In mice, donor leukocyte infusion (DLI) given to established mixed allogeneic chimeras can mediate powerful graft-versus-host (GVH) reactions confined to the lymphohematopoietic system without inducing graft-versus-host disease (GVHD). In a clinical trial attempting to capture this approach to achieve graft-versus-leukemia/lymphoma (GVL) effects without GVHD, we have observed surprisingly powerful antitumor effects of DLI in patients achieving mixed chimerism after nonmyeloablative bone marrow transplantation. This observation led us to hypothesize that host antigen–presenting cells in mixed chimeras might be required to optimally present recipient antigens to the donor lymphocytes, leading to maximal graft-versus-tumor effects. To test this hypothesis, we established mixed and fully allogeneic hematopoietic chimeras in B6 mice and evaluated the effect of DLI on EL4 T-cell lymphoma. DLI administration to mixed chimeras produced dramatically improved leukemia-free survival compared to administration of DLI to full donor chimeras. DLI also converted mixed chimeras to full chimeras without causing GVHD. The magnitude of the GVL effect was dependent on the level of major histocompatibility complex class I expression on recipient hematopoietic cells in mixed chimeras. Thus, the induction of mixed chimerism followed by delayed DLI provides an approach to inhibiting GVHD that optimizes GVL effects.
Introduction
Allogeneic bone marrow transplantation (BMT) following myeloablative conditioning therapy is the only known curative treatment for a variety of hematologic malignancies. This curative effect is based largely on the alloreactivity of donor lymphocytes, which mediate a graft-versus-leukemia (GVL) effect.1,2This GVL effect is associated with the presence of graft-versus-host disease (GVHD)1,2 and is influenced by the degree of major histocompatibility complex (MHC) disparity and the presence of T lymphocytes within the graft, suggesting that it is mediated largely by graft-versus-host (GVH) alloreactive donor T cells.3Unfortunately, this beneficial GVL effect is often counterbalanced by the mortality and morbidity associated with GVHD. However, rodent studies have shown that administration of a donor lymphocyte infusion (DLI) to mixed hematopoietic chimeras can mediate a GVH reaction which is restricted to the lymphohematopoietic system, as indicated by the conversion from mixed chimerism to full chimerism without the concomitant development of GVHD.4 5 This suggested an approach to using the GVH alloresponse to achieve GVL without GVHD.
In humans, GVL effects have been achieved by the administration of DLI to patients with relapsed chronic myelogenous leukemia (CML) after allogeneic BMT.6,7 In a clinical trial at our institution, mixed chimeras induced with nonmyeloablative conditioning have enjoyed striking remissions of advanced, refractory lymphoid malignancies, both with and without the administration of DLI.8,9 Based on these surprisingly powerful GVL effects, we hypothesized that nontolerant donor lymphocytes might result in stronger GVL effects when administered to mixed hematopoietic chimeras than to full donor chimeras. It seemed possible that the presence of host antigen–presenting cells (APCs) in mixed chimeras might allow improved presentation of host antigens to the donor lymphocytes, leading to superior alloactivation of the DLI inoculum. The nonmyeloablative clinical protocol was based on preclinical results obtained with a cyclophosphamide-based nonmyeloablative conditioning regimen that permits the establishment of stable mixed hematopoietic chimerism in mice. In that model, we demonstrated that DLI leads to the conversion from mixed to full donor chimerism without causing GVHD,5,10 consistent with our previous results obtained in mixed chimeras prepared with lethal total body irradiation (TBI).4 To address the question of whether host APCs are critical for the induction of optimal antitumor effects of DLI, it was essential to return to the original lethal TBI regimen. We could not use the nonmyeloablative conditioning regimen because it would not allow comparison between mixed and full donor chimeras—it is not possible to reliably generate full donor chimeras with the CTX-based regimen before administration of DLI. We therefore tested our hypothesis using lethally irradiated B6 mice as recipients of T-cell–depleted (TCD) allogeneic bone marrow cells with or without TCD host-type B6 marrow to produce mixed or full chimeras, respectively. These mice then received the host-type T-cell leukemia/lymphoma, EL4, with or without DLI. The present findings support our hypothesis, revealing a superior GVL effect of DLI in mixed chimeras compared to full chimeras. This effect is shown to be critically dependent on the level of MHC class I expression on host APCs present before DLI administration.
Materials and methods
Animals
B10.A (H-2a: Kk, Ak, Ek, Dd, Ld) female donor mice purchased from Frederick Cancer Research Facility, National Cancer Institute were used at 8 to 12 weeks of age. Female C57BL/6 (B6: H-2b, I-E−) recipient mice were purchased from Frederick Cancer Research Facility, NCI, and used at 10 weeks of age. In some experiments, B6.β2m−/− animals and wild-type control B6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were housed in autoclaved microisolator environments, and all manipulations were performed in a laminar flow hood.
Bone marrow transplantation and DLI administration
We established mixed or full donor hematopoietic chimeras in the fully MHC-mismatched B10.A→C57BL/6(B6) strain combination. B6 (H2b) mice received 10.25 Gy total body irradiation from a cesium 137 (137Cs) irradiator and were reconstituted with either a mixture of T-cell–depleted (TCD) B6 (5 × 106bone marrow cells) plus B10.A (H2a) (15 × 106) bone marrow cells or with TCD B10.A bone marrow cells (15 × 106) alone by intravenous injection 4 hours after irradiation. In vitro depletion of CD4 and CD8 T cells from the bone marrow inoculum was performed using monoclonal antibodies and rabbit complement lysis as previously described.11 To achieve engraftment of B6.β2m−/− marrow in animals receiving B10.A plus B6.β2m−/− bone marrow cells, mice received 150 μg mAb PK136 (NK1.1) on days −6 and −1 before BMT. For preparation of DLI, B10.A spleens were harvested and gently teased in ACK-lysing buffer (BioWhittaker, Walkersville, MD). Single-cell suspensions were filtered through nylon mesh. Donor lymphocytes were administered in the form of 3 × 107 B10.A spleen cells on day 56 after BMT. The C57BL/6-derived T-cell leukemia cell line EL4 (500 cells) was administered intravenously on day 63. Animals were randomized between cages to avoid cage-related bias.
