The capacity of the HIV-1 envelope glycoprotein gp120 to induce intracellular signals is thought to contribute to HIV-1 pathogenesis. Here, we report that gp120 binding resulted in activation of the mitogen-activated protein kinase (MAPK) in CD4+lymphocytes prestimulated through their T-cell receptor (TCR). However, gp120 did not activate this pathway in either freshly isolated quiescent T cells or nonproliferating CD4+ lymphocytes prestimulated with the interleukin-7 (IL-7) cytokine. This response was not solely dependent on proliferation per se because proliferating IL-7–prestimulated umbilical cord (UC)–derived T lymphocytes did not exhibit significant MAPK activation upon gp120 binding. Nevertheless, like peripheral blood lymphocytes, MAPK recruitment was induced by gp120 in UC T cells following TCR prestimulation. The lack of a gp120-mediated signaling response was not due to decreased gp120 receptor levels; CD4 expression was modified neither by IL-7 nor by TCR engagement, and high levels of functional CXCR4 were present on IL-7–treated lymphocytes. In addition to CD4 and CXCR4, recent evidence suggests that glycosphingolipids in raft microdomains serve as cofactors for HIV-1 fusion. The ganglioside GM1, a marker of rafts, was augmented in TCR-stimulated but not IL-7–stimulated T lymphocytes, and disruption of rafts inhibited gp120-induced signaling. Thus, stimulation of a mitogenic pathway by gp120 appears to require receptor binding in the context of membrane microdomains. These studies reveal a mechanism via which gp120 may differentially modulate the fate of activated and quiescent T cells in vivo.
Introduction
HIV-1 infection is initiated by the fusion of the viral envelope with the cell membrane of the target cell. This process involves the binding of the glycoprotein (gp)120 surface component of the viral envelope glycoproteins with CD4 and either the CXCR4 or CCR5 chemokine coreceptor.1-5 Thus, the interaction of gp120 with CD4 and a coreceptor is a critical step in HIV-1 infection. However, some of the adverse effects observed in HIV-1–infected individuals are probably not directly related to viral infection. Indeed, one of the hallmarks of AIDS is the depletion of infected as well as uninfected CD4+ T cells,6 7 raising the question of whether virions and/or viral proteins contribute directly to the pathogenic features of HIV-1.
Notably, the interaction of virion-associated gp120 with cell surface CD4 and CXCR4/CCR5 can occur in uninfected cells. Specifically, gp120/gp41 complexes on infected cells may interact with receptor molecules expressed on noninfected cells. Moreover, gp120 proteins shed from both infected cells and HIV-1 virions may bind to uninfected CD4+ cells.8 A direct role for the HIV-1 envelope glycoprotein in the pathogenesis of the disease has been suggested by studies demonstrating that gp120 can itself transduce intracellular signals.9 These signals are thought to be mediated via interactions of the HIV-1 envelope with both CD4 and CXCR4/CCR5 receptors.
Binding of stromal cell–derived factor 1(SDF-1) to its receptor CXCR4 results in the activation of multiple signaling pathways. SDF-1 treatment elicits activation of G proteins and calcium flux.10 Additionally, stimulation of this chemokine receptor results in a transient activation of MAPK, phosphatidylinositol 3-kinase, Pyk2, and Janus kinases/signal transducers and activators of transcription (Jak/STAT) pathways. Notably, many of these same signaling intermediates are also activated by CXCR4-specific gp120 proteins (see review by Stantchev and Broder11).
Delineation of gp120-induced signaling pathways may provide important insights into the mechanisms of HIV-mediated immune dysregulation. Nevertheless, the vast majority of reported studies have been performed in transformed cell lines, precluding any assessment of whether gp120-induced recruitment of transduction cascades is regulated by the activation status of these T cells. Here, we demonstrate that gp120-mediated signaling is modulated by the activation state of the T cell; gp120-induced MAPK activation is observed in T cells prestimulated through their antigen receptor but not in quiescent T cells. Differential recruitment of a mitogenic pathway in quiescent and activated T lymphocytes may be one mechanism via which HIV-1 contributes to the development of immune dysregulation in HIV-1–infected individuals.
Materials and methods
Antibodies and reagents
The following reagents were used: αCD4 mAb (13B8.2; kindly provided by Sanofi, Montpellier, France); αCD3 mAb (UCHT1; a generous gift from D. Cantrell, Imperial Cancer Research Fund, London, United Kingdom); αCD28 mAb (9.3; kindly provided by C. June, University of Pennsylvania, Philadelphia, PA); αZAP-70 mAb (kindly provided by A. Weiss, University of California, San Francisco); polyclonal antibody (pAb) recognizing the dually phosphorylated T183Y185 form of Erk1/Erk2 (antiactive MAPK; Cell Signaling Technology, Beverly, MA); αErk2 mAb (Transduction Laboratories, Lexington, KY); phycoerythrin (PE)-conjugated αCXCR4 mAb (12G5; Pharmingen, San Diego, CA); fluorescence-conjugated αCD4, αCD25, and isotype-matched control mAbs (Immunotech, Marseille, France); fluorescein isothiocyanate (FITC)–conjugated cholera toxin, methyl-β-cyclodextrin (MβCD), propidium iodide, and EGTA (ethyleneglycoltetraacetic acid; Sigma Chemical, St Louis, MO); stromal cell–derived factor 1 (SDF-1; PeproTech, London, United Kingdom); Bordella pertussis toxin (PTX; Calbiochem, La Jolla, CA); and carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR). Recombinant HIVSF2 envelope glycoprotein gp120 and a goat polyclonal αgp120 were generously provided by Chiron (Emeryville, CA). Interleukin-7 (IL-7) and IL-2 were obtained from PeproTech and Chiron, respectively. All cell culture reagents were obtained from Gibco Invitrogen (Auckland, New Zealand).
Cell isolations and culture conditions
Adult peripheral blood (APB), obtained from healthy adult donors, and umbilical cord (UC) blood, obtained immediately after delivery of full-term infants, were collected in heparinized tubes. CD4+ T cells were purified by negative selection using tetrameric complexes in which one antibody recognizes a surface antigen on B cells, monocytes, natural killer (NK) cells, or CD8+ cells, and the other recognizes glycophorin A on the surface of red blood cells (RosetteSep; StemSep Technologies, Vancouver, BC, Canada). Non-CD4+ T cells were then pelleted upon Ficoll-Hypaque (Sigma, St Louis, MO) separation. The purity of each cell isolation was monitored on a FACScalibur (Becton Dickinson, San Jose, CA) following staining with FITC-conjugated αCD3 and PE-conjugated αCD4 mAbs. Lymphocytes were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), penicillin, and streptomycin in the absence or presence of recombinant IL-7 (10 ng/mL). Alternatively, cells were stimulated with immobilized αCD3 and αCD28 mAbs (1 μg/mL) and after 2 days, cultured in the presence of recombinant IL-2 (100 U/mL). In some experiments, TCR-activated T cells were cultured in the presence of either PTX (100 ng/mL) for 16 hours or EGTA (2 mM) for 20 minutes prior to MAPK activation assays.
