Natural killer (NK)/lymphokine-activated killer (LAK) cell-based immunotherapy could be beneficial against major histocompatibility complex class I–negative tumor residual disease such as neuroblastoma (NB), provided that interleukin 2 (IL-2) or surrogate nontoxic NK cell stimulatory factors could sustain NK cell activation and survival in vivo. Here we show that human monocyte–derived dendritic cells (MD-DCs) promote potent NK/LAK effector functions and long-term survival, circumventing the need for IL-2. This study demonstrates (1) the feasibility of differentiating granulocyte colony-stimulating factor–mobilized hematopoietic peripheral blood stem cells (PBSCs) into high numbers of functional MD-DCs and NK/LAK cells in a series of 12 children with stage 4 neuroblastoma (NB); (2) potent DC-mediated NK cell activation in autologous settings; (3) the reciprocal capacity of NK/LAK cells to turn immature DCs into maturing cells electively capable of triggering NK cell functions; and (4) the unique capacity of maturing DCs to sustain NK cell survival, superior to that achieved in IL-2. These data show a reciprocal interaction between DCs and NK/LAK cells, leading to the amplification of NK cell effector functions, and support the implementation of DC/NK cell–based immunotherapy for purging the graft and/or controlling minimal residual disease after autologous stem cell transplantation.
Introduction
Cell therapy based on autologous stem cell transplantation (ASCT) following myeloablative treatments significantly improves the prognosis of certain types of solid tumors. High-dose chemotherapy (HDC) regimen and ASCT are indicated in children with stage 4 neuroblastoma (NB).1 2 Nonetheless, tumor relapses frequently occur, either from clinically occult residual disease or from contaminating tumor cells in autologous grafts. Postgraft immunointerventions consisting of adoptive transfer of ex vivo–activated and/or antitumor effector cells derived from peripheral blood stem cells (PBSCs) in conjunction with lymphokines remain promising.
In the neuroblastoma model, several lines of evidence support the notion that antitumor immune responses might be clinically beneficial in that (1) spontaneous regressions are observed in Pepper syndrome3; (2) tumor-infiltrating lymphocytes exhibited, in some cases, oligoclonal T-cell repertoire4; and (3) objective responses were reported following interleukin-2 (IL-2) systemic administration.5 However, many in vitro studies pointed to a critical susceptibility of neuroblastoma cell lines to innate effectors, that is, natural killer (NK)/lymphokine-activated killer (LAK) lysis.6,7 In the 1990s, attempts to develop clinical trials using systemic high-dose IL-2 administration with or without LAK cells after HDC plus ASCT have been hampered by severe IL-2–related toxicities reported in such children.8 Alternative strategies are clearly required to boost innate effector functions against residual neuroblastoma.
Besides their pivotal role in promoting naive T-cell activation, dendritic cells (DCs) are capable of triggering innate immune responses in vitro and in vivo. We have initially demonstrated that murine bone marrow–derived DCs (BM-DCs) promote resting NK cell activation, that is, interferon-γ (IFN-γ) secretion and dramatic cytolytic activity against tumor cells. A DC/NK cell contact is necessary and the DC-mediated triggering of NK cells is independent of IL-12 and type I IFNs.9 Interestingly, DC in vivo expansion with the use of Flt3 ligand or adoptive transfer of ex vivo– propagated DCs into palpable tumors led to NK cell–dependent antitumor effects. Whether human monocyte–derived dendritic cells (MD-DCs) could similarly promote NK cell effector functions was also recently adressed by our group and others.10-14 Human MD-DCs derived from healthy donors were shown to enhance proliferation and effector functions of resting or IL-2–activated NK cells through a bidirectionnal cross-talk in which activated NK cells also induce MD-DC activation. Moreover, MD-DC–mediated IFN-γ production and cytolytic activity of NK cells were dependent on cell-to-cell contacts and were independent of soluble factors, that is, IL-12, IL-15, and IL-18.