Phenotyping of chimeras
Chimerism in WBC and bone marrow was assessed by 2-color or 3-color flow cytometry (FCM) using a FACScan cytometer (Becton Dickinson, Mountain View, CA). Peripheral blood was collected into heparinized Eppendorf tubes and subjected to deionized water lysis. For double or triple color staining, 106 cells were incubated in the presence of directly fluorescein-isothiocyanate (FITC)–, phycoerythrin (PE)–, or biotin (Bio)–conjugated monoclonal antibodies (mAbs) for 30 minutes at 4°C. Development of Bio-labeled mAbs was performed by subsequent incubation with phycoerythrin-conjugated avidin (PEA) for 10 minutes. To reduce nonspecific binding of mAbs, 10 μL 2.4G2 (anti–Fcγ-RII receptor, CDw32) hybridoma supernatant12 was added to all tubes. The following antibodies were used for chimerism analyses in various cell lineages: anti–CD4-FITC, anti–CD8β-FITC, anti–B220-FITC (all purchased from PharMingen, San Diego CA), anti–Mac-1-FITC (CalTag, San Francisco, CA), and 34-2-12-Bio (anti–H2-Dd prepared in our laboratory). Nonreactive control mAb HOPC-FITC or HOPC-Bio (mouse IgG2a prepared in our laboratory) and rat IgG2b-PE (PharMingen) were used as negative controls. The percentage of donor cells within each leukocyte population was determined using the following formula: net % donor cells of a particular lineage × 100%/(net % donor cells of a particular lineage + net % host cells of a particular lineage), where net refers to the percentage obtained after subtraction of staining with control antibody. For the H-2 class I allele–specific mAb, the mouse strain (donor or host) not bearing the allele recognized by the mAb was used as the negative control, and the strain expressing the allele was used as the positive control to determine the cutoffs for reactivity with the H2-specific mAb. Exclusion of dead cells was performed by propidium iodide (PI) staining and live gating on PI-negative cells. Ten thousand events were collected and analyzed. The different peripheral blood leukocyte populations were distinguished by their forward scatter (FSC) and side scatter (SSC) properties: FSC low and SSC low (lymphocytes), SSC high (granulocytes), and FSC high and SSC low (monocytes).
EL4 cell culture
EL4 cells were originally obtained from ATCC and were cultured in RPMI 1640 (Biowhittaker) with 10% FCS (Sigma, St Louis, MO). A new vial of a working bank of EL4 cells was thawed for each experiment and was maintained in culture for a maximum of 2 weeks.
Results
Delayed administration of donor lymphocytes to mixed chimeras 8 weeks after BMT led to conversion to full chimerism without causing GVHD
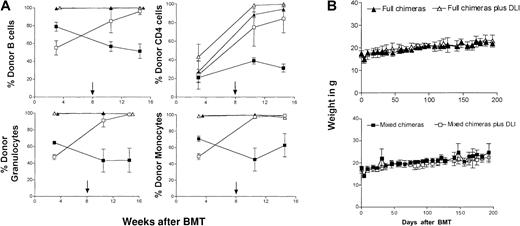
To address our hypothesis that donor lymphocyte infusions might result in stronger GVL effects when administered to mixed chimeras, we produced mixed and full donor hematopoietic chimeras in the fully MHC-mismatched B10.A (H2a)→C57BL/6 (H2b) strain combination. C57BL/6 (B6) recipients were lethally irradiated (1025 cGy total body irradiation) and reconstituted with either TCD B10.A bone marrow alone (B10.A “full” chimeras) or a mixture of TCD B10.A plus TCD B6 bone marrow cells (B6 + B10.A “mixed” chimeras). As discussed above, this myeloablative conditioning approach was pursued because it allowed the generation of mixed and full donor chimeras using identical treatment conditions, without the requirement for GVH-reactive T cells to produce the initial state of full donor chimerism. Peripheral blood chimerism analysis revealed that recipients of B10.A bone marrow cells usually had more than 95% donor cells in all lineages, with the exception of T cells. A high percentage of residual host T cells was initially detectable and declined to less than 20% of T cells at steady state (Figure1A). As expected, B6 + B10.A chimeras reconstituted as mixed chimeras, with substantial donor and host contributions to all lineages studied (Figure 1A). Eight weeks after BMT, 3 × 107 donor-type B10.A spleen cells were injected intravenously. Administration of DLI led to the conversion of mixed to full donor hematopoietic chimerism, as previously reported.4 Full donor chimeras receiving DLI showed conversion to 100% donor in the T-cell lineage (Figure 1A shows one representative experiment). Despite the elimination of host hematopoietic cells, GVHD was not apparent either clinically or histologically. As shown in Figure 1B, the recipient animals maintained their weights, and there was no significant difference between the groups receiving DLI and the corresponding control groups not receiving DLI. Histologic analysis of classical GVHD target organs in animals killed at later than 100 days after DLI showed a complete absence of GVHD manifestations in skin, liver, small intestine, and large intestine in most animals. In one instance, scattered foci of lymphoid infiltrates were detectable in the liver and colon (Table 1).
Conversion from mixed to full donor chimerism without GVHD following DLI.
