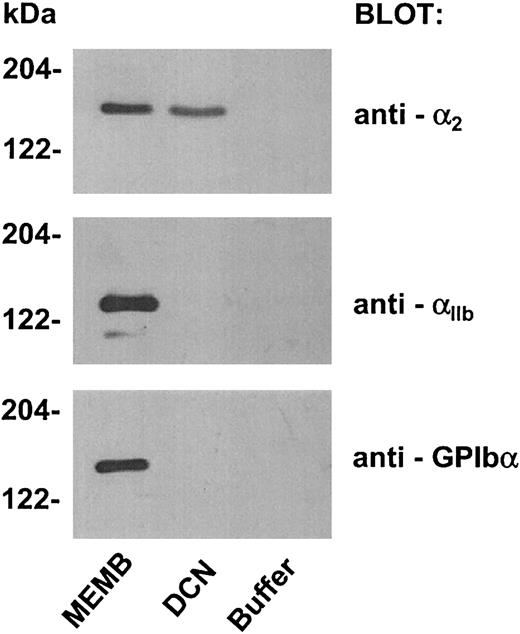
To confirm the involvement of integrin α2β1in platelet adhesion to decorin, we verified the ability of immobilized decorin to pull down this receptor from a membrane glycoprotein preparation, exploiting an experimental strategy recently described by Navdaev et al.35 A preparation of platelet membrane glycoproteins was obtained by affinity chromatography on concanavalin A-Sepharose of lysed platelet membranes. This preparation was incubated with biotinylated decorin and streptavidin-Sepharose or with streptavidin-Sepharose alone as a control. The ability of decorin to bind integrin α2β1 was evaluated by immunoblotting with anti-α2 polyclonal antibody. As shown in Figure 7, we observed specific binding of integrin α2β1 to biotinylated decorin bound to streptavidin-Sepharose, but not to streptavidin-Sepharose alone. Moreover, by testing the same nitrocellulose with antibodies against the GPIbα subunit of the GPIb-V-IX complex or against the αIIb subunit of integrin αIIbβ3, we found that these receptors were not precipitated by immobilized decorin, confirming the specificity of the interaction of the proteoglycan with integrin α2β1 (Figure 7).
Integrin α2β1 binding to biotinylated decorin immobilized on streptavidin-Sepharose.
Glycoproteins were isolated from lysed platelet membranes by affinity chromatography on concanavalin A-Sepharose. Biotinylated decorin was added to an aliquot of platelet glycoproteins and incubated for 2 hours at room temperature. Then, streptavidin-Sepharose was added to the sample and incubated for a further hour. As a negative control, an aliquot of membrane glycoproteins was incubated with buffer instead of biotinylated decorin, followed by addition of streptavidin-Sepharose. Proteins recovered from the control sample (buffer) and from the sample incubated with biotinylated decorin (DCN), together with an aliquot of the membrane glycoproteins preparation (MEMB), were separated by SDS-PAGE on a 7.5% acrylamide gel and transferred to nitrocellulose. Filters were probed with an antibody against the α2 subunit of integrin α2β1 (antibody 1936) and then reprobed with antibodies against the αIIb subunit of integrin αIIbβ3 and against the GPIbα subunit of the GPIb-V-IX complex, as indicated on the right. The positions of molecular weight markers are reported on the left.
Integrin α2β1 binding to biotinylated decorin immobilized on streptavidin-Sepharose.
Glycoproteins were isolated from lysed platelet membranes by affinity chromatography on concanavalin A-Sepharose. Biotinylated decorin was added to an aliquot of platelet glycoproteins and incubated for 2 hours at room temperature. Then, streptavidin-Sepharose was added to the sample and incubated for a further hour. As a negative control, an aliquot of membrane glycoproteins was incubated with buffer instead of biotinylated decorin, followed by addition of streptavidin-Sepharose. Proteins recovered from the control sample (buffer) and from the sample incubated with biotinylated decorin (DCN), together with an aliquot of the membrane glycoproteins preparation (MEMB), were separated by SDS-PAGE on a 7.5% acrylamide gel and transferred to nitrocellulose. Filters were probed with an antibody against the α2 subunit of integrin α2β1 (antibody 1936) and then reprobed with antibodies against the αIIb subunit of integrin αIIbβ3 and against the GPIbα subunit of the GPIb-V-IX complex, as indicated on the right. The positions of molecular weight markers are reported on the left.
