Decreased susceptibility to apoptosis and impaired proliferative control are thought to be responsible for prolonged life span and accumulation of chronic lymphocytic leukemia (B-CLL) cells. The activity of calpains (calcium-dependent, neutral proteases, active in the cells responding to signals inducing a rise of cytoplasmic Ca++) is involved in the regulation of apoptosis of some cell types by interaction with caspase-3. This work verifies the hypothesis of the abnormal activity of calpains and its role in reduced apoptosis of the B-CLL cells. Casein zymography, reverse transcriptase–polymerase chain reaction, and Western blotting were used for identification and quantification of the activity and expression of calpains in B-CLL cells and purified normal B lymphocytes. The activity and expression of μ-calpain (requiring micromolar Ca++ for activation) are significantly higher in the leukemic than in nonmalignant cells. Contrarily, the activity and expression of m-calpain (requiring millimolar Ca++) as well as the expression of calpastatin (an endogenous inhibitor of calpains) are unchanged or reduced in the B-CLL lymphocytes. Correspondingly, the activity of caspase-3 is many times lower in the B-CLL cells than in normal B lymphocytes. Inhibition of overexpressed μ-calpain in living B-CLL cells in vitro results in doubling of the proportion of the cells undergoing spontaneous apoptosis. This observation suggests a possible role for calpains in longer survival of the B-CLL cells and may open new therapeutic possibilities.
Introduction
Chronic lymphocytic leukemia (CLL)—a most common, relatively benign form of lymphoproliferation that occurs mostly in middle-aged and old people—is characterized by accumulation of high numbers of small leukemic lymphocytes in the peripheral blood and in bone marrow. In more than 90% of cases the CLL cells are descendants of the B-cell lineage (hence B-CLL) that expresses surface immunoglobulins (IgM) and HLA-DR but cannot transform into plasma cells or secrete Igs.1-3 Survival times of B-CLL patients are relatively long (especially compared with other types of blood malignancies), and the disease usually does not require intensive chemotherapy for long periods after diagnosis.1
Causes of B-CLL are still not very well understood; the search concentrates around possible genetic aberrations on one side4-7 and biochemical alterations on the other. On average, the life span of a B-CLL cell is significantly longer than that of a normal B lymphocyte, whereas the proliferation rate in the leukemic population is similar or even decreased compared with the nonneoplasmic ancestors.1 These features directed researchers toward resistance to apoptogenic signals as a possible cause of prolonged survival of the B-CLL cells and, in fact, the cause of the disease.8
We propose here that the prolonged survival, reduced apoptosis, and a decreased proliferation rate of B-CLL cells might all depend on an impaired function of common, neutral, cytoplasmic thiol (cysteine) proteases called calpains. Calpains are involved in the control of the level and duration of the transduction signals leading to either proliferation or apoptosis in multiple cell systems (reviewed by Suzuki et al,9 Sorimachi et al,10 and Ono et al11). The role of calpains in the development of leukemias has not been postulated before. Ubiquitous members of the calpain family that can be detected in blood cells are defined according to the concentration of Ca++ required for full activity (millimolar Ca++-dependent [m-] and micromolar Ca++-dependent [μ-] calpains, respectively).9-11 Substrates of calpains include protein kinase C (PKC) and transcription factors such as c-fos, c-jun, nuclear factor of activated T cells (NF-AT), nuclear factor–κB (NF-κB) (and especially its regulator IκB), tyrosine phosphatase calcineurin, calcium channels, Ca-ATPase (“calcium pump”), p53, and many others.9-16 In the blood cells including B lymphocytes, calpains are supposed to participate in the regulation of the PKC, but other substrates of their action in these cells cannot be excluded.17-21 Calpastatins—specific, endogenous inhibitors of calpains—prevent the uncontrolled activity of the proteases, stressing their important and potentially harmful role in the cell.9-11
Calpains are supposedly involved in the regulation of apoptosis of various types of cells. However, their role in the development and course of apoptosis is controversial. Although most of the data seem to indicate a level of collaboration between calpains and caspases in the induction of apoptosis,10,22-24 some groups observed apoptosis induction when cellular calpains were selectively inhibited.16,25,26 We have shown recently that the activity of μ-calpain increases in T cells of old mice that express high activities of a membrane transporter P-glycoprotein (J.M.W. and Miller, manuscript in preparation, 2002). These P-glycoprotein–positive T cells exhibit simultaneously decreased proliferative capability and lower cytokine production that also may suggest lower susceptibility to apoptosis.27
Thus, it is possible that intrinsic changes of the activity of calpain(s) in the B-CLL lymphocytes modulate their signal transduction, leading to impaired susceptibility to apoptosis and thus prolonging their effective life span in an organism. Therefore, we ask if the activity of calpain(s) in the B-CLL differs quantitatively and/or qualitatively from that characteristic for the non-CLL B lymphocytes. Also, we attempt to answer (at least preliminarily) a question of the possible role of calpain activity in the reduced apoptosis of B-CLL cells.
Patients, materials, and methods
Patients
Fifteen B-CLL patients (mean age 54 ± 7 years, range 41-82 years, 7 men and 8 women), 3 acute lymphocytic leukemia (ALL) patients (all of the Department of Hematology, Medical University of Gdansk), and 15 age- and sex-matched healthy volunteers participated in the investigation. The project has been approved by the Independent Committee for Bioethics in Scientific Research at the Medical University of Gdansk, Poland. All participants were informed about the purpose of the investigation and gave their consent according to the Declaration of Helsinki. Patient blood was drawn at the routine follow-up examination. The CLL patients were all diagnosed as B-CLL based on the clinical examination and the results of the morphologic and immunohistochemical analysis of blood and bone marrow specimens. The average patients' leukocytosis was 103.4 g/L (104.3 × 103/mm3). Clonality of leukemic cells has been confirmed by the presence of a single type of Ig light chain (10 cases, κ; 5 cases, λ). According to Rai classification, stage I was found in 4 patients, stage II in 7, stage III in 3, and stage IV in 1 patient. Thirteen patients participated in this study at diagnosis, that is, before the onset of chemotherapy; for the remaining 2, the therapy-free period was 6 and 18 months, respectively. Neither participant suffered from any infectious disease at the time of blood drawing.
Cells
Peripheral venous blood was drawn into the EDTA (ethylenediaminetetraacetic acid)–containing tubes, and the mononuclear cells (PBMCs) were separated on a cushion of Histopaque (Sigma, Deisenhofen, Germany). Remaining red blood cells were eliminated by lysis with Tris (tris(hydroxymethyl)aminomethane)–ammonium chloride solution. Pilot experiments, in which the adherent cells were eliminated from the patients' PBMCs and the resulting cell suspension was tested for calpain activities, did not show a difference in these activities between total patients' PBMCs and the B-CLL cells enriched that way. Thus, no further enrichment of these cells was usually performed. From 90% to 95% of the peripheral blood lymphocytes (PBLs) of all patients was CD19+, CD5+, CD20+, CD22+, CD23+, CD34+, and CD43+, as assessed by both immunochemistry and flow cytometry (FACSCalibur; Becton Dickinson, Sunnyvale, CA).