The Jurkat T-cell line E6-1, passaged for more than 2 years in the laboratory, and the Jurkat clone 77-6.8, generously provided by Dr K. A. Smith (New York, NY), were maintained in RPMI 1640 medium supplemented with 10% FCS.
Flow cytometry for surface markers and cell cycle analysis
To detect expression of CD4, CXCR4 and CD25, cells were incubated for 20 minutes on ice with PE-conjugated αCD4, αCXCR4, and αCD25 mAbs, respectively. Cell surface GM1 levels were assessed using FITC-conjugated cholera toxin. Cells were then analyzed on a FACSCalibur fluorescence-activated cell sorter (Becton Dickinson), and data analysis was performed using Cell Quest software (Becton Dickinson).
The percentage of cells in the S-G2/M phases of the cell cycle was determined by propidium iodide (PI) staining. At the indicated time points, cells were resuspended in PI (50 μg/mL) diluted in phosphate-buffered saline (PBS) with 5% glycerol and 0.1% Triton X-100 and incubated for 15 minutes prior to analysis. Cell cycle was analyzed on the FL2-A wavelength after gating out signals due to cell debris.
CFSE labeling
Freshly isolated CD4+ lymphocytes were washed and resuspended in PBS at a concentration of 2.5 × 106cells/mL for labeling with the fluorochrome CFSE. Cells were incubated with CFSE at a final concentration of 2.5 μM for 3 minutes at room temperature. Labeling was terminated by the addition of FCS (30% of total volume), and cells were washed twice, then cultured as indicated. Division was analyzed on a FACSCalibur on the FL-1 wavelength.
Cell stimulations and Western blot analysis
Prior to stimulation of Jurkat cells and IL-7–treated and TCR-ligated primary CD4+ T lymphocytes, cells were maintained for 16 hours in RPMI 1640 medium with 1% FCS. Cells were then resuspended in serum-free medium (1 × 107/mL) and incubated for 15 minutes at 37°C before stimulation with either αCD3 (2.5 μg/mL) or αCD4 mAbs (10 μg/mL), followed by cross-linking with an anti–mouse F(ab′)2 fragment (40 μg/mL) at 37°C for 5 minutes, or alternatively with recombinant SUgp120 protein (10 μg/mL), followed by cross-linking with an αgp120 pAb (1:100 dilution) or SDF-1 (1 μg/mL) for 1 minute. In some experiments cell membrane cholesterol was depleted essentially as previously described.12 Briefly, cells were incubated in serum-free media supplemented with 10 mM MβCD for 20 minutes at 37°C, washed twice, and stimulated as indicated above. Cells were immediately lysed in ice-cold lysis buffer (20 mM Tris-HCl, pH 7.5, 5 mM EDTA (ethylenediaminetetraacetic acid), 150 mM NaCl, 1% NP40, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 10 mM glycerolphosphate, 20 mM sodium pyrophosphate, 7.5 μg/mL aprotinin, and 200 μM phenylmethylsulfonyl fluoride). Protein lysates were resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred electrophoretically to Protran Nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany). Membranes were blocked for 1 hour at room temperature in Tris-buffered saline (150 mM NaCl, 20 mM Tris, pH 7.5, 0.05% Tween 20) containing 5% milk and incubated with the appropriate primary antibody overnight at 4°C. Blots were then incubated with goat anti–rabbit or anti–mouse secondary antibody linked to peroxidase (Amersham, Arlington Heights, IL), and immunoreactive proteins were visualized using the enhanced chemiluminescence (ECL) detection system (Amersham).
Results
gp120-induced MAPK activation in leukemic Jurkat T-cell lines
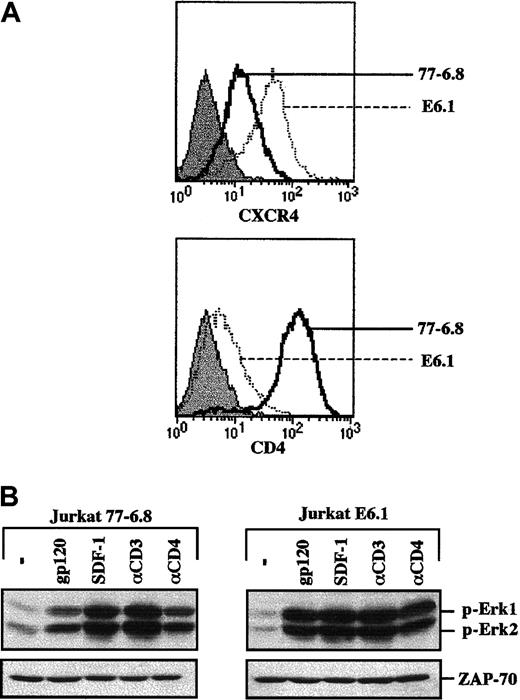
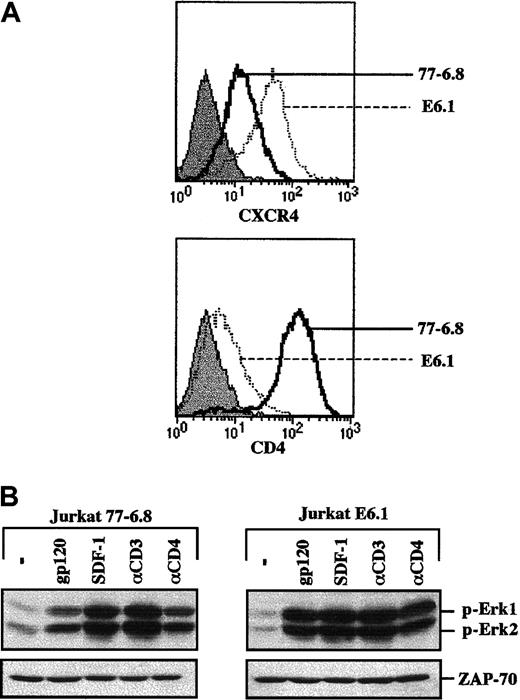
Prior to embarking on studies of HIV-envelope glycoprotein-mediated activation of the MAPK pathway in primary human T cells, the experiments were validated in a leukemic T-cell line. It has previously been demonstrated that recombinant gp120 can activate the MAPK pathway in CD4+ Jurkat T cells but not in a CD4-negative Jurkat clone.13 14 We therefore tested the ability of a recombinant CXCR4-specific gp120 SF2 protein to activate this signaling pathway in 2 different Jurkat clones that express significantly different levels of the CD4 and CXCR4 coreceptor: 77-6.8 and E6.1. Specifically, 77-6.8 Jurkat cells express high levels of both CD4 and CXCR4, whereas E6.1 Jurkats express high levels of CXCR4 but only minimal levels of CD4 (Figure 1A). Notably, gp120 binding resulted in phosphorylation of Erk1 and Erk2 MAPKs in both Jurkat clones (Figure 1B). Moreover, phosphorylation of Erk1/Erk2 was also induced by cross-linking with an αCD4 mAb. Thus, at least in the E6.1 transformed cell background, levels of CD4 that were 1.5 logs lower than those observed in primary CD4+ lymphocytes (compare with Figure2A) are sufficient for both gp120- and CD4-induced MAPK activation. As expected, activation of this cascade was also induced by engagement of the CD3/TCR complex and by the natural ligand of CXCR4, SDF-1 (Figure 1B).
gp120-induced MAPK activation in the transformed Jurkat T-cell line does not correlate with surface CD4 expression.