(A) DLI led to conversion from mixed to full donor hematopoietic chimerism. Peripheral blood multilineage donor chimerism was determined with lineage-specific markers using H2-Dd–specific mAb 34-2-12 in 2-color flow cytometry. Animals were reconstituted either with TCD B10.A BM alone (no DLI, ▴, n = 4) or with a mixture of 15 × 106 TCD B10.A plus 5 × 106 B6 BM (no DLI, ▪, n = 4). Spleen cells (3 × 107 B10.A) were administered to additional groups of mixed and full chimeras on day 56 after BMT. Conversion to full donor chimerism was observed in all mixed chimeras receiving DLI (■, n = 4). Full chimeras remained full donor in all lineages after DLI (▵, n = 5), and T cells converted to full donor chimerism as well. Mean percentage donor chimerism ± SD from one representative experiment is presented. The arrow denotes the time of DLI administration. One representative result of 4 similar experiments is shown. (B) DLI does not cause GVHD. Spleen cells (3 × 107 B10.A) were administered on day 56 after BMT to mixed and full chimeras. DLI recipients (full chimeras ▵, n = 4; mixed chimeras ■, n = 4) had weight curves similar to those of non-DLI recipients (full chimeras ▴, n = 4; mixed chimeras ▪, n = 5) and did not show clinical signs of GVHD. Results from one representative experiment are shown.
Conversion from mixed to full donor chimerism without GVHD following DLI.
(A) DLI led to conversion from mixed to full donor hematopoietic chimerism. Peripheral blood multilineage donor chimerism was determined with lineage-specific markers using H2-Dd–specific mAb 34-2-12 in 2-color flow cytometry. Animals were reconstituted either with TCD B10.A BM alone (no DLI, ▴, n = 4) or with a mixture of 15 × 106 TCD B10.A plus 5 × 106 B6 BM (no DLI, ▪, n = 4). Spleen cells (3 × 107 B10.A) were administered to additional groups of mixed and full chimeras on day 56 after BMT. Conversion to full donor chimerism was observed in all mixed chimeras receiving DLI (■, n = 4). Full chimeras remained full donor in all lineages after DLI (▵, n = 5), and T cells converted to full donor chimerism as well. Mean percentage donor chimerism ± SD from one representative experiment is presented. The arrow denotes the time of DLI administration. One representative result of 4 similar experiments is shown. (B) DLI does not cause GVHD. Spleen cells (3 × 107 B10.A) were administered on day 56 after BMT to mixed and full chimeras. DLI recipients (full chimeras ▵, n = 4; mixed chimeras ■, n = 4) had weight curves similar to those of non-DLI recipients (full chimeras ▴, n = 4; mixed chimeras ▪, n = 5) and did not show clinical signs of GVHD. Results from one representative experiment are shown.
Thus, consistent with our previous observation,4 the GVH reaction induced by delayed DLI given to mixed chimeras was largely restricted to the lymphohematopoietic tissues, converting mixed chimeras to full donor lymphohematopoietic chimeras without the epithelial tissue infiltrations associated with GVHD.
GVL effect of DLI is markedly stronger in mixed than in full chimeras
We next evaluated whether DLI could mediate a GVL effect and whether its magnitude was influenced by the presence of host hematopoietic cells in the recipient at the time of DLI. For this purpose, animals received DLI on day +56 followed by the administration of a lethal dose of the recipient-type (B6) T-cell lymphoma cell line EL4 (500 cells) on day +63. Although we have attempted to elicit GVL effects from allogeneic lymphocytes against already-established EL4 in several models, we have not observed GVL effects against this highly aggressive tumor with this approach (M.S., unpublished data, 1986; M.Y.M., M.S., unpublished data, May 1999). We therefore selected the above approach (DLI before tumor administration), which has been used by other investigators and accepted as a model for minimal residual disease.13 Figure 2 shows the combined data from 4 separate experiments, all of which produced similar results. DLI did not induce GVHD, and all nonleukemic recipients of DLI survived in excellent health (Figure 2A-B). Tumor inoculation caused rapid mortality in all full chimeras (median survival time [MST] 86 days after BMT) and in most mixed chimeras (MST 91 days after BMT) (Figure 2A,C). In all experiments, mixed chimeras receiving EL4 cells without DLI demonstrated slightly prolonged survival compared to the full chimeras receiving only tumor cells (Figure 2A-B). Administration of DLI led to a significant GVL effect in mixed and full chimeras compared with the control groups receiving EL4 cells alone (P < .0001 andP < .0005, respectively). However, full donor chimeras (Figure 2A) that had received DLI displayed only a slight delay in mortality from EL4 leukemia compared to non-DLI controls (MST 91 versus MST 86 days after BMT). In contrast, almost all mixed chimeras receiving DLI were completely protected from leukemic mortality (Figure2B), with 22 of 25 animals still alive at the end of the observation period (ie, more than 100 days after tumor inoculation). The improved survival observed in DLI recipients with mixed hematopoietic chimerism at the time of DLI administration was statistically highly significant (P < .0001) compared to the survival of full chimeras receiving DLI (Figure 2C). Furthermore, mixed chimeras receiving DLI and EL4 did not show tumor infiltration at the time of death. In contrast, tumor infiltration was readily observable in full chimeras receiving DLI and EL4. Two representative animals are depicted in Figure 3. Data are summarized in Table 1.
DLI-mediated GVL effects in mixed and full donor chimeras.