Peripheral blood lymphocytes of healthy controls were enriched by elimination of monocytes by plastic adhesion. Further enrichment of “healthy” B cells was done by magnetic separation. Briefly, cell suspension was incubated for 45 minutes on ice with mouse antihuman CD3 (DAKO, Glostrup, Denmark) alone or together with mouse antihuman CD16 (Becton Dickinson, San Jose, CA) monoclonal antibodies. Then, appropriate numbers of antimouse Ig–conjugated magnetic beads (Advanced Magnetics, Cambridge, MA) were added and the mixture was incubated for another 45 minutes on ice. Strong magnetic field was then applied, and the suspension of cells that did not bind the beads (CD3− and CD3−CD16−, respectively) was drawn out. The cells remaining in this suspension contained from 75% ± 5.9% CD19+ cells (in the CD3− population) to 88% ± 3.7% CD19+cells in the CD3−CD16− population, as shown by flow cytometry.
Epstein-Barr virus (EBV)–transformed human B cells were derived from PBLs of healthy people at the International Institute of Cellular and Molecular Biology in Warsaw, Poland.
Estimation of calpain activity
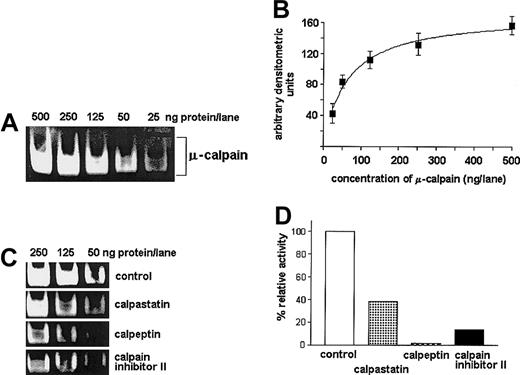
Casein zymography was applied to compare the activities of endogenous calpains in B-CLL cells, PBMCs, and enriched normal B lymphocytes.28-30 The cells were suspended at 8 × 107/mL, and 25 μL aliquots were prepared in either a lysis buffer (50 mM Tris-HCl, 1 mM dithiothreitol [DTT], 5 mM EGTA [ethyleneglycoltetraacetic acid]) or in calcium- and magnesium-free phosphate-buffered saline (PBS) with protease inhibitor cocktail (10 μg/mL aprotinin, 10 μg/mL leupeptin, 1.8 mg/mL iodoacetamide, and 1 μM phenylmethylsulfonyl fluoride [PMSF]; Sigma). Cell lysis was performed by 5 cycles of liquid nitrogen (LN2) flash-freezing, followed by rapid thawing of the sample in a 37°C water bath. Cell nuclei and debris were then removed by short spin at 10 000g, and the resulting lysates were flash-frozen in LN2 and stored at −70°C. Electrophoretic separation of the enzyme from calpastatins influencing its activity was done in a nonreducing, detergent-free buffer in a 12% polyacrylamide gel containing 0.2% milk casein (Sigma) at 126 V. The amount of lysate corresponding to 2 × 106 cells was loaded onto each lane. The activity of calpains in the gel was induced by overnight incubation in a buffer containing 2 mM CaCl2 and 10 mM DTT in 20 mM Tris-HCl, pH 7.4, at room temperature. Casein proteolysis by calpains produced clear, transparent bands in the Coomassie blue–stained gels. The identity of the bands with the μ- and m-calpains was confirmed by simultaneous running of a sample containing a mixture of purified calpains (0.1 μg porcine μ-calpain and 0.1 μg rabbit muscle m-calpain; Calbiochem, Laufelfingen, Switzerland) in the lysis buffer. Casein-digesting activity could only be seen at the levels corresponding to the band positions of these “standard” μ- and m-calpains. The intensity of the bands was quantified by densitometry28-30 after digitization using the UVP gel documentation system (UVP, Cambridge, United Kingdom) and GelBase software (UVP). Densitometric analysis was performed with the ImageQuant 3.3 (Molecular Dynamics, Sunnyvale, CA) software and the raw results were collected as number of “white” pixels in a band. These data were then converted to arbitrary units of the calpain activity, based on the signal obtained from the known amount of standard enzymes. Preliminary experiments had shown a linear dependence between the density of digested bands and the amount of standard calpain in the range of 10 to 250 ng per lane (Figure1). In control samples, 20 μg/mL calpain inhibitor II (N-Acetyl-Leu-Leu-Met-al; specific for both isoforms of the enzyme; Calbiochem); or 20 μg/mL m-calpain inhibitor—rabbit skeletal muscle calpastatin, inhibitory mostly toward m-calpain (calcium-activated neutral protease [m-calpain] inhibitor; Sigma)31; or 1 μM calpeptin (benzyloxycarbonyldipeptidyl aldehyde Z-Leu-Nle-CHO, specific for μ- and m-calpain; Calbiochem) were added and the mixture was incubated on ice for 15 minutes before loading onto the gel.
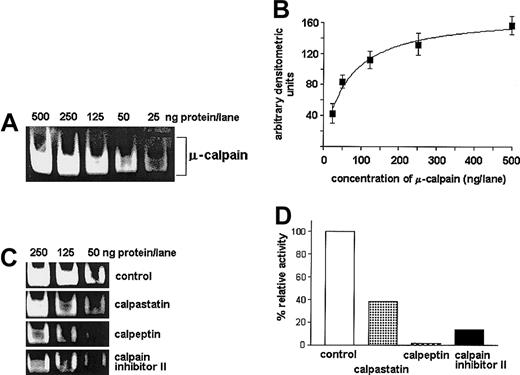
Casein zymography allows for specific, semiquantitative estimation of the μ-calpain activity.
Dilutions of commercial rabbit red blood cell μ-calpain (25, 50, 125, 250, 500 ng per well) were processed by casein zymography with or without preincubation with either calpeptin or calpastatin or calpain inhibitor II at concentrations indicated in “Patients, materials, and methods.” (A) Representative casein zymogram showing reduction of the band intensity proportional to reduced concentration of the enzyme. (B) A “dose-effect” curve relating concentration of the enzyme (x-axis, amount per lane) to the intensity of the casein-cleavage bands measured densitometrically (y-axis, arbitrary units); the relation allows for reliable estimation of relative activity of calpain in the range of 25 to 250 ng per lane. The curve summarizes 3 experiments and shows mean densities (AU) ± SD. (C,D) Inhibitory effect of calpeptin, calpastatin, and calpain inhibitor II on the activity of μ-calpain (50 to 250 ng per lane) estimated by casein zymography: a representative zymogram (C) and a bar graph (D) summarizing 3 experiments for the μ-calpain concentration of 50 ng per lane; bars represent arithmetic means (D).
Casein zymography allows for specific, semiquantitative estimation of the μ-calpain activity.
Dilutions of commercial rabbit red blood cell μ-calpain (25, 50, 125, 250, 500 ng per well) were processed by casein zymography with or without preincubation with either calpeptin or calpastatin or calpain inhibitor II at concentrations indicated in “Patients, materials, and methods.” (A) Representative casein zymogram showing reduction of the band intensity proportional to reduced concentration of the enzyme. (B) A “dose-effect” curve relating concentration of the enzyme (x-axis, amount per lane) to the intensity of the casein-cleavage bands measured densitometrically (y-axis, arbitrary units); the relation allows for reliable estimation of relative activity of calpain in the range of 25 to 250 ng per lane. The curve summarizes 3 experiments and shows mean densities (AU) ± SD. (C,D) Inhibitory effect of calpeptin, calpastatin, and calpain inhibitor II on the activity of μ-calpain (50 to 250 ng per lane) estimated by casein zymography: a representative zymogram (C) and a bar graph (D) summarizing 3 experiments for the μ-calpain concentration of 50 ng per lane; bars represent arithmetic means (D).