(A) Surface expression of CXCR4 and CD4 was analyzed on a FACScan using PE-conjugated monoclonal antibodies. The expression profiles of these 2 proteins in the 77-6.8 and E6.1 Jurkat clones (open histograms) are indicated. The respective immunoglobulin isotype controls (filled histograms) are shown. (B) The 77-6.8 and E6.1 Jurkat clones were either incubated in medium alone (−) or stimulated with gp120 (1 μg), SDF-1 (100 ng), an αCD3 mAb (0.25 μg) or an αCD4 mAb (1 μg). Total cell lysates (1 × 106 cell equivalents) were fractionated on an SDS gel. Membranes were immunoblotted with a polyclonal Ab that recognizes the dually phosphorylated forms of Erk1 and Erk2. The positions of phosphorylated Erk1 (p-Erk1) and Erk2 (p-Erk2) are indicated. Blots were reprobed with an αZAP-70 mAb to assure that expression of a T-cell–specific protein was equivalent in each lane. Results are representative of data obtained in 4 independent experiments.
gp120-induced MAPK activation in the transformed Jurkat T-cell line does not correlate with surface CD4 expression.
(A) Surface expression of CXCR4 and CD4 was analyzed on a FACScan using PE-conjugated monoclonal antibodies. The expression profiles of these 2 proteins in the 77-6.8 and E6.1 Jurkat clones (open histograms) are indicated. The respective immunoglobulin isotype controls (filled histograms) are shown. (B) The 77-6.8 and E6.1 Jurkat clones were either incubated in medium alone (−) or stimulated with gp120 (1 μg), SDF-1 (100 ng), an αCD3 mAb (0.25 μg) or an αCD4 mAb (1 μg). Total cell lysates (1 × 106 cell equivalents) were fractionated on an SDS gel. Membranes were immunoblotted with a polyclonal Ab that recognizes the dually phosphorylated forms of Erk1 and Erk2. The positions of phosphorylated Erk1 (p-Erk1) and Erk2 (p-Erk2) are indicated. Blots were reprobed with an αZAP-70 mAb to assure that expression of a T-cell–specific protein was equivalent in each lane. Results are representative of data obtained in 4 independent experiments.
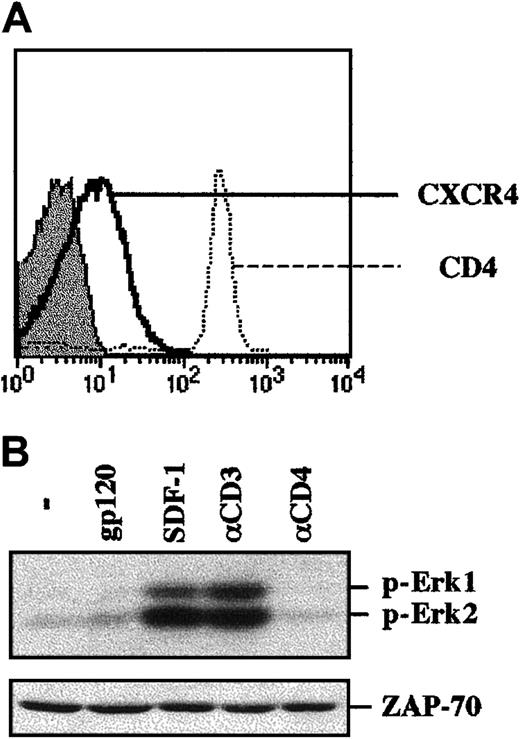
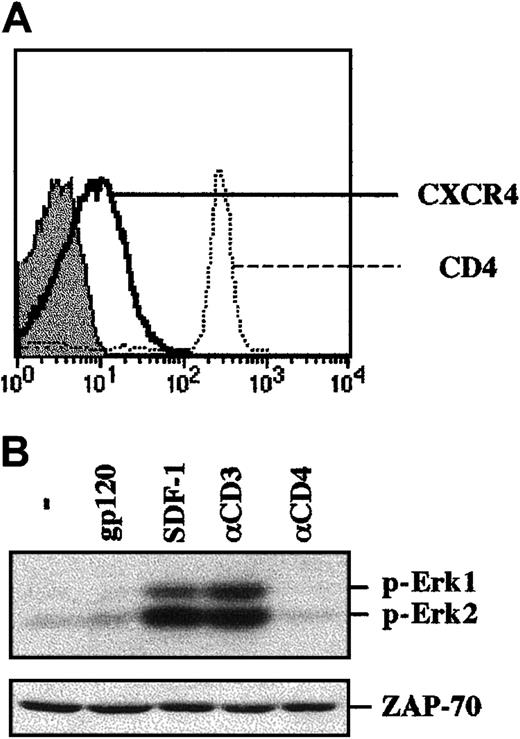
Binding of gp120 to freshly isolated human CD4+ T lymphocytes does not result in MAPK activation.
(A) CD4+ lymphocytes were purified by negative selection and were immediately stained with PE-conjugated anti-CXCR4 or anti-CD4 mAbs. CD4 and CXCR4 staining profiles are shown, and the filled histogram depicts staining with an isotype-matched control mAb. (B) The CD4+ lymphocytes isolated as described in (A) were immediately stimulated with either gp120, SDF-1, an αCD3 mAb, or an αCD4 mAb. Protein lysates from 1 × 106 cells were resolved on an SDS gel and analyzed as described in the legend to Figure 1. The positions of phosphorylated Erk1 and Erk2 are noted. Blots were reprobed with an αZAP-70 mAb to assure that expression of a T-cell–specific protein was equivalent in each lane. Data are representative of results obtained in 4 independent experiments with CD4+ lymphocytes from 4 different donors.
Binding of gp120 to freshly isolated human CD4+ T lymphocytes does not result in MAPK activation.
(A) CD4+ lymphocytes were purified by negative selection and were immediately stained with PE-conjugated anti-CXCR4 or anti-CD4 mAbs. CD4 and CXCR4 staining profiles are shown, and the filled histogram depicts staining with an isotype-matched control mAb. (B) The CD4+ lymphocytes isolated as described in (A) were immediately stimulated with either gp120, SDF-1, an αCD3 mAb, or an αCD4 mAb. Protein lysates from 1 × 106 cells were resolved on an SDS gel and analyzed as described in the legend to Figure 1. The positions of phosphorylated Erk1 and Erk2 are noted. Blots were reprobed with an αZAP-70 mAb to assure that expression of a T-cell–specific protein was equivalent in each lane. Data are representative of results obtained in 4 independent experiments with CD4+ lymphocytes from 4 different donors.
gp120-induced MAPK activation in primary CD4+lymphocytes is not a function of surface CD4 or CXCR4 expression
To study gp120-induced signaling in primary human CD4+lymphocytes, cells were isolated by negative antibody-mediated selection (see “Materials and methods”). This method allows cells to be maintained in an unactivated state (data not shown), reflecting their quiescent condition in vivo. Immediately following isolation, T lymphocytes were activated by either cross-linked gp120, SDF-1, or cross-linked αCD3 or αCD4 mAbs. As expected, phosphorylation of Erk1 and Erk2 was observed in these primary CD4+ lymphocytes following CD3 engagement and SDF-1 treatment (Figure 2B). Nevertheless, MAPK activation was not induced by gp120 ligation. Thus, these data indicate that the outcome of gp120 binding to Jurkat cells and primary T lymphocytes is not equivalent regarding recruitment of the MAPK pathway. This difference does not appear to be due to a suboptimal concentration of gp120, given that increasing concentrations by 4-fold did not modulate this outcome (data not shown).
