Abstract
Wilms tumor gene product WT1 and proteinase 3 are overexpressed antigens in acute myeloid leukemia (AML), against which cytotoxic T lymphocytes can be elicited in vitro and in murine models. We performed this study to investigate whether WT1- and proteinase 3-specific CD8 T cells spontaneously occur in AML patients. T cells recognizing HLA-A2.1-binding epitopes from WT1 or proteinase 3 could be detected ex vivo in 5 of 15 HLA-A2–positive AML patients by interferon-γ (IFN-γ) ELISPOT assay and flow cytometry for intracellular IFN-γ and in 3 additional patients by flow cytometry only. T cells producing IFN-γ in response to proteinase 3 were further characterized in one patient by 4-color flow cytometry, identifying them as CD3+CD8+CD45RA+ CCR7−T cells, resembling cytotoxic effector T cells. In line with this phenotype, most of the WT1- and proteinase-reactive T cells were granzyme B+. These results provide for the first time evidence for spontaneous T-cell reactivity against defined antigens in AML patients. These data therefore support the immunogenicity of WT1 and proteinase 3 in acute leukemia patients and the potential usefulness of these antigens for leukemia vaccines.
Introduction
Leukemia-specific T cells can be generated in vitro from most patients with acute myeloid leukemia (AML),1-3and there is evidence that antileukemic cytotoxicity can play an important role in disease control.4,5 HLA class I antigens, multiple adhesion molecules, and costimulatory molecules are expressed at high levels on AML blasts at diagnosis and at relapse.6 7 These findings illustrate the potential for applying T-cell–based immunotherapy in the treatment of AML.
Two particularly interesting candidate antigens for use in vaccination strategies and adoptive T-cell therapy in AML are the transcription factor WT1 and the serine protease proteinase 3. We and others8,9 found WT1 to be markedly overexpressed in most AML blasts and proteinase 3 to be overexpressed in AML blasts FAB M2 to M5.10 In vitro studies show that the inhibition of either antigen leads to the differentiation of leukemic cells and underline their key role in tumor promotion.11,12 In animal models, vaccination with WT1 peptides or plasmids resulted in vigorous T-cell response induction and rejection of WT1-expressing tumor cells.13-15 HLA-A2.1–restricted epitopes from both antigens were identified, and cytotoxic T cells (CTLs) against these epitopes, which lyse myeloid leukemic blasts, could be generated from healthy controls.16-18
WT1 and proteinase 3 are, like many other tumor-associated antigens, not leukemia specific, but they are expressed at low levels in certain nonmalignant cells.19-21 Therefore, immunologic tolerance to these antigens and induction of autoimmunity caused by cytotoxic T cells are important issues to consider when attempting to elicit immune responses against these antigens.
There is evidence that WT1 is immunogenic in AML because WT1-specific antibodies were identified in 15% to 25% of AML patients.15,22 The demonstration of WT1-specific antibody responses implies that WT1-specific CD4 T-helper cells should be present in these patients. Evidence that proteinase 3 is immunogenic was recently obtained in patients with chronic myeloid leukemia (CML) by tetramer analysis.23
The current study was undertaken to analyze whether WT1- and proteinase 3–specific CD8 T cells naturally occur in patients with AML and in healthy subjects. We report here that CD8 T-cell responses against previously characterized HLA-A2–binding epitopes were detected in a significant proportion of patients in unstimulated peripheral blood mononuclear cells (PBMCs) by 2 independent assays, namely ELISPOT interferon-γ (IFN-γ) secretion assay and flow cytometry detecting intracellular IFN-γ accumulation (IC IFN-γ).
Patients, materials, and methods
Patients and healthy controls
PBMCs from HLA-A2–positive AML patients and from HLA-A2–positive healthy subjects were collected and cryopreserved. Patients were required to have lymphocyte counts of 0.5/nL or higher and an absence of leukemic blasts in peripheral blood smears at the time of sampling. This investigation had been approved by the Institutional Ethics Committee, and informed consent was obtained from all patients and healthy subjects before blood sampling.