Here we analyzed the functionality and relevance of the reciprocal DC/NK cell cross-talk in a preclinical study conducted in children with NB. In a series of 12 children with stage 4 NB, we show that granulocyte colony-stimulating factor (G-CSF)–mobilized PBSCs can be differentiated into high numbers of functional MD-DCs and NK/LAK cells and that coculture of autologous DCs/LAK cells allows reciprocal activation of both subsets, long-term IL-2 deprivation, and sustained NK/LAK antitumor effector functions. These data show that in NK/LAK-based immunotherapy, DCs can substitute for NK cell stimulatory soluble factors, which were not devoid of toxicity.
Patients and methods
Patients and sample collection
Twelve children (2 to 7 years old) with stage 4 NB, treated according to the Société Française d'Oncologie Pédiatrique NB97 protocol were included in this study. Before initiation of treatment, all parents signed an informed consent reviewed by the Hôpital Kremlin Bicêtre ethics comittee. PBSCs, collected after at least 4 courses of conventional chemotherapy to perform the ASCT, were mobilized either by chemotherapy and G-CSF (5 μg/kg/d) (Neupogen; Amgen, Neuilly sur Seine, France) or in steady state with G-CSF alone (10 μg/kg/d). Cryopreservation and thawing of cell suspensions were performed in clinical grade conditions as previously described.20 In addition, peripheral blood mononuclear cells (PBMCs) from paired blood samples were collected before and during mobilization, isolated on Ficoll density gradient, and kept frozen in liquid nitrogen until use. PBSCs contained 49.6% ± 25.3% CD14+CD15+/− cells; 18.7% ± 12.6% CD3+ T lymphocytes; and 6.1% ± 3.0% CD56+CD3− NK lymphocytes. No significant difference in the repartition of these cell subsets was observed between PBSCs and the paired PBMC samples.
Culture procedures
Culture procedures are outlined in Figure1.
Outline of the experimental protocol in 12 children with a stage 4 NB.
PBSCs, collected by cytapheresis, were mobilized by G-CSF with or without chemotherapy and cryopreserved for ASCT. After thawing, samples were split into parallel cultures for MD-DC differentiation and NK/LAK generation, during 1 week. Cocultures of NK/LAK ± MD-DCs were performed in most conditions at a 1:1 ratio for the next 7 days. Cultures were followed by immunophenotyping, cytotoxicity assay, and mixed lymphocyte reaction.
Outline of the experimental protocol in 12 children with a stage 4 NB.
PBSCs, collected by cytapheresis, were mobilized by G-CSF with or without chemotherapy and cryopreserved for ASCT. After thawing, samples were split into parallel cultures for MD-DC differentiation and NK/LAK generation, during 1 week. Cocultures of NK/LAK ± MD-DCs were performed in most conditions at a 1:1 ratio for the next 7 days. Cultures were followed by immunophenotyping, cytotoxicity assay, and mixed lymphocyte reaction.
Monocyte-derived dendritic cell (MD-DC) derivation.
Total leukocytes were incubated at 5 × 106/mL in 75-cm2 culture flasks in AIM-V medium (Gibco-BRL, Paisley, United Kingdom) supplemented with 10% fetal calf serum (FCS; Pan Biotech, Aidenbach, Germany) for 2 hours at 37°C. Nonadherent cells were removed; adherent cells were washed 3 times in 1 × phosphate-buffered saline (PBS; Gibco, Paisley, United Kingdom) and cultured until day 7 in AIM-V with 10% FCS supplemented with recombinant human IL-4 (rhuIL-4, 1000 U/mL; Schering Plough, Kenilworth, NJ) and recombinant human granulocyte-macrophage CSF (rhuGM-CSF, 1000 U/mL; Leucomax, Schering Plough) in order to derive MD-DCs at an immature stage (iDCs). In some experiments, MD-DCs were induced to maturation (mDCs) with the use of lipopolysaccharide (LPS, 20 μg/mL; Sigma Chemical, St Louis, MO) added in cultures on day 5 for 48 hours.
IL-2 activation for NK/LAK generation.
Mononuclear cells were plated in 24-well culture plates at 1 × 106 CD3+ and CD56+lymphocytes per milliliter in RPMI 1640 (Gibco-BRL) supplemented with 10% human AB serum (Institut Jacques Boy, Reims, France). The rhuIL-2 (1000 IU/mL, Proleukin; Chiron, Ratingen, Germany) was added only once, at day 0 of the culture.