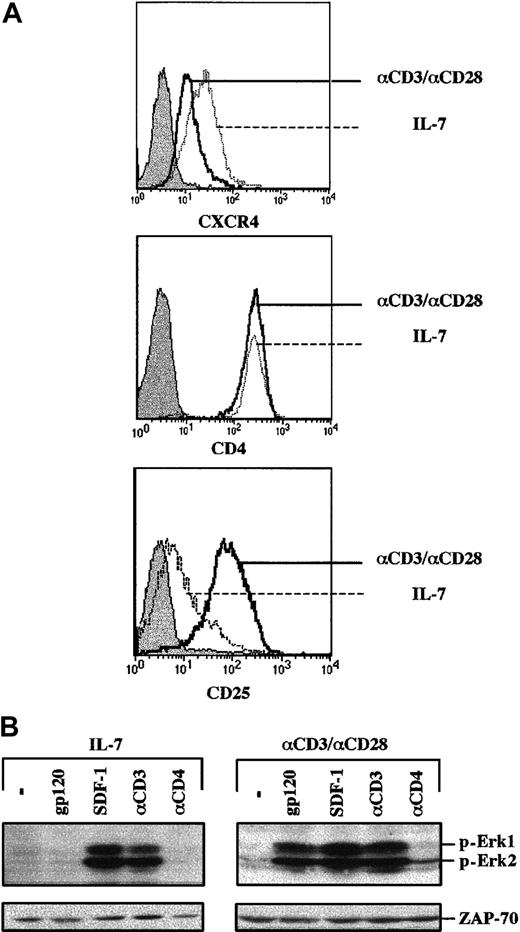
To determine whether increased CXCR4 expression would be sufficient for gp120-induced MAPK activation, cells were prestimulated with the IL-7 cytokine for 4 days prior to gp120 ligation. This treatment was chosen because it has been shown that ex vivo culture is in itself associated with increased surface CXCR4 levels,15,16 and IL-7 is a survival factor for primary lymphocytes in vitro.17-19Importantly, CD4+ lymphocytes from multiple donors demonstrated increased CXCR4 expression following culture in the presence of recombinant IL-7, whereas CD4 levels remained unchanged (Figure 3A and data not shown). Nevertheless, gp120-induced activation of the MAPK cascade was severely limited in these cells (Figure 3B). The lack of gp120-induced signaling did not reflect a global nonresponsiveness as MAPK activation was observed in these IL-7-prestimulated T cells following both CD3 cross-linking and SDF-1 treatment. Thus, higher expression of CXCR4 at the cell surface did not render CD4+ lymphocytes sensitive to gp120-induced recruitment of the MAPK cascade.
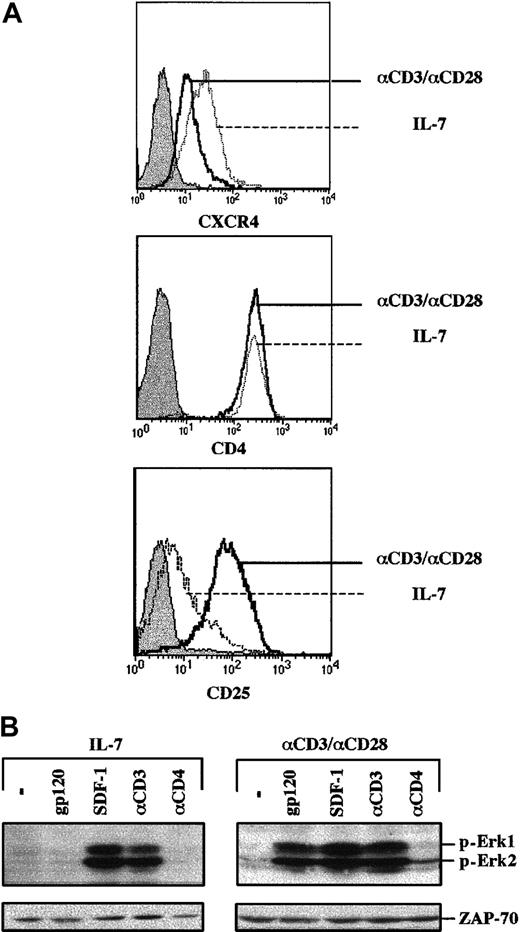
Envelope glycoprotein gp120 induces MAPK activation in TCR-engaged, but not IL-7–prestimulated, primary CD4+ T cells.
(A) CD4+ lymphocytes were prestimulated with either recombinant IL-7 (10 ng/mL) for 4 days or with immobilized αCD3 and αCD28 mAbs for 2 days, followed by a 2-day culture in the presence of recombinant IL-2. CXCR4 and CD4 expression were assessed at day 4 using PE-conjugated mAbs, and the respective histograms are indicated. Expression of the IL-2Rα (CD25) activation marker in these IL-7– and CD3/CD28-stimulated T cells was monitored, and the corresponding histograms are shown. (B) After 4 days of culture, cells were either restimulated with gp120, SDF-1, an αCD3 mAb, or an αCD4 mAb, as indicated. Membranes immunoblotted with a pAb recognizing the dually phosphorylated Erk1/Erk2 are shown. Blots were reprobed with an αZAP-70 mAb to assess expression of a T-cell–specific protein in these differentially stimulated T cells. Results are representative of data obtained in 1 of 3 independent experiments.
Envelope glycoprotein gp120 induces MAPK activation in TCR-engaged, but not IL-7–prestimulated, primary CD4+ T cells.
(A) CD4+ lymphocytes were prestimulated with either recombinant IL-7 (10 ng/mL) for 4 days or with immobilized αCD3 and αCD28 mAbs for 2 days, followed by a 2-day culture in the presence of recombinant IL-2. CXCR4 and CD4 expression were assessed at day 4 using PE-conjugated mAbs, and the respective histograms are indicated. Expression of the IL-2Rα (CD25) activation marker in these IL-7– and CD3/CD28-stimulated T cells was monitored, and the corresponding histograms are shown. (B) After 4 days of culture, cells were either restimulated with gp120, SDF-1, an αCD3 mAb, or an αCD4 mAb, as indicated. Membranes immunoblotted with a pAb recognizing the dually phosphorylated Erk1/Erk2 are shown. Blots were reprobed with an αZAP-70 mAb to assess expression of a T-cell–specific protein in these differentially stimulated T cells. Results are representative of data obtained in 1 of 3 independent experiments.