IFN-γ ELISPOT assay
The assay was performed as described earlier.24 In brief, 1 × 106 PBMCs were incubated in a concentration of 1.67 × 105 cells/well in 6 wells with 10 μg/mL relevant peptides for 20 hours in antihuman IFN-γ antibody (NIB42, 15 μg/mL; BD PharMingen, Hamburg, Germany)–coated plates. All samples were tested against the HLA-A2.1–binding epitopes WT1 126-134 (RMFPNAPYL16,17), WT1 187-195 (SLGEQQYSV16), proteinase 3 169-177 (VLQELNVTV, named PR-118), without peptide (negative control), and with influenza matrix protein 58-66 (GILGFVFTL) and pokeweed mitogen (PWM) 1000 ng/mL, (Sigma, Munich, Germany) as positive controls. The peptides were synthesized and kindly provided by H. G. Rammensee (University of Tubingen, Germany). For the detection of spots, a biotin-labeled antihuman IFN-γ antibody (4S.B3, 0.312 μg/mL; BD PharMingen) was used. Spots were counted using an automated ELISPOT reader (AID, Strassberg, Germany).
Flow cytometric analysis
Analysis was performed as described previously.25 In brief, PBMCs (2 × 106) were incubated without or with 10 μg/mL peptide. After 2 hours, 10 μg Brefeldin A (Sigma, Deisenhofen, Germany) was added, and after 16 additional hours, PBMCs were stained by incubation with fluorescein-conjugated monoclonal antibodies against CD8, CD3, CD45RA, CCR7, and CXCR4 (Becton Dickinson, Heidelberg, Germany). Afterward, FACS Lysing Solution and FACS Permeabilization Solution (Becton Dickinson) were added, and IFN-γ and granzyme B were stained intracellularly using fluorescein-conjugated monoclonal antibodies (Becton Dickinson, Heidelberg; Hoelzel Diagnostica, Cologne, Germany). Data acquisition was performed on FACSCalibur and was analyzed using CellQuest Software (Becton Dickinson).
Statistical analysis
Student t test was calculated to determine whether there was a statistically significant difference in the number of IFN-γ–secreting T cells in response to influenza matrix protein between AML patients and healthy controls.
Results
WT1- and proteinase 3–specific T cells can be detected in some AML patients
Fifteen HLA-A2–positive patients with AML were analyzed for the presence of circulating T cells reactive with WT1 and proteinase 3 using ELISPOT assay. Clinical data are given in Table1. No or few T cells secreted IFN-γ in the absence of peptide. The median number of spots in unstimulated PBMCs (background) was low and in the same range as observed in healthy subjects (AML patients: 10 spots in 106 PBMCs; range, 0-33 spots; Figure 1B,D,F) (healthy subjects: 10.5 spots in 106 PBMCs; range, 1-24 spots; Figure 1A,C,E). A T-cell response was considered positive if the number of spots in peptide-exposed PBMCs was 2-fold or more higher than the number of spots in unstimulated PBMCs and if there was a minimum of 10 peptide-specific spots in 106 PBMCs (after subtracting the number of spots in unstimulated PBMCs). In 5 of 15 patients, a T-cell response to epitopes from WT1 or proteinase 3 was observed with frequencies between 10 and 61 peptide-specific T cells in 106 PBMCs. Figure 1B shows T-cell responses to WT1 126 to 134 (positive responses in patient 2 and 14), Figure 1D to WT1 187 to 195, and Figure 1F to proteinase 3 (positive responses in patients 10, 12, and 13). In contrast, none of 15 HLA-A2–positive healthy subjects had a T-cell response to these antigens (Figure 1A,C,E).
T-cell responses to WT1 and proteinase 3 can be detected by ELISPOT assay.