Specificity of the test was confirmed by the ability of all 3 calpain inhibitors used to decrease the activity of standard μ-calpain (Figure 1) and by significant reduction of the bands digested at the level of μ-calpain and lack of visible m-calpain activity in the lanes containing calpain inhibitor II–treated samples (compare Figure3). The effects of calpeptin and rabbit calpastatin were similar (not shown). No proteolytic activity could be seen in the gels incubated in activation buffer without Ca++.
Estimation of μ-calpain contents by Western blotting
Lysates of cell samples containing 2 × 106 cells each were prepared in a denaturing buffer containing Nonidet P-40 and Triton X-100 detergents. Lysate proteins were electrophoretically resolved using standard sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto nitrocellulose. Rabbit anticalpain serum, shown to be cross-reactive with human and porcine μ-calpain (a kind gift from Dr M. Spencer, The Scripps Institute, CA), was used to detect μ-calpain in the blots. After second incubation with peroxidase-conjugated donkey antirabbit Ig (Amersham, Little Chalfont, United Kingdom), the μ-calpain–containing bands were visualized using the enhanced chemiluminescence kit (ECL, Amersham). Luminescent bands were recorded on the x-ray film and analyzed by densitometry. Samples containing 0.1 μg porcine μ-calpain standard instead of cell lysates were used as positive specificity control. To confirm the uniform loading of cell lysates in each lane, the levels of actin expression in the same volumes of lysates were determined by an analogous Western blot protocol using antiactin antibody (Sigma).
Comparison of the expression of calpains and calpastatin genes in B-CLL and normal B cells
Total RNA was isolated from samples of 1 × 106B-CLL cells using TRI-Reagent (Sigma) according to the manufacturer's protocol. Isolated mRNA was immediately converted to cDNA by reverse transcription with Moloney murine leukemia virus (MMLV) reverse transcriptase (RT) (Promega, Madison, WI) and oligo-dT as starter of the RT reaction. Amplification of gene products of the μ-calpain, m-calpain, and calpastatin genes was performed by polymerase chain reaction (PCR) using 1 μg cDNA as an amplification template in each case. The following primer pairs were used: for μ-calpain: 5′-CCTGCTTGAGAAGGCCTATG-3′ (forward), 5′-GGTCCACGTTGTTCCACTCT-3′ (reverse, product size 389 bp); for m-calpain: 5′-AGGCATACGCCAAGATCAAC-3′ (forward), 5′-GGATGCGGATCAGTTTCTGT-3′ (reverse, product size 306 bp); and for calpastatin: 5′-AAGACCTCGATGATGCCTTG-3′ (forward), 5′-TCACTGGTTTGTCCTGTCCA-3′ (reverse, product size 201 bp). In addition, β-actin (a “housekeeping” gene), was amplified from each cDNA sample, and the amount of product of its amplification (determined densitometrically) served as basis for normalization of the PCR results.
Comparison of the activity of caspase-3 in B-CLL, B-ALL, and EBV-transformed normal B cells
The Caspase-3 Assay Kit (Sigma) was used. Briefly, cell lysates were incubated with the peptide substrate acetyl-Asp-Glu-Val-Asp-(p)nitroanilide (Ac-DEVD-pNA) with or without specific inhibitor of caspase-3. The activity of caspase-3 was calculated from the amount of p-nitroaniline (pNA) released from the substrate, against a standard curve obtained for known amounts of the pNA. Final results were indexed against these obtained for the samples of B-CLL cells.
Analysis of the influence of calpain inhibitors on the spontaneous apoptosis in B-CLL cells
B-CLL lymphocytes were suspended at 1 × 106 cells per milliliter in RPMI 1640 medium, supplemented with 10% fetal calf serum and 2 mM l-glutamine, and incubated with 20 μM membrane-permeable calpain inhibitor (calpeptin; Calbiochem), or 20 μg/mL rabbit muscle calpastatin (calcium-activated neutral protease [m-calpain] inhibitor; Sigma), or protease inhibitor cocktail (10 μg/mL aprotinin, 10 μg/mL leupeptin, 1.8 mg/mL iodoacetamide; Sigma), or without any additions for 24 hours at 37°C. Harvested and washed cells were then stained with the apoptosis detection kit containing fluorescein isothiocyanate (FITC)–annexin V and propidium iodide (PI) (a kind gift from the Nexins Research, The Netherlands) according to the manufacturer's instructions and analyzed by flow cytometry. Cells showing strong binding of the annexin V and no PI staining were considered apoptotic, whereas those with strong PI signal regardless of their annexin staining were considered necrotic.
Results
B-CLL lymphocytes express the activities of both μ- and m-calpain
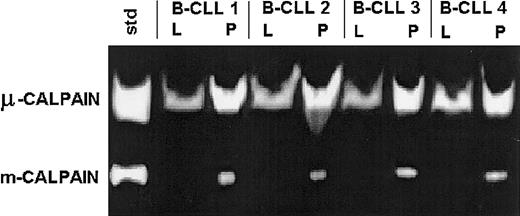
Samples of 2 × 106 B-CLL lymphocytes were lysed in either a lysis buffer containing EGTA and DTT or in plain PBS and processed for casein zymography. In both types of lysates only the proteolytic activity of calpains could be detected, at the levels identical to the bands digested by standard μ- or m-calpain (Figure2).
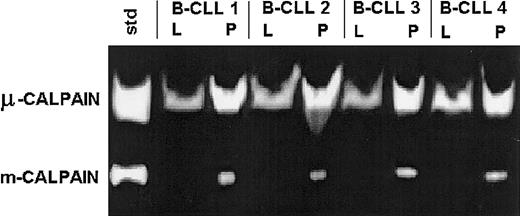
B-CLL cells express the activities of both μ- and m-calpain.
Lysates of B-CLL cells of 4 patients (B-CLL 1 through 4) were prepared in either lysis buffer (L) or PBS (P) as in “Patients, materials, and methods” and tested by casein zymography. A mixture of 0.1 μg each of commercial μ- and m-calpain was processed alongside and served as activity standard (std). The activity of m-calpain can only be seen in the lysates prepared in PBS in which the activity of μ-calpain also is relatively enhanced.
B-CLL cells express the activities of both μ- and m-calpain.
Lysates of B-CLL cells of 4 patients (B-CLL 1 through 4) were prepared in either lysis buffer (L) or PBS (P) as in “Patients, materials, and methods” and tested by casein zymography. A mixture of 0.1 μg each of commercial μ- and m-calpain was processed alongside and served as activity standard (std). The activity of m-calpain can only be seen in the lysates prepared in PBS in which the activity of μ-calpain also is relatively enhanced.
The activity of μ-calpain was strong; partially inhibited by calpain inhibitor II (Figure 3), calpeptin, or rabbit calpastatin (not shown); and somewhat higher in the samples lysed in PBS than in the lysis buffer (Figure 2). On the other hand, the activity of m-calpain was detectable (as digested bands) only in the lysates prepared in PBS and absent in the lysates made in the EGTA- and DTT-containing medium. This activity was completely absent from the samples treated with calpain inhibitor II (Figure 3), calpeptin, or rabbit calpastatin (not shown).