Coculture of MD-DCs with NK/LAK.
At day 7 or 8, IL-2–activated bulk NK/LAK cells (or immunopurified CD3− and CD3+ lymphocyte subsets) and day-7 immature autologous MD-DCs were collected, washed twice, and resuspended in supplemented RPMI 1640 medium with 10% human AB serum at a concentration of 1 × 106 cells per milliliter. Each cell suspension (in 0.1 mL) was plated in 96-well round-bottomed culture plates at a 1:1 iDC/NK cell ratio. Control cultures of bulk NK/LAK cells or iDCs were also used. Immunopurifications of CD3− and CD3+ lymphocyte subsets from bulk NK/LAK cell cultures were carried out by means of magnetic cell separation after a 20-minute incubation step with an anti-CD3 monoclonal antibody (mAb) used at saturating conditions and a second incubation step with Dynabeads coated with sheep antimouse immunoglobulin G (IgG) antibodies (Dynal, Oslo, Norway) or by means of the NK cell isolation kit (Miltenyi Biotec, Bergisch-Gladbach, Germany). To dissect the DC/NK interactions, further cocultures of day-7 iDCs with or without LPS (10 μg/mL) or mDCs (as described above with the use of LPS added at day 5 for 48 hours) with 1 × 106 immunopurified day-7 NK/LAK cells per milliliter were performed at 3 NK/MD-DC ratios (1:1, 5:1, and 10:1). Control cultures included NK/LAK cells alone with or without IL-2 (1000 IU/mL). At days 3, 5, and 7 of coculture, viable lymphocytes were counted after trypan blue exclusion of dead cells, and the percentages of CD56+CD3− NK/LAK cells were determined by flow cytometry. Survival values were determined as the percentages of live CD56+CD3− total numbers at days 3, 5, and 7 compared with day 0 of coculture. In addition, NK/LAK cells were assayed for cytotoxicity, and IFN-γ and IL-12–p70 heterodimer production was assessed on supernatants of cocultures with the use of enzyme-linked immunosorbent assay (ELISA) kits (Immunotech, Marseilles, France).
Immunophenotyping
Three- and 4-color immunostainings were performed with the use of fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, peridinin chlorophyll A protein (PerCP)– and allophycocyanin-conjugated mAbs (all purchased from Becton Dickinson, Pont de Claix, France) in the following combinations: day 0, PBSCs using CD14/CD56/CD3/CD45 and, in some experiments, CD15/CD14/CD3/CD56; day 7, MD-DC cultures using CD40/CD83/HLA-DR, CD86/CD1a/human leukocyte antigen–DR (HLA-DR), and CD80/CD14/HLA-DR; day 7, NK/LAK cultures using CD4/CD8/CD3/CD45, CD25/CD56/CD3/CD45, and HLA-DR/CD69/CD3/CD45; and days 3 and 7, NK/LAK with or without MD-DC cocultures using CD86/CD83/HLA-DR/CD45, CD16/CD56/CD3/CD45, and CD16/CD3/CD8/CD56. Direct stainings were performed by adding the combinations on 0.3 × 106 washed cells for 15 minutes at 4°C. Analyses were performed after washing twice and fixing in 1 × PBS with 0.1% paraformaldhehyde. In some experiments, immunostainings of cocultures with a panel of unconjugated anti-NK receptor mAbs (all kindly given by Dr Eric Vivier, Centre d'Immunologie de Marseille-Luminy, Marseilles, France) were performed, including EB6 (anti-p58.1), GL183 (anti-p58.2), FSTR (anti–killer cell inhibitory receptor–2DS4 [anti-KIR2DS4] or anti-p58.3), DEC66 (anti-KIR3DL2 or anti-p140), CD94, ZIN270 (anti-NKG2A), 191B8 (anti-NKRP1), and BAB281 (anti-NKp46). Indirect immunostainings were performed on 0.2 × 106 cells for 30 minutes at 4°C. Cells were then washed twice in 1 × PBS with 0.5% bovine serum albumin and incubated with a PE-conjugated goat antimouse anti-IgG for 20 minutes at 4°C. After a 30-minute saturation step at 4°C in 1 × PBS with 10% mouse serum, a direct staining with CD16-FITC, CD56–cyanine-5, and CD3-allophycocyanin was performed as described above. Controls included isotype-matched immunoglobulins. Fluorescence-activated cell sorter (FACS) analysis was performed on a FACSCalibur cytometer (Becton Dickinson, BDIS, San Jose, CA) by means of Cellquest software (Becton Dickinson). Ten thousand cells were acquired according to standard forward scatter (FSC)/side scatter (SSC) criteria. Phenotypic analyses were determined within CD45+ gating for optimal exclusion of residual red cells and debris.