Cumulative data from 4 similar experiments are presented. DLI were administered on day 56 after BMT followed by intravenous injection of 500 EL4 cells on day 63 (↓). (A) Full chimeras receiving DLI and EL4 cells (▪, n = 20) showed a slight, but statistically significant, prolongation of survival compared to full chimeras receiving tumor cells only (■, n = 12; P < .001). Full chimeras receiving (▾, n = 14) or not receiving DLI (▿, n = 7) showed no treatment-related mortality. (B) Mixed chimeras receiving (♦, n = 11) or not receiving (⋄, n = 8) DLI showed no treatment-related mortality. Mixed chimeras receiving DLI and EL4 cells (●, n = 25) had significantly improved survival compared with mixed chimeras receiving EL4 alone (○, n = 14; P < .0001). (C) Mixed chimeras receiving DLI and EL4 cells (●, n = 25) had significantly improved survival compared with full chimeras receiving EL4 + DLI (▪, n = 20; P < .0001).
DLI-mediated GVL effects in mixed and full donor chimeras.
Cumulative data from 4 similar experiments are presented. DLI were administered on day 56 after BMT followed by intravenous injection of 500 EL4 cells on day 63 (↓). (A) Full chimeras receiving DLI and EL4 cells (▪, n = 20) showed a slight, but statistically significant, prolongation of survival compared to full chimeras receiving tumor cells only (■, n = 12; P < .001). Full chimeras receiving (▾, n = 14) or not receiving DLI (▿, n = 7) showed no treatment-related mortality. (B) Mixed chimeras receiving (♦, n = 11) or not receiving (⋄, n = 8) DLI showed no treatment-related mortality. Mixed chimeras receiving DLI and EL4 cells (●, n = 25) had significantly improved survival compared with mixed chimeras receiving EL4 alone (○, n = 14; P < .0001). (C) Mixed chimeras receiving DLI and EL4 cells (●, n = 25) had significantly improved survival compared with full chimeras receiving EL4 + DLI (▪, n = 20; P < .0001).
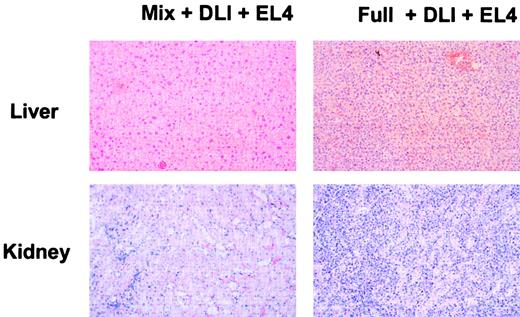
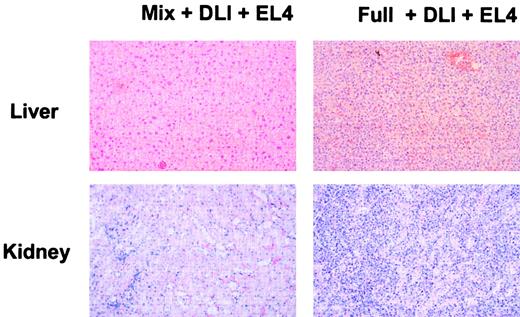
Absence of leukemic organ infiltration or GVHD in mixed chimeras receiving DLI and EL4.
Histologic analysis of mixed chimeras and full chimeras receiving DLI and EL4 was performed on animals that had succumbed to EL4 leukemia or on animals killed at the end of the observation period. On necropsy, overt infiltration of liver and kidney by leukemic cells was evident in full chimeras receiving DLI and EL4. In contrast, mixed chimeras receiving DLI and EL4 were killed on day 169 after BMT and showed no tumor cell infiltration. Histologic sections from 2 representative chimeras are presented. Liver and kidney from a full chimera receiving DLI and EL4 showed severe infiltration with leukemic cells. In contrast, mixed chimeras receiving DLI showed normal tissue morphology.
Absence of leukemic organ infiltration or GVHD in mixed chimeras receiving DLI and EL4.
Histologic analysis of mixed chimeras and full chimeras receiving DLI and EL4 was performed on animals that had succumbed to EL4 leukemia or on animals killed at the end of the observation period. On necropsy, overt infiltration of liver and kidney by leukemic cells was evident in full chimeras receiving DLI and EL4. In contrast, mixed chimeras receiving DLI and EL4 were killed on day 169 after BMT and showed no tumor cell infiltration. Histologic sections from 2 representative chimeras are presented. Liver and kidney from a full chimera receiving DLI and EL4 showed severe infiltration with leukemic cells. In contrast, mixed chimeras receiving DLI showed normal tissue morphology.