There are multiple differences between Jurkat cells and primary T cells, but 2 parameters that were studied in more detail, as related to gp120-induced signaling intermediates, were the activation state and cell cycle progression. With regard to the former, IL-7 serves as a T-cell survival factor but does not induce T-cell activation,17,19,20 as monitored by low expression of the IL-2Rα (CD25) activation marker in IL-7–treated cells (Figure 3A). In contrast, T lymphocytes activated through their cognate antigen receptor (TCR) demonstrate a distinct activation profile with significantly higher IL-2Rα expression (Figure 3A). However, as previously noted, TCR activation is associated with decreased cell surface CXCR4.15 16 These cells were used to assess whether gp120 can induce a signal in an activated primary cell. Notably, gp120 binding to TCR-prestimulated lymphocytes activated the MAPK pathway, as demonstrated by Erk1 and Erk2 phosphorylation (Figure3B). This phenomenon was observed despite consistently lower levels of CXCR4 expression in TCR-stimulated lymphocytes than in their IL-7–stimulated counterpart (Figure 3A).
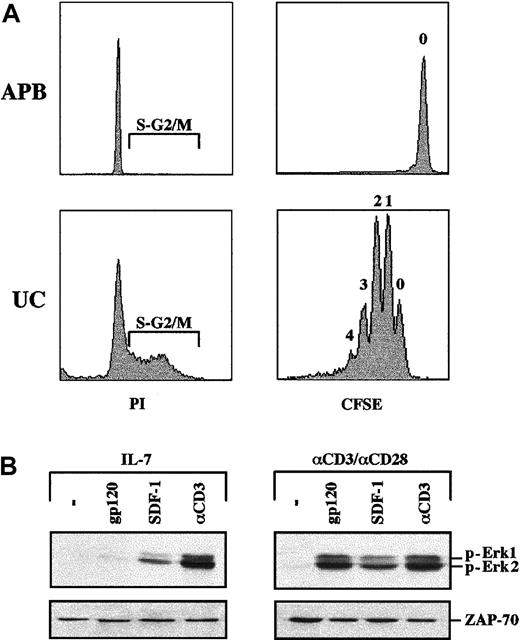
As indicated above, one significant difference between Jurkat cells and primary lymphocytes is that the former are permanently cycling, whereas the latter are almost all quiescent, in the G0 phase of the cell cycle. It was therefore important to discern whether the ability of gp120 to induce MAPK activation in TCR-stimulated T cells was a function of the cell cycle entry of these cells or, alternatively, the result of a more global activation state. We have previously demonstrated that although IL-7 stimulation induces no, or little, proliferation of CD4+ peripheral T cells, this cytokine induces proliferation of neonatal CD4+ lymphocytes isolated from UC blood.21 Indeed, IL-7–treated UC-derived CD4+ lymphocytes demonstrate significant cell cycle entry and division, whereas adult peripheral blood T cells are essentially all in the G0/G1 phase of the cell cycle and do not undergo division (Figure 4A). Nevertheless, even in proliferating IL-7–prestimulated UC lymphocytes, gp120 binding did not induce significant phosphorylation of the Erk1/Erk2 MAPKs. Notably though, in both UC and adult CD4+ T cells, activation markers are not induced by IL-7 treatment (Figure 3A and data not shown). Under conditions where activation was induced by TCR engagement (as demonstrated by expression of CD25 and CD69 markers; data not shown), UC CD4+ lymphocytes, like adult peripheral blood CD4+ lymphocytes, demonstrated gp120-induced Erk1/Erk2 phosphorylation (Figure 4B). As expected, MAPK was phosphorylated in response to SDF-1 and CD3 ligation in both IL-7– and TCR-prestimulated UC T cells (Figure 4B). Variations in the levels of MAPK phosphorylation were observed between different UC samples but globally, phosphorylation was equivalent to that observed in adult T cells (Figure 4B and data not shown). The ensemble of these results strongly suggests that gp120-induced MAPK activation depends on the activation state of primary T lymphocytes rather than the absolute levels of CD4/CXCR4 or cell cycle entry/proliferation.
IL-7–induced proliferation does not sensitize CD4+ lymphocytes to gp120-induced MAPK activation.
(A) CD4+ T cells were isolated from both UC blood and adult peripheral blood (APB) and cultured for 6 days in the presence of recombinant IL-7. Cell cycle entry was monitored at day 6 by assessing the DNA content of PI-stained cells on FACS. Cells in the S and G2/M phases of the cell cycle are indicated in each histogram. To directly assess cell division/proliferation, cells were labeled with CFSE and analyzed for CFSE intensity by FACS. The number shown above each peak indicates the number of cell divisions. (B) MAPK activation in proliferating IL-7–treated UC CD4+ lymphocytes was assessed following stimulation with gp120, SDF-1, or an αCD3 mAb, as indicated. Blots were reprobed with an αZAP-70 mAb to assure that expression of a T-cell–specific protein was equivalent in each lane. Data are representative of results obtained in 2 independent experiments.
IL-7–induced proliferation does not sensitize CD4+ lymphocytes to gp120-induced MAPK activation.
(A) CD4+ T cells were isolated from both UC blood and adult peripheral blood (APB) and cultured for 6 days in the presence of recombinant IL-7. Cell cycle entry was monitored at day 6 by assessing the DNA content of PI-stained cells on FACS. Cells in the S and G2/M phases of the cell cycle are indicated in each histogram. To directly assess cell division/proliferation, cells were labeled with CFSE and analyzed for CFSE intensity by FACS. The number shown above each peak indicates the number of cell divisions. (B) MAPK activation in proliferating IL-7–treated UC CD4+ lymphocytes was assessed following stimulation with gp120, SDF-1, or an αCD3 mAb, as indicated. Blots were reprobed with an αZAP-70 mAb to assure that expression of a T-cell–specific protein was equivalent in each lane. Data are representative of results obtained in 2 independent experiments.
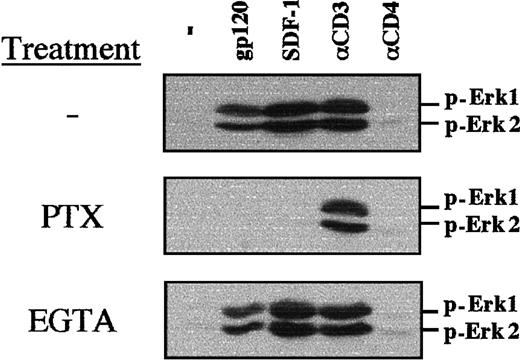
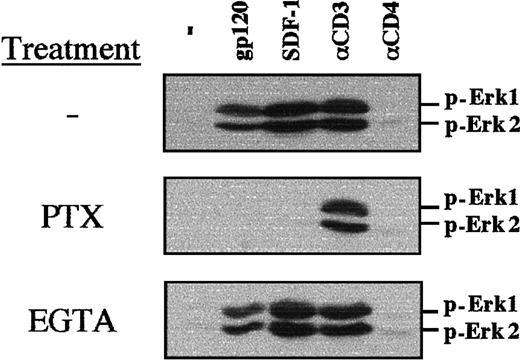
SDF-1–mediated PI-3 kinase activation and calcium flux has been shown to be dependent on a Gαi protein–mediated mechanism in primary T lymphocytes.22 Using PTX, which uncouples Gαi proteins from G protein–linked 7 transmembrane receptors, we determined that both gp120- and SDF-1–induced MAPK phosphorylation requires Gαi protein–mediated signaling in TCR-prestimulated lymphocytes (Figure5). As expected, PTX did not inhibit CD3-induced MAPK phosphorylation (Figure 5). Calcium influx was not required for gp120-, SDF-1–, or αCD3-induced MAPK phosphorylation, as chelation of extracellular calcium with EGTA did not block this process (Figure 5).