Unstimulated PBMCs were analyzed for recognition of specific epitopes WT1 126-134 (A), WT1 187-194 (B), and proteinase 3 (C) by IFN-γ ELISPOT assay in 15 HLA-A2+ healthy subjects and in 15 AML patients. Values are expressed as number of spots per 106 PBMCs (white bars, number of spots in unstimulated PBMCs; black/striped bars, number of spots in response to peptide). Responses, which exceeded 2-fold or more the number of spots in unstimulated PBMCs and had a minimum of 10 peptide-specific spots in 106 PBMCs (after subtracting the number of spots in unstimulated PBMCs) were considered peptide specific and were shown as striped bars. The proportion of CD3 CD8 T cells in whole PBMCs, as determined by flow cytometry analyses, is indicated in AML patients (B).
T-cell responses to WT1 and proteinase 3 can be detected by ELISPOT assay.
Unstimulated PBMCs were analyzed for recognition of specific epitopes WT1 126-134 (A), WT1 187-194 (B), and proteinase 3 (C) by IFN-γ ELISPOT assay in 15 HLA-A2+ healthy subjects and in 15 AML patients. Values are expressed as number of spots per 106 PBMCs (white bars, number of spots in unstimulated PBMCs; black/striped bars, number of spots in response to peptide). Responses, which exceeded 2-fold or more the number of spots in unstimulated PBMCs and had a minimum of 10 peptide-specific spots in 106 PBMCs (after subtracting the number of spots in unstimulated PBMCs) were considered peptide specific and were shown as striped bars. The proportion of CD3 CD8 T cells in whole PBMCs, as determined by flow cytometry analyses, is indicated in AML patients (B).
WT1- and proteinase 3–specific T cells are present in the CD3 CD8 T-cell fraction and are CD45RA+CCR7− granzyme B+ CXCR4−
We next analyzed T-cell responses to each WT1 and the proteinase 3 peptide by intracellular IFN-γ accumulation detected by flow cytometry (IC IFN-γ) in 12 of 15 AML patients. In the remaining 3 patients, not enough material was available to perform IC IFN-γ. In unstimulated PBMCs, 0.04% to 0.64% (median, 0.05%) of the CD3 CD8 T cell subpopulation contained intracellular IFN-γ. A response was considered positive if the percentage of peptide-specific IFN-γ–producing CD3 CD8 T cells was 2-fold or more higher compared with the percentage of IFN-γ–producing CD3 CD8 T cells in the absence of peptide and if there was a minimum of 0.05% peptide-specific IFN-γ–producing CD3 CD8 T cells in 106PBMCs (after subtracting the percentage of IFN-γ–producing CD3 CD8 T cells in the absence of peptide).
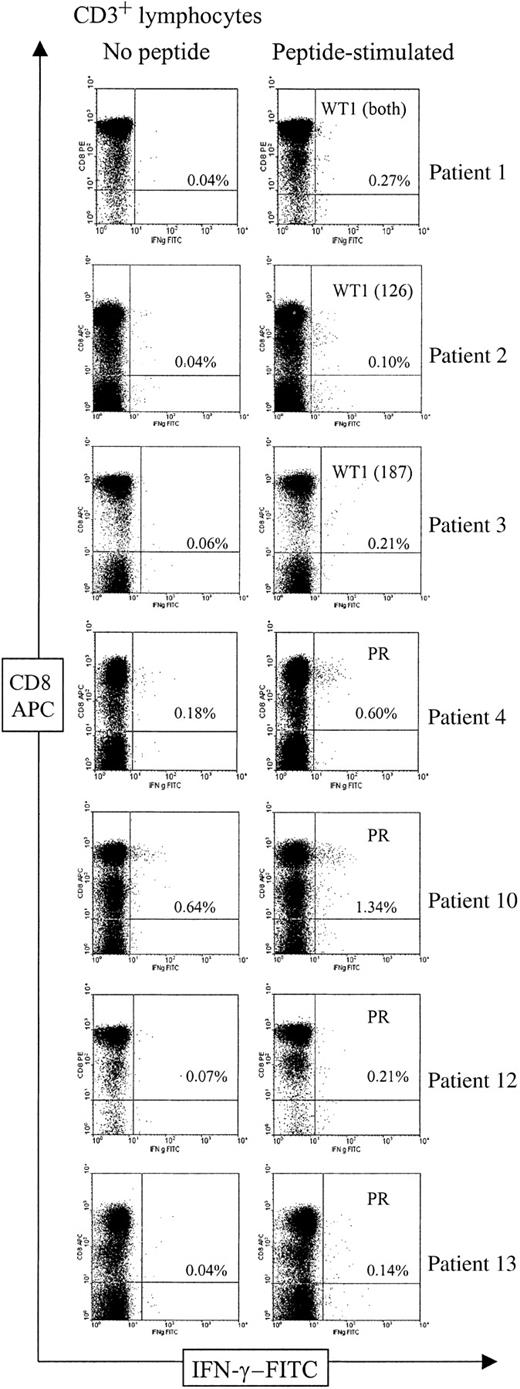