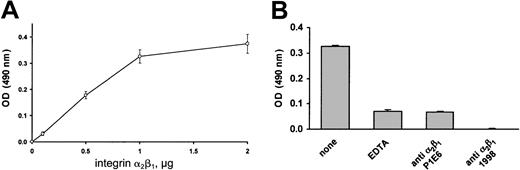
Finally, we analyzed the ability of purified integrin α2β1 to directly interact with immobilized decorin. Decorin-coated 96-well plates were incubated with increasing amounts of purified integrin α2β1, and the binding of the receptor was visualized by an immunoenzymatic assay. Figure 8A shows that purified integrin α2β1 was actually able to bind to immobilized decorin in a dose-dependent manner. The specificity of this interaction is documented in Figure 8B. Binding of purified integrin α2β1 to decorin was found to be almost completely prevented either by addition of EDTA or in the presence of the blocking antibodies P1E6 and 1998. In conclusion, these results indicate that integrin α2β1 is a platelet receptor for decorin.
Binding of purified integrin α2β1 to immobilized decorin.
(A) Increasing amounts of purified integrin α2β1 (0, 0.5, 1, 1.5, 2 μg) were added to decorin-coated wells of a microtiter plate in the presence of 2 mM MgCl2. As a negative control, the same amount of purified integrin α2β1 was incubated with BSA-coated wells. Upon incubation for 2 hours, bound integrin α2β1 was revealed by a colorimetric reaction after incubation with an antibody against the α2subunit of integrin α2β1 (1936) and a peroxidase-conjugated antimouse antibody. Data are reported as absorbance at 490 nm of the wells coated with decorin upon subtraction of the values measured in corresponding wells coated with BSA and represent the mean ± SD of 3 separate experiments. (B) Purified integrin α2β1 (1 μg) was added to decorin-coated wells in the presence of 2 mM MgCl2, 2 mM EDTA, 1 μL P1E6 (ascitic fluid), or 1 μg/mL anti-integrin α2β1 monoclonal antibody 1998. Upon incubation for 2 hours, bound integrin α2β1was revealed as described for Figure 8A. Results are the mean ± SD of 3 separate experiments.
Binding of purified integrin α2β1 to immobilized decorin.
(A) Increasing amounts of purified integrin α2β1 (0, 0.5, 1, 1.5, 2 μg) were added to decorin-coated wells of a microtiter plate in the presence of 2 mM MgCl2. As a negative control, the same amount of purified integrin α2β1 was incubated with BSA-coated wells. Upon incubation for 2 hours, bound integrin α2β1 was revealed by a colorimetric reaction after incubation with an antibody against the α2subunit of integrin α2β1 (1936) and a peroxidase-conjugated antimouse antibody. Data are reported as absorbance at 490 nm of the wells coated with decorin upon subtraction of the values measured in corresponding wells coated with BSA and represent the mean ± SD of 3 separate experiments. (B) Purified integrin α2β1 (1 μg) was added to decorin-coated wells in the presence of 2 mM MgCl2, 2 mM EDTA, 1 μL P1E6 (ascitic fluid), or 1 μg/mL anti-integrin α2β1 monoclonal antibody 1998. Upon incubation for 2 hours, bound integrin α2β1was revealed as described for Figure 8A. Results are the mean ± SD of 3 separate experiments.
Discussion
In the present work we have demonstrated that the small proteoglycan decorin is able to promote platelet adhesion and activation. These results represent the first evidence for a direct interaction of a member of the small leucine-rich proteoglycan family of the subendothelial matrix with circulating platelets and indicate that, in addition to its role as a regulator of matrix assembly and cell growth, decorin may also be involved in hemostasis and thrombosis. Decorin is composed of a protein core and a glycosaminoglycan side chain. We found that interaction with platelets is mediated by the decorin protein core. We consistently found a higher percentage of platelet adhesion to the isolated decorin protein core than to the intact proteoglycan. It is known that the glycosaminoglycan side chain linked to the N-terminal region of decorin has a high molecular mass, even greater than that of the protein core. Therefore, it is possible that in the native proteoglycan, the glycosaminoglycan chain may mask some platelet binding sites on the protein core, thus reducing the efficiency of the interaction.