PTX blocks gp120-induced MAPK phosphorylation in TCR-engaged primary T cells.
CD4+ lymphocytes were prestimulated with immobilized αCD3 and αCD28 mAbs for 3 days, followed by a 16-hour culture in the presence of PTX (100 ng/mL) or a 20-minute culture in the presence of EGTA (2 mM). Nontreated, PTX-treated, and EGTA-treated cells were then either stimulated with gp120, SDF-1, an αCD3 mAb, or an αCD4 mAb, as indicated. Membranes immunoblotted with a pAb recognizing the dually phosphorylated Erk1/Erk2 are shown.
PTX blocks gp120-induced MAPK phosphorylation in TCR-engaged primary T cells.
CD4+ lymphocytes were prestimulated with immobilized αCD3 and αCD28 mAbs for 3 days, followed by a 16-hour culture in the presence of PTX (100 ng/mL) or a 20-minute culture in the presence of EGTA (2 mM). Nontreated, PTX-treated, and EGTA-treated cells were then either stimulated with gp120, SDF-1, an αCD3 mAb, or an αCD4 mAb, as indicated. Membranes immunoblotted with a pAb recognizing the dually phosphorylated Erk1/Erk2 are shown.
Raft integrity is required for gp120-mediated MAPK activation
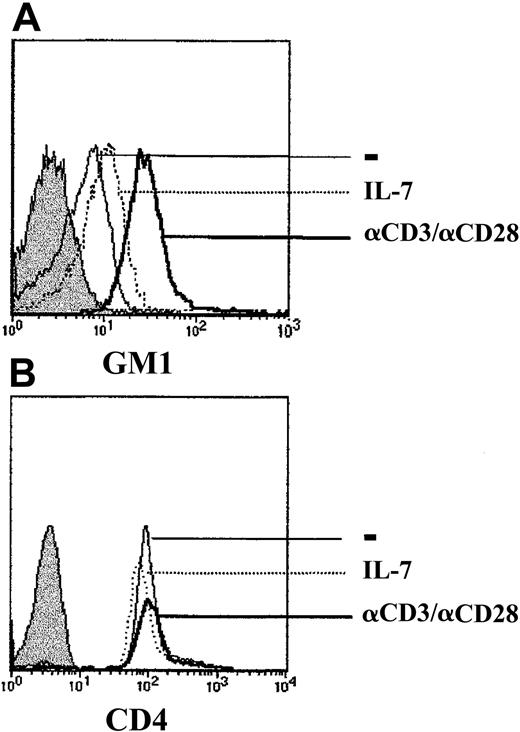
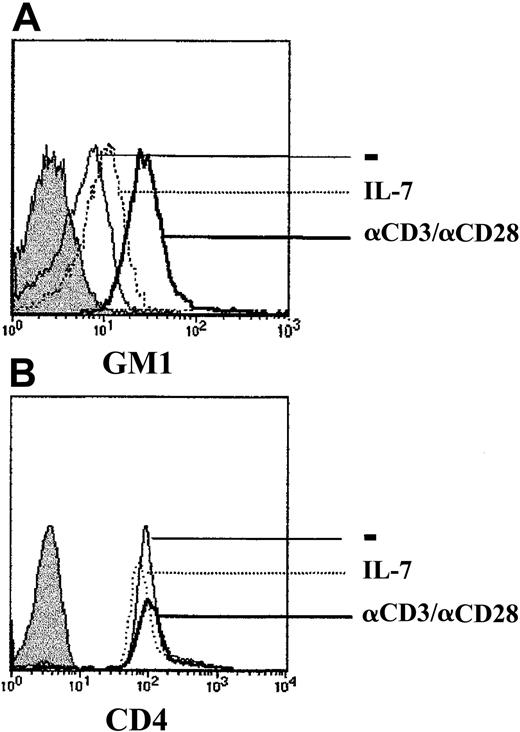
Much recent data indicate that the integrity of lipid rafts is required for the initiation of multiple signaling cascades at the cell surface.23 Rafts are domains of lateral lipid clusters that are enriched in sphingolipids, cholesterols, and glycosylphosphatidylinositol (GPI)-anchored proteins, as well as signal transduction assemblies.24 Moreover, Tuosto and colleagues have recently shown that activation of T cells through the T-cell receptor results in increased raft domains, as demonstrated by augmented levels of the raft glycosphingolipid GM1.25 As HIV-1 infection is dependent on raft integrity,26 27 it was of interest to determine whether expression of the GM1 raft marker correlated with gp120-induced MAPK activation. GM1 expression increased within 24 to 48 hours of TCR activation and remained elevated for the next 96 hours (Figure 6 and data not shown). In contrast, IL-7 stimulation did not result in significantly augmented GM1 expression, compared with either freshly isolated CD4+ lymphocytes or CD4+ lymphocytes cultured during the same period in media without any exogenous stimuli (Figure 6and data not shown). The increased GM1 expression in TCR-stimulated lymphocytes was not related to the large size of activated cells, as expression of the CD4 surface marker was equivalent under all 3 culture conditions (Figure 6). Thus, increases in GM1 levels correlated with gp120-induced signaling in primary CD4+ lymphocytes.
Up-regulation of the raft marker GM1 in TCR-stimulated but not IL-7–stimulated CD4+ lymphocytes.
CD4+ T cells were cultured for 4 days in the presence of media alone (−), recombinant IL-7 (IL-7), or immobilized αCD3 and αCD28 mAbs. Expression of the ganglioside GM1 was assessed using FITC-conjugated cholera toxin B. Unstained control cells are shown by a filled histogram. CD4 surface staining was performed using an αCD4-PE mAb, and staining of cells in each of the 3 culture conditions is shown. Histograms are representative of data obtained in 3 independent experiments.
Up-regulation of the raft marker GM1 in TCR-stimulated but not IL-7–stimulated CD4+ lymphocytes.
CD4+ T cells were cultured for 4 days in the presence of media alone (−), recombinant IL-7 (IL-7), or immobilized αCD3 and αCD28 mAbs. Expression of the ganglioside GM1 was assessed using FITC-conjugated cholera toxin B. Unstained control cells are shown by a filled histogram. CD4 surface staining was performed using an αCD4-PE mAb, and staining of cells in each of the 3 culture conditions is shown. Histograms are representative of data obtained in 3 independent experiments.