CD3 CD8 T cells specifically producing IFN-γ when exposed to WT1 or proteinase 3 epitopes were detected in 7 patients; frequencies were between 0.06% and 0.70% of the CD3 CD8 T-cell population (Figure2, Table2). None of the patients had a positive response to all 3 peptides from the WT1 and proteinase analyzed. Therefore, the peptides to which no T-cell responses were seen served as additional negative controls in all patients (Table 2). The 7 patients with a positive response in IC IFN-γ included all 4 patients with specific T-cell responses to the same peptides detected by ELISPOT assay in which enough material for IC IFN-γ was available (patients 2, 10, 12, 13; Figure 1, Table 1). Strikingly, frequencies detected by IC IFN-γ were severalfold higher in these 4 patients (median, 10-fold; range, 6- to 35-fold) compared with those detected by ELISPOT assay. Two of the 3 other patients with positive results in IC IFN-γ also had a borderline response in the ELISPOT assay with 25 WT1 187-195–specific spots, but only 1.8-fold higher than background (patient 3, Figure 1B), and with 7 proteinase 3–specific spots (patient 4, Figure 1C). In the 5 remaining patients (patients 5, 7, 8, 9, 11), no specific T cells could be detected by IC IFN-γ (Table 2), confirming the negative results obtained by the ELISPOT assay. Taken together, positive and negative T-cell responses to WT1 and proteinase 3 epitopes detected by ELISPOT assay and ICC were highly concordant with a severalfold higher sensitivity of the ICC, making nonspecific effects unlikely. (Tables 1, 2; Figures 1, 2).
Flow cytometric analysis of T-cell responses to WT1 126-134, WT1 187-195, and proteinase 3 in AML patients.
Analysis of PBMCs was performed by 3-color flow cytometry. Figures show the CD8/IFN-γ profile of CD3-gated lymphocytes in unstimulated (left) and peptide-exposed (right) PBMCs in the 7 patients with specific T-cell responses to the indicated peptides. The T-cell response in patient 1 was analyzed against both WT1 peptides in one sample. Frequencies of CD3 CD8 T cells that produced IFN-γ are shown as percentages of the total number of CD3 CD8 T cells. APC indicates allophycocyanin; FITC, fluorescein isothiocyanate.
Flow cytometric analysis of T-cell responses to WT1 126-134, WT1 187-195, and proteinase 3 in AML patients.
Analysis of PBMCs was performed by 3-color flow cytometry. Figures show the CD8/IFN-γ profile of CD3-gated lymphocytes in unstimulated (left) and peptide-exposed (right) PBMCs in the 7 patients with specific T-cell responses to the indicated peptides. The T-cell response in patient 1 was analyzed against both WT1 peptides in one sample. Frequencies of CD3 CD8 T cells that produced IFN-γ are shown as percentages of the total number of CD3 CD8 T cells. APC indicates allophycocyanin; FITC, fluorescein isothiocyanate.