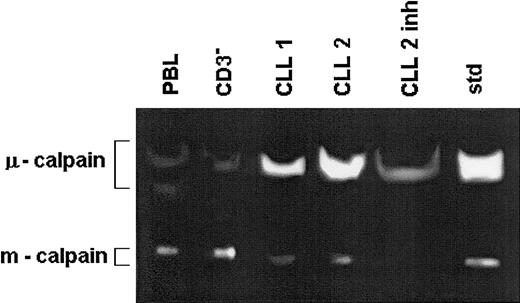
The activity of μ-calpain is increased while that of m-calpain is reduced in the B-CLL cells compared with B cells of healthy people.
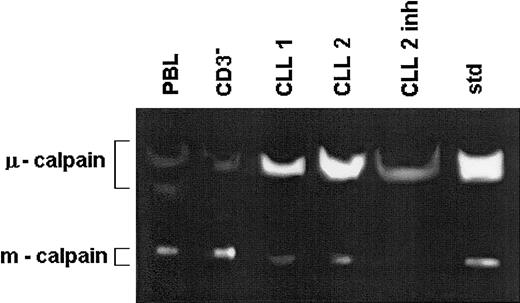
Lysates of peripheral blood lymphocytes and immunomagnetically enriched B cells (CD3−) of a healthy 67-year-old and of the same number of B-CLL cells from 2 age-matched patients (CLL 1, CLL 2) were prepared in PBS and tested by zymography. The lysate treated with calpain inhibitor II served as specificity control (CLL 2 inh). Std indicates the activities of standard μ- and m-calpain as in Figure 1. This is a representative zymogram of 1 of 5 experiments giving similar results. The inhibitory effects of calpain inhibitor II, calpeptin, and rabbit muscle calpastatin were similar.
The activity of μ-calpain is increased while that of m-calpain is reduced in the B-CLL cells compared with B cells of healthy people.
Lysates of peripheral blood lymphocytes and immunomagnetically enriched B cells (CD3−) of a healthy 67-year-old and of the same number of B-CLL cells from 2 age-matched patients (CLL 1, CLL 2) were prepared in PBS and tested by zymography. The lysate treated with calpain inhibitor II served as specificity control (CLL 2 inh). Std indicates the activities of standard μ- and m-calpain as in Figure 1. This is a representative zymogram of 1 of 5 experiments giving similar results. The inhibitory effects of calpain inhibitor II, calpeptin, and rabbit muscle calpastatin were similar.
The activity of μ-calpain is higher and that of m-calpain is lower in the B-CLL cells than in nonmalignant B lymphocytes
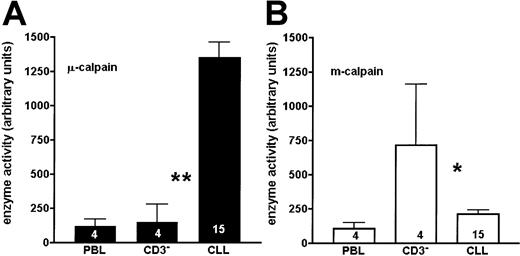
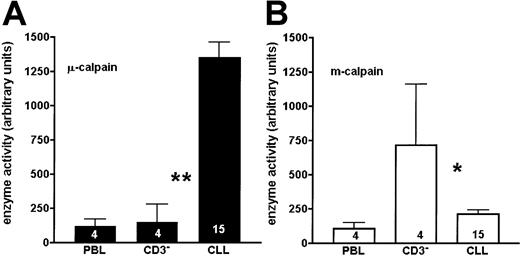
When the activity of calpains was simultaneously assessed in the lysates of B-CLL lymphocytes and of age-matched PBL and CD3− (B-enriched) cells of healthy people, the activity of μ-calpain was significantly higher in the leukemic cells than in either control (Figures 3 and 4A). The activity of m-calpain was significantly lower in the chronic leukemia cells than in matched, nonmalignant B lymphocytes (CD3-depleted cells), whereas it was similar in B-CLL lymphocytes and in the age-matched PBL cells (Figures 3 and 4B). There was no difference between the results obtained for the CD3− and CD3−CD16− cells, indicating that participation of the natural killer (NK) cells in the overall activity of calpains in human lymphocytes is negligible (not shown). Therefore, the 2 proteolytic activities of μ- and m-calpain behave reciprocally in the B-CLL cells compared with normal B-cell control. No differences in the activity of either calpain were observed in relation to the advancement of the disease (Rai classification).
The activity of μ-calpain is significantly higher, whereas that of m-calpain is reduced in the B-CLL cells compared with B cells of healthy people.
The graphs show a summary of results obtained from 15 B-CLL patients and PBLs or enriched CD3− cells of 4 age-matched healthy people. Bars correspond to mean activities of μ-calpain (A) and m-calpain (B) expressed as arbitrary densitometric units ± SD. The numbers of cases tested are shown on the bars. Asterisks indicate statistical significance of difference (Student t test) between the adjacent bars (CLL, CD3−) at the level ofP < .01 (**) and P < .05 (*).
The activity of μ-calpain is significantly higher, whereas that of m-calpain is reduced in the B-CLL cells compared with B cells of healthy people.
The graphs show a summary of results obtained from 15 B-CLL patients and PBLs or enriched CD3− cells of 4 age-matched healthy people. Bars correspond to mean activities of μ-calpain (A) and m-calpain (B) expressed as arbitrary densitometric units ± SD. The numbers of cases tested are shown on the bars. Asterisks indicate statistical significance of difference (Student t test) between the adjacent bars (CLL, CD3−) at the level ofP < .01 (**) and P < .05 (*).
Lysates of normal, nonmalignant peripheral blood lymphocytes had shown the presence of both μ- and m-calpain activities (Figure 5). However, when the PBLs were depleted of T (CD3+) cells by magnetic separation, the activity of μ-calpain disappeared and that of m-calpain remained at only a slightly lower level. The removal of CD16+ cells resulted in no further change in m-calpain activity (Figure 5A). Interestingly, when the activity of μ-calpain was assessed for the PBLs of healthy people, it was strong for young people aged 25 to 34 years, clearly decreased for middle-aged individuals aged 40 to 50 years, and almost none for the elderly above 65 years (Figure 5B), whereas the proportion of T:B:NK cells among PBLs of people of various age did not differ. These results suggest that in nonmalignant lymphocytes the activity of μ-calpain is associated almost exclusively with T cells (for which it is clearly age dependent), whereas it is very low in the B lymphocytes regardless of the age of the individual. On the other hand, the activity of m-calpain is present at a similar level in all populations of PBLs regardless of age (not shown).
In nonmalignant lymphocytes, the activity of μ-calpain is concentrated mostly in the T cells and decreases with age.
PBLs and immunomagnetically enriched CD3− and CD3−CD16− cells of a healthy young person were lysed in PBS and analyzed by casein zymography. Proteolytic bands corresponding to the activities of both isoenzymes were strongly seen only in the total peripheral blood lymphocyte (PBL) lysates, while the μ-calpain activity disappeared from the CD3−and CD3−CD16− cell lysates; std indicates the activities of standard μ- and m-calpain as in Figure 1 ([A] A representative zymogram of 1 of 3 similar experiments). Comparison of the activities of μ-calpain in the PBLs of 2 young, 2 middle-aged, and 2 old individuals shows a strong, age-related decrease of this activity ([B] Representative zymogram shows results of 1 of 3 similar experiments.
In nonmalignant lymphocytes, the activity of μ-calpain is concentrated mostly in the T cells and decreases with age.