Superior GVL effect of DLI given to mixed chimeras is dependent on MHC class I expression on host hematopoietic cells
The improved GVL effect observed when DLI was administered to mixed compared to full chimeras suggested that host hematopoietic cells, particularly antigen-presenting cells (APCs), might play a critical role in driving GVL effects. Because we have previously shown that the GVL effect against EL4 is completely dependent on donor CD8 cells and independent of CD4 cells,14 15 we hypothesized that direct allorecognition of MHC class I antigens on host hematopoietic cells might be responsible for the superior DLI-mediated GVL effect in mixed chimeras. To address this possibility, we established mixed chimerism in B6 mice, which were reconstituted following lethal irradiation with TCD BMC from allogeneic B10.A donors plus TCD BMC from syngeneic class I–deficient beta-2-microglobulin (β2m)−/− B6 mice (B6.β2m−/− + B10.A chimeras). The resultant mixed chimeras reconstituted following lethal conditioning had normal MHC class I expression on nonhematopoietic tissues but were deficient in MHC class I expression on hematopoietic cells of B6 origin (ie, host-type). We compared the DLI-mediated GVL effect in B6.β2m−/− + B10.A mixed chimeras to those in wild-type B6 + B10.A mixed chimeras and to those in full B10.A chimeras. In an initial experiment, NK-cell–mediated resistance led to the rejection of B6.β2m−/− BMC, so that recipients of TCD B6.β2m−/− + TCD B10.A marrow reconstituted with predominant B10.A hematopoiesis (data not shown). To circumvent this problem, we injected the recipients in a subsequent experiment with 150 μg anti-NK1.1 mAb PK136 on days −6 and −1 before BMT. Using this modification, B6.β2m−/− BMC engrafted well, and varying levels of chimerism were achieved. As is shown in Figure4, DLI administered to B6 + B10.A mixed chimeras again resulted in a markedly superior GVL effect compared to that in full chimeras. Mixed B6.β2m−/− + B10.A chimeras showed an intermediate result, with 3 of 7 animals succumbing to the leukemia. However, the leukemia-induced mortality in that group occurred exclusively in animals that showed relatively low levels of B6.β2m−/− hematopoiesis and had predominantly B10.A hematopoiesis. Compared to the surviving animals from that group, donor (B10.A) chimerism before DLI was significantly higher in the animals that succumbed to the leukemia (P < .05 for B cells;P < .003 for monocytes; P < .01 for granulocytes). Nonparametric (Spearman) correlation analysis also demonstrated a significant correlation between the level of B6.β2m−/− hematopoiesis and the duration of survival in all lineages tested (data not shown). The relationship between chimerism in a representative lineage (monocytes) and survival is indicated in Figure 4B. When animals were stratified according to the level of chimerism, those with high levels of allogeneic hematopoietic chimerism and, hence, low numbers of B6.β2m−/−hematopoietic cells (eg, less than 40% B6.β2m−/−monocytes) had poorer survival than animals with higher levels of B6.β2m−/− hematopoiesis. In the B6.β2m−/− + B10.A mixed chimeras with low B6.β2m−/− hematopoiesis that received DLI (Figure 4B), leukemia-induced mortality was virtually identical to that in full chimeras receiving DLI. In contrast, the B6.β2m−/−+ B10.A mixed chimeras with higher levels of B6.β2m−/−hematopoiesis showed indistinguishable survival compared to wild-type mixed chimeras.
Superior DLI-mediated GVL effects in mixed chimeras are dependent on host MHC class I expression.
To evaluate the effect of expression of host MHC class I on hematopoietic cells on the GVL effect mediated by DLI, we established full donor chimeras (⋄, full), mixed chimeras (▿, B6+B10.A), or mixed chimeras in which host-type hematopoietic cells were deficient in host MHC class I expression (○, B6.β2m−/−+ B10.A). Following BMT, chimeric animals received DLI with or without EL4 leukemia cells (full + DLI [∗, n = 4]; B6 + B10.A + DLI [×, n = 3]; B6.β2m−/− + B10.A + DLI [+, n = 3]). Chimeras receiving no additional treatment after BMT were used as controls (⋄, full [n = 3]; ▿, B6 + B10.A [n = 3]; ○, B6.β2m−/− + B10.A [n = 2]). DLI was administered on day 56 followed by EL4 leukemia administration on day 63. (A) Survival of all groups. Leukemic wild-type mixed chimeras receiving DLI demonstrated significantly longer survival (▾, 9 of 10 surviving) compared to full donor chimeras (♦, 0 of 9 surviving MST 91 days). Leukemic B6.β2m−/− + B10.A chimeras revealed an intermediate result (●, 4 of 7 animals surviving). (B) Survival analysis in leukemic B6.β2m−/− + B10.A chimeras stratified according to peripheral blood chimerism levels in the monocytic lineage. Animals with high (▪, more than 40% B6.β2m−/− monocytes) or low (■) B6.β2m−/− hematopoiesis receiving DLI+EL4 are compared to wild-type B6 + B10.A chimeras receiving DLI + EL4 (▾). Full chimeras receiving DLI + EL4 (♦) are also shown for comparison. B6.β2m−/− + B10.A mixed chimeras with low B6.β2m−/− hematopoiesis receiving DLI and EL4 showed a significantly poorer survival than animals with high levels of B6.β2m−/− hematopoiesis (P < .0002).
Superior DLI-mediated GVL effects in mixed chimeras are dependent on host MHC class I expression.
To evaluate the effect of expression of host MHC class I on hematopoietic cells on the GVL effect mediated by DLI, we established full donor chimeras (⋄, full), mixed chimeras (▿, B6+B10.A), or mixed chimeras in which host-type hematopoietic cells were deficient in host MHC class I expression (○, B6.β2m−/−+ B10.A). Following BMT, chimeric animals received DLI with or without EL4 leukemia cells (full + DLI [∗, n = 4]; B6 + B10.A + DLI [×, n = 3]; B6.β2m−/− + B10.A + DLI [+, n = 3]). Chimeras receiving no additional treatment after BMT were used as controls (⋄, full [n = 3]; ▿, B6 + B10.A [n = 3]; ○, B6.β2m−/− + B10.A [n = 2]). DLI was administered on day 56 followed by EL4 leukemia administration on day 63. (A) Survival of all groups. Leukemic wild-type mixed chimeras receiving DLI demonstrated significantly longer survival (▾, 9 of 10 surviving) compared to full donor chimeras (♦, 0 of 9 surviving MST 91 days). Leukemic B6.β2m−/− + B10.A chimeras revealed an intermediate result (●, 4 of 7 animals surviving). (B) Survival analysis in leukemic B6.β2m−/− + B10.A chimeras stratified according to peripheral blood chimerism levels in the monocytic lineage. Animals with high (▪, more than 40% B6.β2m−/− monocytes) or low (■) B6.β2m−/− hematopoiesis receiving DLI+EL4 are compared to wild-type B6 + B10.A chimeras receiving DLI + EL4 (▾). Full chimeras receiving DLI + EL4 (♦) are also shown for comparison. B6.β2m−/− + B10.A mixed chimeras with low B6.β2m−/− hematopoiesis receiving DLI and EL4 showed a significantly poorer survival than animals with high levels of B6.β2m−/− hematopoiesis (P < .0002).