The highest T-cell frequency was observed in patient 10 against proteinase 3. This allowed us further phenotypic characterization of specific T cells for expression of CD45RA, CCR7, CXCR4, and granzyme B by costaining with the corresponding monoclonal antibodies simultaneously to the measurement of IFN-γ production. As illustrated in Figure 3, in 4 parallel assays, between 0.47% and 0.66% of all CD3 CD8 T cells specifically produced IFN-γ in response to the proteinase 3 peptide (sample taken 8 months after diagnosis, analyzed in 4 parallel assays). Most proteinase 3–specific IFN-γ–producing CD3 CD8 T cells had the phenotype CD45RA+CCR7− (Figure 3D,F), which was recently shown to characterize differentiated effector T cells able to exert direct lytic activity.26 27 In accordance with this phenotype, a considerable fraction of these T cells contained intracellular granzyme B (Figure 3B). Similarly, in patients 2 and 3, more than 90% of the CD3 CD8 T cells secreting IFN-γ in response to the WT1 peptides contained granzyme B (data not shown). The proteinase 3-specific T cells in patient 10 did not express CXCR4, the receptor for the chemokine stromal cell-derived factor (SDF-1) (Figure 3H).
Most CD3 CD8 T cells specifically producing IFN-γ in response to proteinase 3 peptide are Granzyme B+CD45RA+ CCR7− CXCR4−.
Analysis of PBMCs was performed by 4-color flow cytometry in patient 10. (A, B) Granzyme B (Gra B)/IFN-γ profile of CD3/CD8-gated lymphocytes. (C, D) CD45RA/IFN-γ profile of CD3/CD8-gated lymphocytes. (E, F) CCR7/IFN-γ profile of CD3/CD8-gated lymphocytes. (G, H) CXCR4/IFN-γ profile of CD3/CD8-gated lymphocytes. Frequencies of CD3 CD8 T cells that produced IFN-γ are shown in each quadrant as a percentage of the total number of CD3 CD8 T cells.
Most CD3 CD8 T cells specifically producing IFN-γ in response to proteinase 3 peptide are Granzyme B+CD45RA+ CCR7− CXCR4−.
Analysis of PBMCs was performed by 4-color flow cytometry in patient 10. (A, B) Granzyme B (Gra B)/IFN-γ profile of CD3/CD8-gated lymphocytes. (C, D) CD45RA/IFN-γ profile of CD3/CD8-gated lymphocytes. (E, F) CCR7/IFN-γ profile of CD3/CD8-gated lymphocytes. (G, H) CXCR4/IFN-γ profile of CD3/CD8-gated lymphocytes. Frequencies of CD3 CD8 T cells that produced IFN-γ are shown in each quadrant as a percentage of the total number of CD3 CD8 T cells.
In patient 10, proteinase 3–specific T cells could be repeatedly demonstrated by IC IFN-γ, with frequencies between 0.15% and 0.70% of the CD3 CD8 T-cell population in 3 samples for 4 months while the patient was in ongoing complete remission (Figure4).
Follow-up of CD3 CD8 T cells reactive with the proteinase 3 peptide in patient 10.
Analysis of PBMCs was performed by 3-color flow cytometry in patient 10 at 3 time points as indicated. Figures show the CD8/IFN-γ profile of CD3-gated lymphocytes. Frequencies of CD3 CD8 T cells that produced IFN-γ are shown as percentages of the total number of CD3 CD8 T cells.
Follow-up of CD3 CD8 T cells reactive with the proteinase 3 peptide in patient 10.
Analysis of PBMCs was performed by 3-color flow cytometry in patient 10 at 3 time points as indicated. Figures show the CD8/IFN-γ profile of CD3-gated lymphocytes. Frequencies of CD3 CD8 T cells that produced IFN-γ are shown as percentages of the total number of CD3 CD8 T cells.