PBLs and immunomagnetically enriched CD3− and CD3−CD16− cells of a healthy young person were lysed in PBS and analyzed by casein zymography. Proteolytic bands corresponding to the activities of both isoenzymes were strongly seen only in the total peripheral blood lymphocyte (PBL) lysates, while the μ-calpain activity disappeared from the CD3−and CD3−CD16− cell lysates; std indicates the activities of standard μ- and m-calpain as in Figure 1 ([A] A representative zymogram of 1 of 3 similar experiments). Comparison of the activities of μ-calpain in the PBLs of 2 young, 2 middle-aged, and 2 old individuals shows a strong, age-related decrease of this activity ([B] Representative zymogram shows results of 1 of 3 similar experiments.
The amount of μ-calpain protein is higher in B-CLL cells than in normal CD3 lymphocytes
The calpain protein amount was estimated by Western blot in detergent lysates from the same numbers of cells as used for casein zymography. The amount of μ-calpain protein was evidently increased in the B-CLL cells compared with the B (CD3−) lymphocytes from age-matched healthy individuals (representative blot is shown in the Figure 6A). When the amount of the enzyme was estimated in the same amount of total cellular protein and compared in B-CLL samples versus normal PBLs and CD3− cells, not only was the difference between B-CLL and CD3− cells highly significant, but total PBLs also contained less than half the amount of the enzyme present in the B-CLL (Figure 6B).
The amount of μ-calpain is significantly higher in the B-CLL cells than in healthy lymphocytes.
The expression of μ-calpain was assessed by Western blotting in the samples of B-CLL cells and of PBLs and enriched CD3− cells of healthy controls. (A) A representative blot of 1 of 3 experiments performed (containing data from 3 healthy people [CD3− 1 through CD3− 3] and 3 different, age-matched B-CLL patients [B-CLL1 through B-CLL3]). Upper row: expression of μ-calpain; lower row: expression of F-actin in the same material as described in “Patients, materials, and methods.” (B) Graphical summary of all 3 experiments. Bars represent arithmetical means of the amount of protein in the μ-calpain bands, expressed as arbitrary densitometric units per milligram of cellular protein ± SEM. Asterisks indicate statistical significance of difference (Student t test) between the adjacent bars (B-CLL, CD3−) at the level of P < .01 (*).
The amount of μ-calpain is significantly higher in the B-CLL cells than in healthy lymphocytes.
The expression of μ-calpain was assessed by Western blotting in the samples of B-CLL cells and of PBLs and enriched CD3− cells of healthy controls. (A) A representative blot of 1 of 3 experiments performed (containing data from 3 healthy people [CD3− 1 through CD3− 3] and 3 different, age-matched B-CLL patients [B-CLL1 through B-CLL3]). Upper row: expression of μ-calpain; lower row: expression of F-actin in the same material as described in “Patients, materials, and methods.” (B) Graphical summary of all 3 experiments. Bars represent arithmetical means of the amount of protein in the μ-calpain bands, expressed as arbitrary densitometric units per milligram of cellular protein ± SEM. Asterisks indicate statistical significance of difference (Student t test) between the adjacent bars (B-CLL, CD3−) at the level of P < .01 (*).
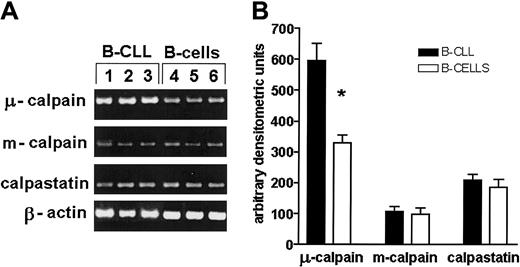
The expression of μ-calpain gene is significantly higher in B-CLL than in normal B lymphocytes
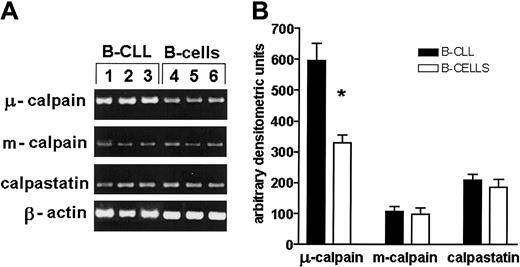
Analysis of the expression of genes for μ- and m-calpain as well as for calpastatin was done using RT-PCR. The amount of the amplification product of μ-calpain gene from B-CLL cells was twice as big as that obtained from cDNA derived from normal B cells, whereas the expression of m-calpain and calpastatin genes did not differ (Figure7).
The expression of gene for μ-calpain, but not that of m-calpain and calpastatin, is significantly increased at the transcriptional level compared with healthy B cells.
(A) Representative results of an RT-PCR estimation of the activities of μ-, m-calpain, and calpastatin genes in 3 B-CLL samples and 3 samples of enriched B cells of healthy, age-matched individuals. The β-actin gene product was used to assure that an equal amount of total mRNA was analyzed. (B) Mean results of 3 experiments similar to those shown in panel A. The difference (Student t test) between the amounts of gene product for μ-calpain was statistically significant at the level of P < .01 (*).
The expression of gene for μ-calpain, but not that of m-calpain and calpastatin, is significantly increased at the transcriptional level compared with healthy B cells.
(A) Representative results of an RT-PCR estimation of the activities of μ-, m-calpain, and calpastatin genes in 3 B-CLL samples and 3 samples of enriched B cells of healthy, age-matched individuals. The β-actin gene product was used to assure that an equal amount of total mRNA was analyzed. (B) Mean results of 3 experiments similar to those shown in panel A. The difference (Student t test) between the amounts of gene product for μ-calpain was statistically significant at the level of P < .01 (*).
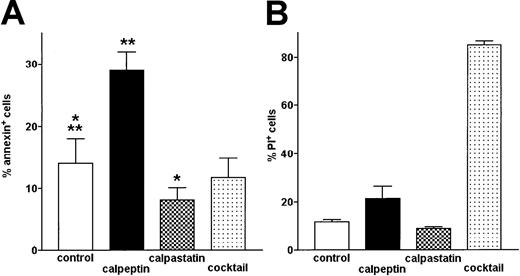
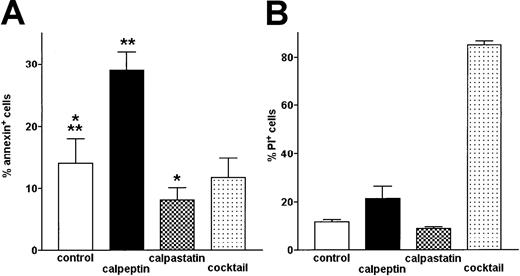
Inhibition of μ-calpain increases the level of spontaneous apoptosis in B-CLL cells
The level of apoptosis was determined by flow cytometry using FITC–annexin V to detect the apoptotic and PI necrotic cells. Upon treatment with calpeptin resulting in the significant inhibition of μ-calpain (Figure 1), the proportion of apoptotic (annexin+) cells increased twice compared with untreated B-CLL cells and this increase was highly statistically significant (Figure 8A). Inhibition of m-calpain by rabbit calpastatin had a reciprocal, weaker (but still significant) effect: a decreased percentage of cells binding annexin. Inhibition of other cellular proteases by a protease inhibitor cocktail had no effect on the same parameter (Figure 8A). Neither calpeptin (specific for both μ- and m-calpain) nor rabbit calpastatin (calcium-activated neutral protease inhibitor, relatively specific for m-calpain under experimental conditions used) had a significant effect on the viability of B-CLL cells (proportion of PI+ cells), whereas protease inhibitor “cocktail” very significantly reduced their viability (Figure 8B). Thus, action of calpeptin and calpastatin influencing the susceptibility of B-CLL cells to apoptosis seems to be related to their modulation of activities of calpains in these cells.