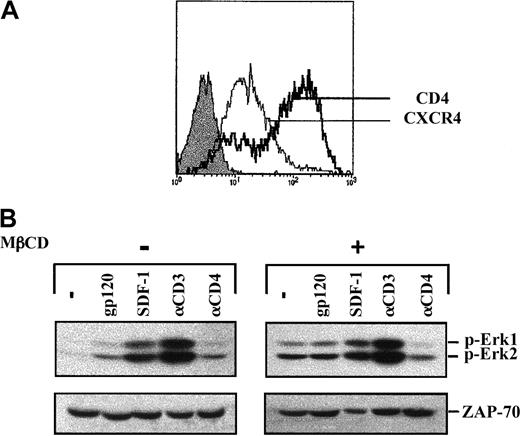
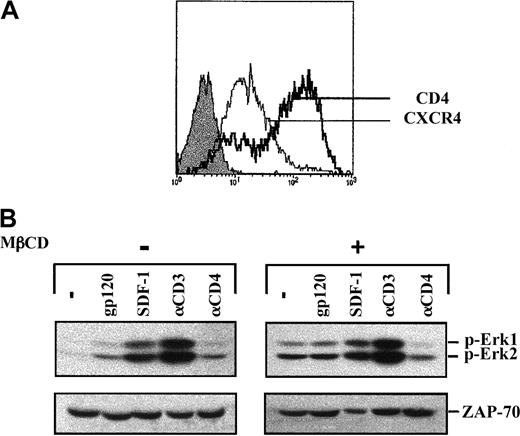
To investigate the importance of lipid rafts in gp120 signaling, lipid rafts were disrupted by treatment with MβCD, an agent that depletes cellular cholesterol.28 Previous work demonstrated that in Jurkat cells, lipid rafts are disrupted following a 20-minute treatment of cells with 10 mM MβCD.29 These conditions were therefore used in the experiments described here. Notably, both CD4 and CXCR4 were expressed at the cell surface following MβCD treatment (Figure 7A). As reported,29we observed that treatment of cells with MβCD was in itself sufficient to induce activation of the MAPK pathway (Figure 7B). In MβCD-treated cells, the level of Erk1/Erk2 phosphorylation was further augmented following both CD3 engagement and SDF-1 treatment, albeit at higher levels in the former. In marked contrast, no increase in Erk1/Erk2 phosphorylation was observed following gp120 binding (Figure 7B). Thus, raft integrity appears to be required for further recruitment of the MAPK signaling cascade by gp120.
Lack of gp120-induced MAPK activation following lipid raft disruption.
(A) Jurkat 77-6.8 cells were treated with 10 mM MβCD for 20 minutes at 37°C, washed, and then resuspended in serum-free media. Surface expression of CD4 and CXCR4 was analyzed on a FACScan using PE-conjugated mAbs. The expression profiles of these 2 proteins were then assessed using PE-conjugated αCD4 and αCXCR4 mAbs as indicated. Isotype control staining is shown by a filled histogram. (B) Untreated and MβCD-treated cells were then stimulated with gp120, SDF-1, an αCD3 mAb, or an αCD4 mAb, as described in the legend to Figure 1. Membranes immunoblotted with the pAb recognizing dually phosphorylated Erk1/Erk2 are shown. Blots were reprobed with an αZAP-70 mAb to assess expression of a T-cell–specific protein. Results are representative of data obtained in 1 of 2 independent experiments.
Lack of gp120-induced MAPK activation following lipid raft disruption.
(A) Jurkat 77-6.8 cells were treated with 10 mM MβCD for 20 minutes at 37°C, washed, and then resuspended in serum-free media. Surface expression of CD4 and CXCR4 was analyzed on a FACScan using PE-conjugated mAbs. The expression profiles of these 2 proteins were then assessed using PE-conjugated αCD4 and αCXCR4 mAbs as indicated. Isotype control staining is shown by a filled histogram. (B) Untreated and MβCD-treated cells were then stimulated with gp120, SDF-1, an αCD3 mAb, or an αCD4 mAb, as described in the legend to Figure 1. Membranes immunoblotted with the pAb recognizing dually phosphorylated Erk1/Erk2 are shown. Blots were reprobed with an αZAP-70 mAb to assess expression of a T-cell–specific protein. Results are representative of data obtained in 1 of 2 independent experiments.
Discussion
The stimulation of signal transduction cascades, mediated by the HIV-1 envelope glycoprotein gp120, has been postulated to influence HIV-1 replication, as well as viral-associated cytopathicity and apoptosis.30 Nevertheless, the vast majority of studies assessing the role of gp120 in the recruitment of signaling intermediates have been performed in transformed cell lines,13,14 31-36 precluding any assessment of the role of the T-cell activation state on gp120-induced effects. The data presented here demonstrate that the ability of gp120 to trigger the MAPK signaling pathway is regulated by the activation state of primary T cells. Specifically, gp120 binding did not result in the phosphorylation of Erk1/Erk2 MAPKs in either freshly isolated quiescent T cells or in T cells prestimulated with the IL-7 cytokine.
To determine whether MAPK recruitment was dependent on cell cycle entry, gp120-induced phosphorylation was also assessed in IL-7–prestimulated CD4+ T cells isolated from UC blood because, in contrast with adult peripheral blood lymphocytes, UC T cells proliferate in response to IL-7.21 37 Nevertheless, even in proliferating IL-7–prestimulated UC T cells, the MAPK pathway was not significantly activated by gp120 ligation. In a large number of parameters tested in the context of this study, gp120-induced recruitment of the MAPK pathway was observed only in primary T lymphocytes that were prestimulated through their TCR. Importantly, levels of the CD4 receptor were approximately equivalent in TCR-prestimulated and freshly isolated lymphocytes, whereas the gp120 coreceptor CXCR4 was expressed at comparatively lower levels on TCR-stimulated lymphocytes. TCR-stimulated cells differ from both freshly isolated and IL-7–stimulated T cells in that only TCR-mediated signals induce an activated phenotype associated with cytokine secretion and a high level of proliferation. Thus, the global activation state induced by TCR engagement appears to be required for gp120-induced triggering of the MAPK cassette.
We determined that in these primary T cells, gp120-induced MAPK activation, like SDF-1–induced MAPK activation,22 is dependent upon signaling through Gαi proteins. Of note, gp120-induced MAPK recruitment in macrophages is not regulated at the level of Gαi proteins, at least not by those inhibited via PTX.38 In contrast, calcium influx is required for gp120-induced MAPK recruitment in macrophages38 but not in primary lymphocytes. As previously suggested by these studies and earlier work,38 39 gp120, as well as chemokine-induced coupling of G proteins, may differ in distinct cell types.