We have also provided convincing evidence that integrin α2β1 is the membrane receptor mediating the interaction of platelets with decorin. In fact, we have demonstrated that 2 different antibodies against this integrin receptor specifically inhibited platelet adhesion to decorin. Moreover, immobilized decorin is able to specifically bind integrin α2β1from a membrane glycoprotein preparation, and purified integrin α2β1 is able to bind immobilized decorin. It has been known for years that integrin α2β1 is a collagen receptor on the platelet surface.42-44 Although decorin can specifically interact with collagen,2-5 we can rule out the possibility than the observed integrin α2β1-dependent platelet adhesion to decorin is due to contaminating collagen. We have provided evidence that the decorin preparations used in this study are essentially free of contaminating collagen: (1) N-terminal sequencing of the decorin preparations revealed the presence of a single type of protein and was identical to the N-terminal sequence of decorin; (2) amino acid analysis failed to detect any hydroxyproline, and the amount of measured proline was consistent with known data on decorin sequence; (3) an antibody against collagen type I, able to detect amounts of collagen as low as 10 ng, did not react with the decorin preparations used in this study; and (4) recombinant decorin was as efficient as purified decorin in supporting platelet adhesion. In light of this body of evidence, we conclude that the observed adhesion of platelets to decorin cannot be ascribed to collagen contamination and that decorin is actually a new physiological ligand for integrin α2β1. The ability of this integrin receptor to bind both collagen and decorin is intriguing. In the subendothelial matrix, decorin is associated with collagen fibrils to modulate their assembly. Therefore, under physiological conditions both collagen and decorin are exposed to platelets as a macromolecular complex rather than as single molecules. In this context, the same platelet receptor, the integrin α2β1, may contribute to platelet adhesion to the exposed subendothelial matrix by recognizing simultaneously both components of the collagen-decorin fibrils. Further studies are required to verify whether the presence of decorin along the collagen fibrils might actually improve or regulate platelet adhesion to this molecule.
In this work we have also found that platelet adhesion to decorin is increased upon activation of these cells. This may reflect a positive regulation of the receptor function of integrin α2β1 by platelet agonists. In this regard, it is interesting to note that it has recently been demonstrated that platelet activation converts integrin α2β1into an active form with increased affinity for soluble collagen.45,46 Recent data suggest that this activation of integrin α2β1 is mediated by ADP secreted from stimulated platelets.47 A similar mechanism may also regulate integrin α2β1-dependent platelet adhesion to decorin. Our results demonstrate that exogenous ADP may actually lead to a 2-fold increase of platelet adhesion to immobilized decorin.
We have also demonstrated that adhesion to decorin leads to platelet activation through the stimulation of protein tyrosine phosphorylation. However, we have found that addition of soluble decorin to a platelet suspension did not cause detectable aggregation (data not shown). We have found that the tyrosine kinase Syk and the lipid metabolizing enzyme PLCγ2 are tyrosine phosphorylated (ie, activated) in decorin-adherent platelets. It is known that in collagen or von Willebrand factor–treated platelets, the tyrosine kinase Syk is directly involved in the phosphorylation and activation of PLCγ2, which, in turn, leads to the production of the intracellular second messengers IP3 and DAG responsible for Ca++mobilization and protein kinase C activation, respectively.37-40 Our results suggest that a similar signal transduction pathway responsible for platelet activation may be stimulated upon binding to decorin. Because we have found that platelet adhesion to decorin is mediated by integrin α2β1, it is likely that this receptor may mediate the subsequent activation of Syk and PLCγ2. Although integrin α2β1 is also involved in platelet adhesion to collagen, a different receptor, the GPVI/Fc receptor γ-chain, has been shown to play a more relevant role in platelet activation induced by this adhesive protein.37 These findings have raised the question of the role of integrin α2β1 as a cell-activating receptor. However, several works have recognized the ability of this integrin receptor to transmit signals inside the platelet.39,46 48 Although we cannot rule out the possibility that platelet activation by decorin is mediated by a receptor different from that involved in adhesion, our results support a link between recruitment of integrin α2β1and activation of intracellular tyrosine kinases.
In conclusion, this work demonstrated for the first time a novel role for the small proteoglycan decorin in platelet adhesion and activation. Decorin is a member of a related family of small leucine-rich proteoglycans expressed in the subendothelial matrix, including the highly homologous biglycan. Further studies will be required to verify whether other members of this family of proteoglycans might also be involved in the regulation of platelet function.
Supported by grants from Consiglio Nazionale delle Ricerche (CNR, target project biotechnology), CIB (Consorzio Interuniversitario Biotecnologie), and the University of Pavia (Progetto di Ateneo).
G.G. and A.B. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mauro Torti, Department of Biochemistry, University of Pavia, via Bassi 21, 27100 Pavia, Italy; e-mail:mtorti@unipv.it.