The proportion of spontaneously apoptotic B-CLL cells is increased by treatment with calpeptin.
Apoptotic (FITC-annexin–binding) cells were detected by flow cytometry. Bars represent means from 3 separate experiments ± SD. (A) Proportion of apoptotic, annexin-binding cells in untreated control (control) and cells treated with calpeptin (calpeptin), rabbit muscle m-calpain inhibitor (calpastatin), and protease inhibitor cocktail (cocktail), as in “Patients, materials, and methods.” The differences between proportions of apoptotic cells in control and inhibitor-treated samples are statistically significant (**calpeptin, paired t test, P < .0001; *calpastatin, paired t test, P < .02). (B) Proportion of necrotic cells staining with propidium iodide (PI+) in the same samples shows high toxicity of protease inhibitor cocktail but not of selective calpain inhibitors.
The proportion of spontaneously apoptotic B-CLL cells is increased by treatment with calpeptin.
Apoptotic (FITC-annexin–binding) cells were detected by flow cytometry. Bars represent means from 3 separate experiments ± SD. (A) Proportion of apoptotic, annexin-binding cells in untreated control (control) and cells treated with calpeptin (calpeptin), rabbit muscle m-calpain inhibitor (calpastatin), and protease inhibitor cocktail (cocktail), as in “Patients, materials, and methods.” The differences between proportions of apoptotic cells in control and inhibitor-treated samples are statistically significant (**calpeptin, paired t test, P < .0001; *calpastatin, paired t test, P < .02). (B) Proportion of necrotic cells staining with propidium iodide (PI+) in the same samples shows high toxicity of protease inhibitor cocktail but not of selective calpain inhibitors.
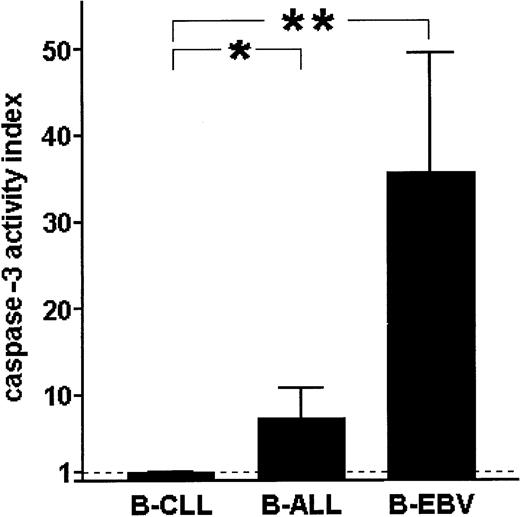
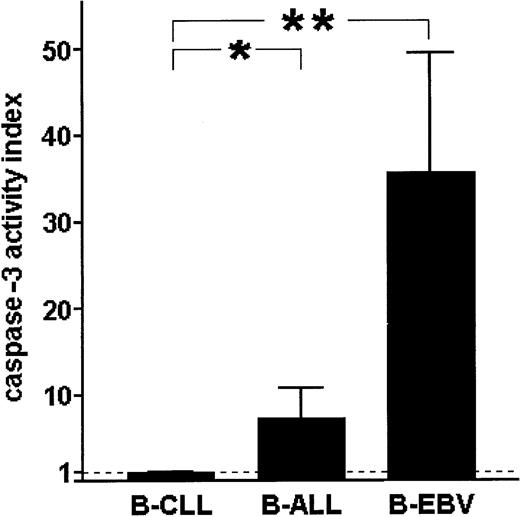
The activity of μ-calpain in normal and leukemic B cells reciprocally correlates with the activity of caspase-3
Comparison of caspase-3 activities in B-CLL cells, acute leukemia B lymphocytes, and EBV-immortalized B cells shows that the activity of this caspase is almost absent in B-CLL lymphocytes, clearly detectable in the ALL cells, and relatively very high in the EBV-transformed, vigorously proliferating B lymphocytes (Figure9). This result suggests a reciprocal relation between the activity of caspase-3 and μ-calpain in normal B and B-CLL lymphocytes.
Spontaneous activity of caspase-3 in B-CLL cells is extremely low compared with acute-leukemia B cells (B-ALL) and B cells of healthy individuals immortalized with EBV (B-EBV).
The activity of caspase-3 was measured as in “Patients, materials, and methods” and the results indexed against these obtained for B-CLL cells. Bars show mean indexed values ± SD of 3 separate experiments. Statistically significant differences between the caspase-3 activities are marked * for P < .05 and ** forP < .001.
Spontaneous activity of caspase-3 in B-CLL cells is extremely low compared with acute-leukemia B cells (B-ALL) and B cells of healthy individuals immortalized with EBV (B-EBV).
The activity of caspase-3 was measured as in “Patients, materials, and methods” and the results indexed against these obtained for B-CLL cells. Bars show mean indexed values ± SD of 3 separate experiments. Statistically significant differences between the caspase-3 activities are marked * for P < .05 and ** forP < .001.
Discussion
Activities of 2 isotypes of calcium-activated neutral proteases— μ- and m-calpains—are detectable and significantly modified in the B-CLL cells as compared with nonmalignant B lymphocytes. Of these, the activity of μ-calpain in the leukemic cells increases 8-fold, whereas that of m-calpain is on average 3 times lower in the malignant than in normal lymphocytes. This very significant increase of the available activity of μ-calpain in B-CLL is associated with only a 2 to 3 times higher amount of the enzyme protein in these cells, suggesting that apart from the increased amount, other factor(s) may lead to the increased activity of the protease in leukemic cells. Our RT-PCR data indicate that the increase of available activity of μ-calpain in B-CLL is probably at least partially dependent on transcriptional up-regulation of its gene. It is worth stressing that available activity of μ-calpain in the lymphocytes of healthy people decreases with age and in people over 65 years is almost undetectable, thus increasing the apparent difference between μ-calpain activity in healthy and B-CLL cells for this age group.
The level of transcription of calpastatin gene in the B-CLL cells was in our study similar to that exhibited by normal B cells. This observation suggests that the increased activity of μ-calpain does not depend on lower endogenous inhibition. Interestingly, the activity of m-calpain in B-CLL cells is much lower than that of μ-calpain and can only be demonstrated in the lysates prepared in the PBS without the addition of calcium chelator EGTA or DTT. This effect is most probably due to the requirement of a relatively high (supraphysiological and available only in vitro) concentration of Ca++—and, possibly, partial autocleavage—for activation of this isoenzyme. Also, we did not see any difference between transcription levels of the m-calpain gene in B-CLL and healthy B cells. Thus, the role of m-calpain in B-CLL pathogenesis is probably minor.