A small number of studies have assessed the effects of gp120 ligation in primary lymphocytes, rather than in a transformed cell background. In one reported study performed using quiescent T lymphocytes, isolated by a negative selection method such as that reported here, recruitment of the MAPK cascade was also not detected following binding of the gp120 envelope40 (and B. Rossi, personal oral communication, February 2002). Nevertheless, in the majority of studies performed with primary CD4+ lymphocytes, T cells were isolated by sheep red blood cell (SRBC) rosetting.41-44 The rosetting method is based on the interaction of SRBC with the T-cell–specific CD2 glycoprotein, a molecule that plays a role in T-cell signaling and lymphocyte adhesion.45 This interaction is largely responsible for the augmented responses of SRBC-isolated T cells to suboptimal doses of mitogens.46 Intriguingly, T cells isolated by SRBC rosetting demonstrate increased levels of CXCR4 at the cell surface as compared with T cells isolated by negative antibody-mediated selection (S.K. and F.B., unpublished observations, July 2001). Thus, the isolation protocol is a parameter that needs to be taken into account when interpreting gp120-induced signaling data in primary lymphocytes.
One of the important questions raised by this study concerns the differential signaling responses of CD4+ lymphocytes to gp120 ligation as compared with interaction with the natural ligand of CXCR4, SDF-1. While gp120-induced MAPK activation was observed only in TCR-prestimulated lymphocytes, SDF-1 activated this pathway under all conditions; freshly isolated lymphocytes as well as lymphocytes prestimulated via IL-7 or the TCR. Concerning SDF-1 stimulation, it is interesting to note that SDF-1–mediated effects could be differentiated from gp120-mediated effects in at least 2 other experimental systems. Kaul and Lipton reported that gp120-induced neuronal apoptosis is prevented by a tripeptide that inhibits macrophage activation, whereas SDF-1–mediated neuronal apoptosis is not sensitive to this tripeptide.47 In a second elegant study using primary T lymphocytes, Weissman and colleagues demonstrated that activation of cells with a CXCR4-tropic envelope protein did not result in calcium flux, whereas SDF-1 was a potent stimulus. Interestingly, these authors showed that the CXCR4-specific envelope induced calcium flux in a tumor-infiltrating cell line (B10) that expresses significantly lower levels of CXCR4 than primary lymphocytes.48 Thus, the data reported by Weissman et al48 support the proposition generated by this study, namely, the global cellular environment rather than receptor expression per se regulates responsiveness to gp120 ligation. Moreover, these 2 studies,47 48 together with the data reported here, strongly suggest that the recruitment of signaling intermediates by SDF-1 and gp120 is not equivalent.
Our data also indicate that signaling through gp120 and CD4 is not equivalent. CD4-induced MAPK phosphorylation was not detected in primary T cells, irrespective of their activation status. Intriguingly, MAPK phosphorylation was observed in primary lymphocytes upon binding of the CD4 mAb to beads (not shown), suggesting that in these cells, extensive cross-linking is required for activation. Thus, the presence of CD4 in lipid rafts49 50 does not in itself appear to be sufficient for recruitment of the MAPK pathway, at least in the context of primary lymphocytes.
The organization of functional rafts at the cell surface appears to be crucial for the regulation of host cell functions via the activation of signaling cascades.23 Indeed, many signaling molecules are abundantly present in lipid raft structures, including GPI-anchored proteins, Src, Lck, Fyn, Lyn, LAT, Grb-2, Ras, and GTP-binding proteins.12,51-58 Moreover, it appears that the formation of lipid rafts in primary T lymphocytes is induced by TCR activation.25 In this regard, it is notable that the SDF-1 signaling response, a parameter that was not modulated by T-cell engagement, proceeds via CXCR4, a receptor that, according to one report, is absent from lipid rafts.49
Unlike CXCR4, a high proportion of CD4 is localized in lipid rafts following T-cell activation.49,50 T-cell tropic gp120 interacts with CXCR4 only after undergoing a conformational change induced by its binding to CD4.59 One apparent distinction between primary quiescent T lymphocytes and the transformed Jurkat cell line vis-à-vis CD4 localization is that in the former, a significant proportion of CD4 is outside the lipid rafts,49,50 whereas in the latter, it is located almost exclusively in raft structures.60 61 It is indeed likely that this localization is one of the factors accounting for the differential gp120-mediated signaling responses observed in nonactivated primary T lymphocytes and Jurkat cells. Likewise, it should be noted that CD4 cross-linking induced MAPK activation in Jurkat cells but not in identically treated primary CD4+ T lymphocytes.
Recently, 2 groups have identified at least 2 glycosphingolipids (GSLs), globotriaosyl-ceramide (Gb3) and the ganglioside GM3, that serve as functional fusion cofactors for both X4 and R5 HIV-1 isolates.62,63 Hammache et al62 have hypothesized that GSLs recognized by both CD4 and CXCR4 induce the formation of a trimolecular CD4/GSL/gp120 complex in such rafts,63 whereas work from Kozak et al49suggests that upon interaction of multivalent gp120-CD4 complexes to CXCR4, there might be an emigration of CD4 out of rafts. Irrespective of the exact mechanism(s) controlling gp120-mediated fusion, the plethora of evidence indicates that lipid rafts play an important role in this process.64 Indeed, disruption of lipid rafts in target cells results in a significantly decreased level of HIV-1 infection.26 27 Together, these results suggest that disruption of lipid rafts inhibits gp120-induced signaling as well as HIV-1 infection.
In conclusion, we demonstrate that gp120 induces the recruitment of the MAPK cascade in primary lymphocytes under specific activation conditions. These data reveal a strategy via which gp120 may differentially modulate the fate of activated and quiescent T cells. Moreover, other studies have shown that gp120 also induces the MAPK cassette in primary human macrophages38 and arterial smooth muscle cells.65 The relevance of all these data remains to be determined, but the ensemble of these reports suggests that multivalent virus-associated gp120 as well as soluble gp120 play a role in the dysregulated physiological responses observed in HIV-1–infected individuals. It is clear that gp120 is not the sole actor in this process, as other HIV-1 regulatory proteins also induce multiple signaling responses. Very recently, it has been shown that Nef activates the MAPK pathway in primary T cells.66 Nef also blocks apoptosis by inducing Bad phosphorylation,67 as well as by inhibiting apoptosis signaling regulating kinase 1 (ASK1).68 Thus, HIV-1 has evolved in such a manner that several of the proteins encoded by this virus contribute to the modulation of mitogenic and apoptotic pathways in various hematopoietic and nonhematopoietic cell types.
We are indebted to the entire maternity staff at Clinique St Roch, without whose assistance this study would not have been possible. We also are grateful to Drs M. Sitbon, J. Fantini, S. Manes, and A. Viola for their scientific input, and to B. Rossi for graciously communicating unpublished results. We thank M. Burdjanadze, V. Dardalhon, S. Jaleco, N. Noraz, M. Steinberg, and L. Swainson for their assistance and support throughout the course of this study.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2002-03-0819.
Supported by funding from the Association National pour la Recherche contre le Sida (N.T.); a fellowship from the European community (HPMF-CT-2000-01035) (S.K.); the CHU de Montpellier (F.B.); the Association France-Israel pour la Recherche en Science et Technologie (C.M.); and INSERM (N.T.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Naomi Taylor, Institut de Génétique Moléculaire de Montpellier, 1919 Route de Mende, 34293 Montpellier, Cedex 5, France; e-mail:taylor@igm.cnrs-mop.fr.