In contrast, in wild-type mixed chimeras, survival was not influenced by the level of host-type chimerism. Animals with relatively low levels of host chimerism showed complete protection from leukemia-induced mortality (eg, 6 of 6 animals with less than 40% host monocytes survived). The one mixed chimera that died showed relatively high levels of host chimerism before DLI, with more than 60% host monocytes, more than 50% granulocytes, and more than 30% B cells.
In a similar experiment, 5 of 6 B6 β2m−/−+ B10.A chimeras receiving DLI and EL4 died of leukemia, whereas 9 of 10 B6+B10.A chimeras receiving DLI and EL4 survived longer than 100 days after DLI (data not shown), confirming the importance of MHC class I expression on host hematopoietic cells for DLI-mediated GVL effects.
Discussion
GVL and GVHD are induced largely by the alloreactivity of donor T cells, and this alloresponse is critically influenced by the presence of host APCs. Shlomchik et al16 recently demonstrated that the development of GVHD was dependent on the presence of host APCs, and they proposed depletion or inactivation of host APCs as an approach to preventing GVHD. However, those studies did not address the potential impact of host APC depletion on GVL effects. Based on clinical results, we hypothesized that host APCs might play a critical role in inducing the most potent DLI-mediated GVL effects. To address this hypothesis, we compared the potency of DLI-mediated GVL effects in mixed or full donor chimeras. The data presented here are consistent with the necessity of host APCs in inducing potent GVH alloresponses, and they suggest that eliminating such APCs would have the undesired consequence of mitigating GVL effects. GVL activity was markedly superior in mixed chimeras compared to full chimeras, demonstrating the importance of host-type APCs for the induction of maximal GVL effects. This effect was dependent on MHC class I expression on host APCs because the reduced levels of host-type MHC class I on B6.β2m−/−hematopoietic cells, given with allogeneic BMT to wild-type B6 mice, were associated with markedly reduced GVL effects, which could be compensated for in part by the presence of high proportions of class I low host-type hematopoietic cells.
We show here that the maximal GVL effects observed in mixed chimeras can occur without GVHD, despite mediation by a potent GVH alloresponse. We believe that the avoidance of GVHD following DLI requires 2 preconditions. The first precondition is sufficient time for the recipient to recover from conditioning-induced inflammation that may promote the migration of T cells into GVHD target tissues. In the lethal TBI model used here to permit the production of mixed and full chimeras with the same regimen, we allowed 8 weeks between conditioning and DLI because this interval has been shown to allow DLI to convert mixed chimerism to full chimerism without GVHD.4 In contrast, the donor lymphocyte numbers used in our DLI produce rapidly lethal GVHD in freshly irradiated mice. The second precondition is an absence of GVH-reactive T cells in the initial donor transplant. The presence of such early GVH alloreactions, in combination with early conditioning-induced inflammation, might produce clinical or subclinical GVHD, with target tissue inflammation, that could lead to severe GVHD following DLI. In the model used here, T-cell depletion of the initial allogeneic inoculum avoids any such GVH reactions from the initial marrow transplant. In other nonmyeloablative murine models that are more relevant to the nonmyeloablative clinical transplants in which we observed potent antitumor effects in association with DLI following the induction of mixed chimerism, we have seen similarly potent GVL effects of DLI without the induction of GVHD. Thus, in mixed chimeras (B10.A→B6) generated with the CTX-based nonmyeloablative conditioning regimen on which our clinical studies are based, DLI given at 5 weeks led to powerful GVL effects against EL4 leukemia/lymphoma and also against the myeloid leukemia MMB3.19 (M.Y.M. et al, manuscript in preparation; Y.-M.K., M.Y.M., and M.S., unpublished data, May 2000). We have previously established that GVL effects in the EL4 leukemia model used here are completely dependent on GVH alloreactivity15 and are more potent when greater histoincompatibilities exist between donor and host.17 This GVL effect is mediated by CD8+ T cells without any apparent role for CD4+cells. The present studies show that the GVH alloresponses induced by host APCs can be exploited to mediate powerful GVL effects without GVHD.
Previous studies from our laboratory4 and the present results demonstrate that the strong host alloantigen–driven activation of a nontolerant donor T-cell population through host APCs, which results in elimination of normal and leukemic host-type hematopoietic cells, does not initiate GVHD when the administration of the DLI is delayed until 8 weeks after BMT. Similar observations were made by Kolb et al18 in a large animal model using lethal irradiation and TCD DLA-matched BMT. We previously demonstrated that DLI led to a loss of third-party CTL alloresponses when given to mixed chimeras and that this effect was dependent on the presence of host-type lymphohematopoietic cells because it did not occur when DLI were given to full chimeras.4 This result provided an indication that recipient APCs play an important role in inducing lymphohematopoietic GVH reactions (LGVHR) mediated by delayed DLI. The present studies document the capacity of this host APC-induced LGVHR to lead to powerful GVL effects.