Our understanding of changes of the proliferation control in the B-CLL is still elusive and remains multifaceted. Suggested causes of the derailment of the cell cycle include various mutations, especially in the molecules controlling the course of the cell cycle, such as p53, c-myc, Rb, and others.32,33 Proliferation control of B-CLL cells comprises both the mechanisms of decreased division rate and prolonged survival. The latter may be due to the cells escaping the signals for apoptosis. In fact, decreased susceptibility of the B-CLL cells to apoptosis has been recently discussed as one of the factors deciding of their accumulation during the disease.34,35Proteolytic enzymes are clearly involved in the induction and progression of apoptosis; of these, apopains (caspases) are the best-known group.23 Participation of calpains in programmed cell death of blood cells (although not of the B-CLL cells so far) is also postulated.23,24 Our observation of increased apoptosis in B-CLL cells treated with a calpain inhibitor (calpeptin) suggests an antiapoptotic role of the enzyme activity. It also must be stressed that currently there are no inhibitors specific for either μ- or m-calpain. Thus, calpeptin inhibits both μ-calpain at ID50 (median effective dose) 52 nM and m-calpain at 34 nM; calpain inhibitor II acts in a similar way. Rabbit muscle calpastatin is described as inhibitory against mostly the m-calpain, although specificity of calpastatins against specific isoforms of calpains depends to some extent on their phosphorylation level and cleavage.36 On the other hand, we show in this work that an overexpression and overactivity of the μ isoform predominates in the B-CLL cells over much weaker activity of m-calpain. It is thus conceivable that the proapoptotic effect of calpain inhibitors that we observe depends mostly on the inhibition of μ-calpain and not of the minute activities of m-calpain in these cells.
Most of the current reports suggest a positive relation between the activity of calpains and the induction of apoptosis. Thus, for example, calpains were found to participate in the apoptosis of human leukemic cell lines, where their activity was necessary for increased GADD153 gene expression at the onset of apoptosis.37 GADD153 encodes CHOP 10, a nuclear protein acting as a negative modulator of CCAAT (enhancer of expression of some transcription factors), thus inhibiting cell cycle progression and inducing apoptosis.38 On the other hand, Juin et al38find no participation of calpains in nuclear apoptosis induced by 5 mM Ca++ (a concentration of calcium sufficient to activate both μ- and m-calpain).
Considering the spectrum of calpain substrates, including inter alia PKC and transcription factors c-fos, c-jun, NF-AT, NF-κB (and its regulator IκB), calcineurin, calcium channels, and Ca-ATPase,9-15 we propose that the reported changes in activities of both calpain isoforms might be important for the behavior of B-CLL cells, especially their prolonged survival and lower proliferation rate. Recently, Schuh and coworkers demonstrated a constitutive nuclear translocation and accumulation of NF-ATp and of NF-κB2/p52 in the B-CLL cells.39 They also found a constitutive activation of other transcription factors, including JunD, c-fos, and FosB in the cells of several B-CLL patients.39 Accumulation of NFκB suggests its activation and release from IκB. It is known that calpain-dependent cleavage of IκB can be an alternative way of NFκB activation in addition to the better-known way involving IκB phosphorylation and ubiquitination.12,13,40 It seems possible that increased activity of μ-calpain in B-CLL cells participates in hyperactivation of NFκB, c-Fos, and possibly other factors. In this scenario, higher than usual amounts of activated NFκB would bind the Rel protein and in this complexed form inhibit apoptosis, as in the case of WEHI231 cells described by Miyamoto et al.21
One of the causes for lower susceptibility of the B-CLL cells to apoptosis might be an increased Bcl-2/Bax ratio, due to significantly and reciprocally altered levels of both factors, observed in the malignant cells of both treated and untreated B-CLL patients.41 In a recent work, Wood et al show that calpain in the leukemic HL-60 cell line can selectively cleave the Bax protein.42 Thus, acting that way in the B-CLL cells, calpain could increase the Bcl-2/Bax ratio and actually prevent rather than induce apoptosis. Recent reports indicate that apoptosis in the B-CLL is prevented mostly by increased levels of the Bcl-2 with a simultaneous decrease of bax.34,35,43 The level of Bcl-2 can be increased by α-interferon.44,45 It is not known whether the activity of α-interferon can influence the level of calpain(s) in the cytoplasm, although γ-interferon was found to increase the level of calpain mRNA and protein in monocytic cell lines U-937 and THP-1.46 In our opinion, these observations point toward a possible link between increased activity of μ-calpain in B-CLL lymphocytes and their lower susceptibility to apoptosis. This is also in agreement with our data showing an increased level of spontaneous apoptosis in the B-CLL cells treated with a μ-calpain inhibitor.
Finally, our results indicate very low immediately available activity of the caspase-3 in the B-CLL cells. The activity of caspase-3 (and apoptosis) in/of the B-CLL cells can be induced by multiple factors, suggesting the possibility of impaired regulation of the gene for caspase-3 but not of its defect.47,48 The possibility of caspases being directly inhibited by calpain action had been recently demonstrated.49 50 Thus, it is feasible that calpain in B-CLL cells is either directly or, more likely, indirectly responsible for their lack of caspase-3 activity and, consequently, reduced apoptosis.
Another question related to the possible participation of calpains in the prevention of apoptosis in B-CLL cells is posed by our observation of reciprocal behavior of the μ- and m-calpains. Interestingly, we have demonstrated here a decrease in the activity of the m-calpain that requires millimolar Ca++ for activation and is probably only active in the cells in which calcium is (almost) freely entering the cytoplasm (as in the case of late apoptotic and necrotic cells). It cannot be said at the moment if these 2 isoforms of the enzyme could reciprocally control apoptosis in the B-CLL cells—the μ- form being antiapoptotic and the m- form proapoptotic—however, such a possibility is tempting in light of our results. Also, it was recently demonstrated that m-calpain could induce apoptosis by activating the caspase-351; so, low activity of this isoenzyme in the B-CLL cells may lower their susceptibility to programmed death.
Although modulation of p53 antioncogene had been shown not to participate directly in the B-CLL resistance to drug-induced apoptosis,31 it was recently shown that mutations and impaired function of p53 occur in a proportion of chronic B-cell leukemias.52 Calpain has been shown to cleave p53, and thus it could prevent this pathway of apoptosis induction in the B-CLL.16
Garban et al reported recently that the PKC is down-regulated in the B-CLL and that this reduced activity is accompanied by lack of Ca++ signal, at least in response to stimulation through HLA-DR.53 This modulation of the PKC may be attributed to increased calpain activity; it has been shown that both μ- and m-calpain cleave and ultimately down-regulate PKC in other cell types.9-11 17
Increased activity of μ-calpain that we observe in B-CLL cells may be related to at least some of the mutations found in the genome of these leukemic cells. Genetic disorders associated with B-CLL concern mostly the 11 and 13 chromosomes, whereas the genes for μ- and m-calpains are located, respectively, in the 11th (11q13) 5-8,54 and first55 chromosome. In fact, Brizard et al find in the B-CLL rare translocations and deletions precisely in the 11q13 band of the 11th chromosome containing the calpain gene.56 Translocations in this chromosome region of B-CLL cells were also reported by Cuneo et al.57 Thus, the relation between mutations typically seen in the B-CLL and modulation of calpain gene expression in the B-CLL cells cannot be excluded.
In conclusion, our observation of significant increase of the available activity of μ-calpain in the B-CLL lymphocytes is in agreement with both the cells' lower proliferation and increased survival phenotype and with genetic markers of the disease. The actual role of high activity of the protease and the exact mechanism(s) through which it may control the survival of B-CLL cells remain to be investigated. Finally, our finding of the increased spontaneous apoptosis level upon μ-calpain inhibitor treatment may suggest a possibility for potential therapeutic action of specific calpain inhibitors in chronic B-cell leukemia.