T-cell responses to influenza in patients with AML and in healthy subjects
The T-cell response to the HLA-A2.1–restricted influenza matrix protein epitope 58-66 was analyzed by ELISPOT assay to assess potential T-cell immunodeficiency in the AML patients (Figure5). We could detect influenza-specific T-cell responses in 8 of 14 AML patients, with a median frequency of 46 peptide-specific IFN-γ–secreting T cells per 106 PBMCs (range, 20-258; n = 8). This was not significantly different from the healthy control group, in which 10 of 15 subjects had a detectable T-cell response with a median frequency of 49 peptide-specific IFN-γ–secreting T cells per 106 PBMCs (range, 13-307; n = 10; P = .4), suggesting that the AML patients had neither an unspecific up-regulation nor a general deficiency in T-cell function following cytotoxic therapy.
T-cell responses against influenza matrix protein epitope 56-64 in AML patients and healthy subjects.
Unstimulated PBMCs were analyzed for the recognition of influenza matrix protein 58-66 peptide by IFN-γ ELISPOT assay in 14 HLA-A2+ AML patients and 15 HLA-A2+ healthy subjects. Values are expressed as number of spots per 106 PBMC (white bars number of spots in unstimulated PBMCs; black/striped bars number of spots in response to peptide). Responses, which exceeded the number of spots in unstimulated PBMCs by 2-fold or more and had a minimum of 10 peptide-specific spots in 106 PBMCs (after subtracting the number of spots in unstimulated PBMCs) were considered peptide specific and are shown as striped bars.
T-cell responses against influenza matrix protein epitope 56-64 in AML patients and healthy subjects.
Unstimulated PBMCs were analyzed for the recognition of influenza matrix protein 58-66 peptide by IFN-γ ELISPOT assay in 14 HLA-A2+ AML patients and 15 HLA-A2+ healthy subjects. Values are expressed as number of spots per 106 PBMC (white bars number of spots in unstimulated PBMCs; black/striped bars number of spots in response to peptide). Responses, which exceeded the number of spots in unstimulated PBMCs by 2-fold or more and had a minimum of 10 peptide-specific spots in 106 PBMCs (after subtracting the number of spots in unstimulated PBMCs) were considered peptide specific and are shown as striped bars.
AML patients with WT1- or proteinase 3–specific T cells have no evidence for hematopoietic or renal dysfunction
Six of 8 patients with specific T cells (patients 1, 2, 3 to WT1, and patients 4, 10, 12 to proteinase 3) were in complete remission at time of analysis (Table 1). Remarkably, the other 2 patients (patients 13, 14) with a T-cell response to proteinase 3 and WT1, respectively, had newly diagnosed secondary AML with 20% to 40% blasts in bone marrow and absence of blasts in PBMCs at the time of T-cell analysis.
Because WT1 is transiently expressed at low levels in normal hematopoietic progenitor cells and proteinase is transiently expressed at low levels in cells of the granulocytic and monocytic lineage, it is important to note that all 6 patients in complete remission with T-cell responses to WT1 or proteinase 3 had normal WBC counts and differentials. Furthermore, considering the low-level expression of WT1 in renal cells, it is of relevance that 4 patients with WT1-specific T cells had no evidence of renal dysfunction.
Discussion
This study provides evidence that CD8 T-cell responses to the leukemia-associated antigens WT1 and proteinase 3 can develop spontaneously in AML patients. The absence of such T-cell responses in healthy subjects suggests that the T cells were elicited in response to leukemic blasts. Our observation is in accordance with findings of 2 other recent studies in AML patients showing that antibodies to WT1 could be detected in 15% to 25% of AML patients but only in 2% of healthy subjects.15,22 To our knowledge, other groups have not studied the immunity to proteinase 3 in AML patients so far. However, data from CML patients suggest that proteinase 3 is highly immunogenic because specific T-cell responses to proteinase 3 could be identified using HLA-A2 peptide tetramers in most patients with a cytogenetic response.23
Whether the WT1- and proteinase 3–reactive T cells detected by assays measuring specific IFN-γ production mediate antileukemic cytotoxicity cannot directly be assessed ex vivo. We could, however, provide further insight into this question by phenotypically characterizing the ex vivo proteinase 3–reactive T cells in one patient, identifying them as mostly CD45RA+CCR7−. Recent reports have defined CD45RA+CCR7− T cells as differentiated effector T cells expressing receptors for migration to inflamed tissues and able to exert vigorous target cell lysis.26,27 The cytotoxic potential of the proteinase 3–reactive T cells is further supported by the presence of intracellular granzyme B. Proteinase 3–reactive T cells, however, lacked CXCR4, a chemokine receptor mediating homing of hematopoietic stem cells to bone marrow,28 which is also found on a subset of differentiated T cells.29 The ability of the circulating proteinase 3–specific T cells to migrate to the bone marrow is therefore unclear.