To explain the confinement of GVH reactions to the lymphohematopoietic system in mixed chimeras receiving delayed DLI, we postulate that the conditioning regimen–induced inflammatory cascade (expression of adhesion molecules, chemokines, and proinflammatory cytokines) plays an important role in promoting the migration of GVH alloreactive donor T cells activated by host APCs in the lymphohematopoietic system into nonhematopoietic epithelial target tissues. The presence of host APCs at the time of conditioning and BMT contributes to the development of GVHD by this alloresponse.16 However, in the absence of such proinflammatory stimuli (ie, weeks or months following conditioning and T-cell–depleted BMT), the presence of host APCs and mixed hematopoietic chimerism permits the recipient to enjoy maximal DLI-mediated GVL effects not associated with GVHD. Apparently, the conditions at this late time are less conducive to the migration of activated alloreactive T cells into the GVHD target tissues. There is evidence in humans that the incidence of GVHD following DLI is inversely correlated to the time interval between BMT and DLI administration. However, in contrast to rodent study findings, in the clinical setting the development of GVHD is still one of the most prominent and feared complications following delayed administration of DLI. Several factors might account for this discrepancy. DLI studies in humans have often involved recipients of myeloablative conditioning, with or without T-cell depletion of the initial stem cell inoculum. Humans and large animals might display a more prolonged proinflammatory milieu following conditioning than rodents. In addition, a lack of depletion or incomplete depletion of human donor T cells in these studies would fail to satisfy the second condition discussed above for the ability to give DLI without GVHD (ie, the absence of T-cell alloreactivity from the initial stem cell transplant). Furthermore, dosage of T cells is an important issue. Mackinnon et al19showed that the administration of increasing numbers of DLI T cells allowed identification of a window whereby DLI could mediate GVL responses without causing overt GVHD. Finally, regulatory cells have been recently suggested to contribute to the resistance against development of GVHD in mice,20 21 a phenomenon that might not be operative or as effective in humans. However, studies we have performed with DLI in mixed chimeras prepared with nonmyeloablative conditioning do not demonstrate a role for regulatory cells in conferring resistance to GVHD following DLI (M.Y.M., M.S., manuscript in preparation), so the absence of a proinflammatory environment may in itself be sufficient to confer such resistance.
The above discussion underscores the importance of delaying DLI administration until the conditioning-induced proinflammatory milieu has disappeared and of ensuring the avoidance of GVH alloreactivity from the initial transplant to avoid GVHD from DLI. Although these principles from animal models have not yet been shown to allow the administration in humans of DLI to mixed chimeras prepared across HLA barriers without GVHD, published studies in HLA-identical transplants8 and preliminary data involving T-cell–depleted nonmyeloablative haploidentical transplants at our center are encouraging in this regard. However, treatment strategies relying on cell therapy alone or using nonmyeloablative conditioning followed by delayed DLI should be most effective in the treatment of more indolent malignancies because the tumor cytoreduction used in milder conditioning regimens might not be sufficient to contain the disease until DLI can be given after a sufficient delay to allow inflammation to subside.
Proof of the principle that DLI can mediate powerful GVL effects during relapse after myeloablative BMT was provided by the potent antitumor responses observed in rodents22,23 and in humans with relapsed CML6,7 receiving DLI following allogeneic BMT. In some patients, DLI has been shown to mediate GVL effects without inducing GVHD.19 However, other hematologic malignancies have been less amenable to GVL effects of DLI under similar circumstances. We postulate that the capacity of CML cells to function as professional APCs and the failure of other tumor types to do so may account for these observations. Our studies suggest that the provision of a host-type professional APC population in the form of mixed chimerism could augment the ability to achieve GVL effects from DLI in patients with tumors that have poor APC capacity. Results in patients with refractory lymphomas treated in our center following nonmyeloablative BMT that induces mixed chimerism are consistent with this possibility (see below).
Most currently used nonmyeloablative conditioning regimens result in the development of full donor chimerism early after hematopoietic cell transplantation.24,25 This result probably reflects the inclusion of T cells in the donor stem cell inoculum given immediately after conditioning. These T cells mediate early GVH responses and frequently induce GVHD,24-26 which may be a consequence of allowing the GVH response to occur in the proinflammatory, periconditioning period. Based on the results presented here, early achievement of full donor chimerism may not allow maximal GVL effects to be achieved. In contrast to the regimens cited above, mixed chimerism is routinely achieved in a nonmyeloablative regimen based on our murine nonmyeloablative protocol5 that includes in vivo T-cell depletion of the donor marrow in addition to the recipient.8,9 The goal of this protocol is to give DLI later, when conditioning-induced inflammation has subsided, to achieve GVL without GVHD. This outcome has been achieved in some patients on this protocol, despite the fact that the in vivo depletion of donor T cells has been incomplete.8,9 Striking remissions have been achieved in patients with advanced chemorefractory malignancies receiving DLI or slowly converting spontaneously to full chimerism after receiving this nonmyeloablative conditioning regimen.8 9 Alternative strategies to capitalize on the capacity of host APCs to induce maximal GVL effects could include approaches to restoring mixed chimerism in established full chimeras or the administration of host dendritic cells before DLI.
In conclusion, our data have demonstrated the advantage of establishing mixed chimerism to maximize DLI-dependent GVL effects (while avoiding GVHD) in the treatment of hematologic malignancies.
We thank Drs Henry Winn and David Scadden for critical reading of the manuscript.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2002-01-0023.
Supported by a research fellowship from the Deutsche Forschungsgemeinschaft (DFG-Ma 1664/2-1) (M.Y.M.) and by National Cancer Institute grant RO1 CA 79989.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Megan Sykes, Transplantation Biology Research Center, Bone Marrow Transplantation Section, Massachusetts General Hospital-MGH-East Bldg 149-5102, Harvard Medical School, Boston, MA 02129; e-mail: megan.sykes@tbrc.mgh.harvard.edu.