Prepublished online as Blood First Edition Paper, May 24, 2002; DOI 10.1182/blood-2001-11-0073.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jacek M. Witkowski, Department of Pathophysiology, Medical University of Gdansk; Debinki 7, 80-211 Gdansk, Poland; e-mail:jawit@amedec.amg.gda.pl.

![Fig. 5. In nonmalignant lymphocytes, the activity of μ-calpain is concentrated mostly in the T cells and decreases with age. / PBLs and immunomagnetically enriched CD3− and CD3−CD16− cells of a healthy young person were lysed in PBS and analyzed by casein zymography. Proteolytic bands corresponding to the activities of both isoenzymes were strongly seen only in the total peripheral blood lymphocyte (PBL) lysates, while the μ-calpain activity disappeared from the CD3−and CD3−CD16− cell lysates; std indicates the activities of standard μ- and m-calpain as in Figure 1 ([A] A representative zymogram of 1 of 3 similar experiments). Comparison of the activities of μ-calpain in the PBLs of 2 young, 2 middle-aged, and 2 old individuals shows a strong, age-related decrease of this activity ([B] Representative zymogram shows results of 1 of 3 similar experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/5/10.1182_blood-2001-11-0073/3/m_h81723046005.jpeg?Expires=1768334890&Signature=K6u5GyNE-ieF8Dqvr9h~Lo0UqDK17FyCRYmwBnKQK3z7SEigUarAnshi2G4FogFV3jxFtSpNEWeI~Aaqz~dUCJafG8WA~y1SKVHWqRVTIWNEOelMbWS2oBdEJQ-zXGxU3e0keEOSwCib6s8KFWkW9crkFjnSkRGTWl9igWfM1g~WP8hUCUP8420dRrfPwC~Vt6l2G~zOtM-E-rUB8kV9wiq3NexnWWU6b5zPGqB7jVGEwJDx1IIgM4rfYgkkrJfydj-P1VhnDFVIzdo~dGRLnw3F80GkUdLfvrSfc7hHrNpcNXN3fuw9jiNAMEkq4vsIAHhWGa2wtOA8YQ6jQWFeuQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. The amount of μ-calpain is significantly higher in the B-CLL cells than in healthy lymphocytes. / The expression of μ-calpain was assessed by Western blotting in the samples of B-CLL cells and of PBLs and enriched CD3− cells of healthy controls. (A) A representative blot of 1 of 3 experiments performed (containing data from 3 healthy people [CD3− 1 through CD3− 3] and 3 different, age-matched B-CLL patients [B-CLL1 through B-CLL3]). Upper row: expression of μ-calpain; lower row: expression of F-actin in the same material as described in “Patients, materials, and methods.” (B) Graphical summary of all 3 experiments. Bars represent arithmetical means of the amount of protein in the μ-calpain bands, expressed as arbitrary densitometric units per milligram of cellular protein ± SEM. Asterisks indicate statistical significance of difference (Student t test) between the adjacent bars (B-CLL, CD3−) at the level of P < .01 (*).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/5/10.1182_blood-2001-11-0073/3/m_h81723046006.jpeg?Expires=1768334890&Signature=prRpTgREJAMbRLJJdbL4cXQeccJasxbo-2SQVcf51scjg0fnHWp6GDG8~BgdIvqjuYz95YB6OLgB5x7e42wueig8tVKPQ2mP~sTQrXn92EojbZr3XRbEC1qFP1r7RdQ7oa6tlgsTSj2U0oq6vPdizFc8yn-fugKngOpRdj~SRl91sQugyGggDbf3OkFNz~wUdbkKp5aHLfZbxvU9yzIo7ZYlv5tS4RiSTEbljUR2hiBmAdtCFQV0Z5XZb9it-SyLHXhrLyMeopOvm7eSI1MT2hynZ0sbhohjVzqegVXPEiCZnqvqb0EN8L5ePK6UiONwdgJXqOajSz7QtSdbwiszpA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 5. In nonmalignant lymphocytes, the activity of μ-calpain is concentrated mostly in the T cells and decreases with age. / PBLs and immunomagnetically enriched CD3− and CD3−CD16− cells of a healthy young person were lysed in PBS and analyzed by casein zymography. Proteolytic bands corresponding to the activities of both isoenzymes were strongly seen only in the total peripheral blood lymphocyte (PBL) lysates, while the μ-calpain activity disappeared from the CD3−and CD3−CD16− cell lysates; std indicates the activities of standard μ- and m-calpain as in Figure 1 ([A] A representative zymogram of 1 of 3 similar experiments). Comparison of the activities of μ-calpain in the PBLs of 2 young, 2 middle-aged, and 2 old individuals shows a strong, age-related decrease of this activity ([B] Representative zymogram shows results of 1 of 3 similar experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/5/10.1182_blood-2001-11-0073/3/m_h81723046005.jpeg?Expires=1768851384&Signature=47GmqzLifGXoC84DYdKa9IrSH4cLfeykW887t89onlHpNRjNQhMbP114eh3OvvqyevqVPunrdmxg9UsXwgCOOyUsUiRx0Xqtk9lC02o0R0k5Rkd-aD216wQPxugLFEMc7x5TUJ5434cIwI1ep-HqfFsc~tMl9I1LCOezcfENjw1qLVntLHHbD4C5C3Cpw0kPgm-14~Sjrg7U8vBJyqe-5HW6mDhqbe8W-iXxJQ~Ys~4Q4W~zARCsp7UgYIZYqv0BaPwxOHzAa1IvBfGFQ8OMe1PdbaHr7r~6Wd1p1U3H61ncgpUFE2iyLPw0vWteteVxLAaTT2qTihE-~aZvHsdkcw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. The amount of μ-calpain is significantly higher in the B-CLL cells than in healthy lymphocytes. / The expression of μ-calpain was assessed by Western blotting in the samples of B-CLL cells and of PBLs and enriched CD3− cells of healthy controls. (A) A representative blot of 1 of 3 experiments performed (containing data from 3 healthy people [CD3− 1 through CD3− 3] and 3 different, age-matched B-CLL patients [B-CLL1 through B-CLL3]). Upper row: expression of μ-calpain; lower row: expression of F-actin in the same material as described in “Patients, materials, and methods.” (B) Graphical summary of all 3 experiments. Bars represent arithmetical means of the amount of protein in the μ-calpain bands, expressed as arbitrary densitometric units per milligram of cellular protein ± SEM. Asterisks indicate statistical significance of difference (Student t test) between the adjacent bars (B-CLL, CD3−) at the level of P < .01 (*).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/5/10.1182_blood-2001-11-0073/3/m_h81723046006.jpeg?Expires=1768851384&Signature=nkzznPkEs~wp-BoqLlE991PFGqPCDHIcwHlj~abFVHOuY3sizNeJoyySHkYhCvnNhgn-TnsXnUnkD~5-lg-XbSSYy-~K5jsNsLi20ElbRBYgSnWvuD4Jk25gagu6LbgLOmVuruuYneBLV9mqG7VKJ0wmC4ZpnWyNDJOupoHFQjYf7cdf20n86renqpy4eQVAG1Gme2Nkcs68rx195XipwWOYvwd7dP4YalnBaCTTT4ILb8WsVhWAPxWbcSHh7dZev4jBqC2oXJa2wf7lXcRS-kZWNUnRak4qsLx0eMyMBvnqZ8oRUvH8XpdXLS31recYINxABMEzPDiS6PXJ4JgdNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)