Frequencies of T cells secreting IFN-γ in response to WT1 or proteinase 3 determined by ELISPOT assay were severalfold lower than those detected by flow cytometry. This finding is in contrast to earlier results from our group25 showing corresponding frequencies of influenza-reactive T cells determined by ELISPOT assay and IC IFN-γ flow cytometry. One possible explanation for these discrepancies may be a lower sensitivity of the ELISPOT assay to detect T cells, producing low amounts of IFN-γ. This hypothesis is supported by our observation that the mean fluorescence intensity of intracellular IFN-γ of the influenza-specific T cells in this previous study25 was approximately 2- to 3-fold higher than the mean fluorescence intensity of intracellular IFN-γ of the WT1- or proteinase 3–specific T cells in this study. One possible explanation for this lower amount of IFN-γ produced per cell might be a lower functional avidity of T cells, as suggested by a recent study.30
Immune responses in leukemia patients may be hampered by leukemia-specific immunosuppression and general immunosuppression, attributed to T-cell dysfunction induced by leukemia blasts31 and by cytotoxic therapy.32 Patients with advanced tumors often show a diminished T-cell response. Compared with healthy subjects, patients with advanced melanoma and colorectal cancer had lower precursor frequencies to an influenza peptide.33 34 Remarkably, in our study, similar ex vivo T-cell responses to influenza are detected in healthy subjects and AML patients, suggesting that no severe general suppression of T-cell reactivity is present in our patients with no or low leukemic burden despite recent chemotherapy.
When vaccinating against antigens, which are also expressed at low levels in nonmalignant cells, the concern arises that CTLs may damage healthy tissue. Proteinase 3 is found in cells of the monocytic and granulocytic lineage and is the target of anticytoplasmic antibodies detected in Wegeners granulomatosis.19 WT1 is expressed during fetal development, but low levels of expression are also found in some adult tissue, including renal and germ line cells20 and a transient expression in CD34+hematopoietic stem cells.21 In our study no evidence for autoimmunity against hematopoietic or renal cells in patients with WT1 and proteinase 3 responses was found. This is in accordance with several other preclinical and clinical reports. In animal models in which WT1-specific T cells could be induced by vaccination, no signs of auto-aggression were observed.13-15 WT1-specific and proteinase 3–specific CTLs were shown to selectively lyse leukemic blasts but not normal hematopoietic progenitor cells.16-18In addition, in leukemia patients with antibodies to WT1 or T-cell responses to proteinase 3, no auto-immune phenomena were reported.22 23 Taken together these data suggest that the expression level of WT1 and proteinase 3 in normal cells is not sufficient to elicit specific T-cell responses or to be recognized by specific T cells. This second conclusion has to be drawn with caution, however, because we do not know whether the WT1- or proteinase 3–specific T cells detected in our study were able to lyse leukemic blasts or normal cells in vivo. In summary the results of our study provide further evidence for the immunogenicity of WT1 and proteinase 3 and support the potential usefulness of these antigens for T-cell immunotherapy.
We thank Sandra Bauer for excellent technical assistance.
Prepublished online as Blood First Edition Paper, May 24, 2002; DOI 10.1182/blood-2002-01-0163.
Supported by Deutsche Forschungsgemeinschaft grant Sche 478/1-3.
C.S. and A.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ulrich Keilholz, Universitätsklinikum Benjamin-Franklin, Medizinische Klinik III, Hämatologie, Onkologie und Transfusionsmedizin, Hindenburgdamm 30, 12200 Berlin, Germany.