![Fig. 4. Superior DLI-mediated GVL effects in mixed chimeras are dependent on host MHC class I expression. / To evaluate the effect of expression of host MHC class I on hematopoietic cells on the GVL effect mediated by DLI, we established full donor chimeras (⋄, full), mixed chimeras (▿, B6+B10.A), or mixed chimeras in which host-type hematopoietic cells were deficient in host MHC class I expression (○, B6.β2m−/−+ B10.A). Following BMT, chimeric animals received DLI with or without EL4 leukemia cells (full + DLI [∗, n = 4]; B6 + B10.A + DLI [×, n = 3]; B6.β2m−/− + B10.A + DLI [+, n = 3]). Chimeras receiving no additional treatment after BMT were used as controls (⋄, full [n = 3]; ▿, B6 + B10.A [n = 3]; ○, B6.β2m−/− + B10.A [n = 2]). DLI was administered on day 56 followed by EL4 leukemia administration on day 63. (A) Survival of all groups. Leukemic wild-type mixed chimeras receiving DLI demonstrated significantly longer survival (▾, 9 of 10 surviving) compared to full donor chimeras (♦, 0 of 9 surviving MST 91 days). Leukemic B6.β2m−/− + B10.A chimeras revealed an intermediate result (●, 4 of 7 animals surviving). (B) Survival analysis in leukemic B6.β2m−/− + B10.A chimeras stratified according to peripheral blood chimerism levels in the monocytic lineage. Animals with high (▪, more than 40% B6.β2m−/− monocytes) or low (■) B6.β2m−/− hematopoiesis receiving DLI+EL4 are compared to wild-type B6 + B10.A chimeras receiving DLI + EL4 (▾). Full chimeras receiving DLI + EL4 (♦) are also shown for comparison. B6.β2m−/− + B10.A mixed chimeras with low B6.β2m−/− hematopoiesis receiving DLI and EL4 showed a significantly poorer survival than animals with high levels of B6.β2m−/− hematopoiesis (P < .0002).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/5/10.1182_blood-2002-01-0023/3/m_h81723073004.jpeg?Expires=1769561662&Signature=a68o57d5Is17iXDFwgfcSv0DreTeKe7e~lbC0xlWRWWBLC820f4nhV6TRdr2YYtuyii4E1d6F19t3t2uOb1wZ6521Zp39XtvoeXfTdxUp8fLdd9R1AhmV4BWnqRruilwXBohLEWqItsIoUB2voZL7rTIdMPwLNIWTFE~D-S6BQKVtYQ2ea78yVRp8wqgHNxNxL2BIOyRHFMNmx7mftE90n4bJYG85GNzFjbqk~3ORKt4p~mpctIq2Iob7n1uNGQm9B1LiMwcxWAmkOm1j6f4E7gBKkyO-apOsxYNJIJN1fyU5gWcVAulaynXNLNlujQmaTfasCnfGUvvpyPNr~BbzA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 4. Superior DLI-mediated GVL effects in mixed chimeras are dependent on host MHC class I expression. / To evaluate the effect of expression of host MHC class I on hematopoietic cells on the GVL effect mediated by DLI, we established full donor chimeras (⋄, full), mixed chimeras (▿, B6+B10.A), or mixed chimeras in which host-type hematopoietic cells were deficient in host MHC class I expression (○, B6.β2m−/−+ B10.A). Following BMT, chimeric animals received DLI with or without EL4 leukemia cells (full + DLI [∗, n = 4]; B6 + B10.A + DLI [×, n = 3]; B6.β2m−/− + B10.A + DLI [+, n = 3]). Chimeras receiving no additional treatment after BMT were used as controls (⋄, full [n = 3]; ▿, B6 + B10.A [n = 3]; ○, B6.β2m−/− + B10.A [n = 2]). DLI was administered on day 56 followed by EL4 leukemia administration on day 63. (A) Survival of all groups. Leukemic wild-type mixed chimeras receiving DLI demonstrated significantly longer survival (▾, 9 of 10 surviving) compared to full donor chimeras (♦, 0 of 9 surviving MST 91 days). Leukemic B6.β2m−/− + B10.A chimeras revealed an intermediate result (●, 4 of 7 animals surviving). (B) Survival analysis in leukemic B6.β2m−/− + B10.A chimeras stratified according to peripheral blood chimerism levels in the monocytic lineage. Animals with high (▪, more than 40% B6.β2m−/− monocytes) or low (■) B6.β2m−/− hematopoiesis receiving DLI+EL4 are compared to wild-type B6 + B10.A chimeras receiving DLI + EL4 (▾). Full chimeras receiving DLI + EL4 (♦) are also shown for comparison. B6.β2m−/− + B10.A mixed chimeras with low B6.β2m−/− hematopoiesis receiving DLI and EL4 showed a significantly poorer survival than animals with high levels of B6.β2m−/− hematopoiesis (P < .0002).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/5/10.1182_blood-2002-01-0023/3/m_h81723073004.jpeg?Expires=1769561663&Signature=tMsMpVzWO8Z1llhAIpjevvgE-Ru8wUir80CyzsrXm2OKGl10sLmqMvGkNkq5LvyQenMZnOKjXU8ljytKiXVvj9P-9oC4ftdOlX2bOyQvNpSKFA9n7zDSZ74LuvHuvMcvOAv1PB53oFEBtGfEOU2gWqOr~yObcVylHFa5D3h81W6eg~RPaaVjraLmcBkCn9SCN2DrngPqtRD2MDLUkPtbpmRxyv2THz6X5hvQ-HHqWeupP1GYejwZk9nGKc3OrEI3FkJamX69ckw715Aqda4vuFXUvtAvrT73HgWex6OfVeoml4k9wPOO~O8R~YvOL5sKiyfNHj6vRNw5O~yBfWyYjA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)