Functional assays
Cytotoxicity assays.
The cytolytic activity of NK/LAK cell cultures was measured in a standard 4-hour 51Cr-release assay against K562 and Daudi cell line targets. In addition, the lytic activity was also tested against 2 established NB cell lines, IGR-NB1 (NB-91) and IGR-NB2 (GAU). Furthermore, the susceptibility of freshly isolated NB cells to autologous NK/LAK killing was assessed with the use of short-term NB cell lines (fewer than 3 passages and referred as AUTO NB) established from invaded bone marrow samples. Briefly, target cells (106) were labeled with 150 μCi (5.55 MBq) Na251CrO4 (1 mCi/mL [37 MBq/mL]; Dupont, Boston, MA) and 2 × 103target cells per well were incubated with effector cells at different effector-target (E/T) ratios for 4 hours at 37°C. Experimental, spontaneous, and maximal releases of 2 × 103 labeled target cells were counted on a microplate scintillation counter (TopCount-NXT; United Technologies, Packard Laguna Hills, CA). For each E/T ratio, the percentage of lysis was calculated conventionally as follows: % lysis = (experimental cpm − spontaneous lysis cpm)/(maximal lysis cpm − spontaneous lysis cpm). All determinations were made in triplicate cultures, and data were calculated as mean ± SEM.
Mixed lymphocyte reactions.
T-cell allostimulatory functions of immature or LPS-treated MD-DCs were assessed in triplicate cocultures of 2 × 105allogeneic mononuclear cells with a decreased number of MD-DCs. Proliferative capacity was determined after overnight pulsing with [3H]thymidine (1 μCi [0.037 MBq] per well; NEN, Paris, France) at day 5 of the coculture. Cells were harvested onto 96-well Unifilter microplates and dried overnight, and the radioactivity was counted on a microplate scintillation counter (Topcount-NXT). All determinations were made in triplicate wells, and data were calculated as mean ± SEM.
Results
PBSCs from 12 children with a stage 4 NB were collected after mobilization by G-CSF with or without chemotherapy and thawed for the study with the clinical procedures used for ASCT. After the gating of mononuclear cells to exclude contaminating granulocytes, CD45+ PBSCs contained 49.6% ± 25.3% CD14+CD15 ± cells, 18.7% ± 12.6% CD3+ T lymphocytes, and 6.1% ± 3.0% CD3−CD56+ NK lymphocytes. No significant difference in the repartition of these cell subsets was observed between PBSCs and the paired PBMC samples; even so, CD14+ cells were systematically found enriched in PBSCs (data not shown).
PBSCs from children with NB are a source of functional MD-DCs
MD-DCs were derived from the adherent fractions of PBSCs by culture in AIM-V medium with 10% FCS and IL-4 plus GM-CSF for 1 week. It is noteworthy that starting CD14+ cells in PBSCs were distributed in 2 phenotypes according to CD15 expression: CD14+CD15− (21.6% ± 6.8% true monocytes) and CD14+CD15+ (78.4% ± 6.8%). From day 5, cultures contained a majority of floating veiled cells with a typical morphology of MD-DCs. At day 7, the mean MD-DC yield (mean recovery of 42.6 ± 35.1 × 106 MD-DCs) was 10.1% ± 2.1% of the starting PBSCs (367.0 ± 284.0 × 106 cells).
Differentiation, assessed by immunophenotyping at day 7, revealed that MD-DCs exhibit an immature phenotype (iDCs; CD14−, HLA-DRdim, CD40+, CD80dim, CD86dim, CD1abright, CD83−) (Figure 2A). Induction of maturation using a 48-hour LPS stimulation resulted in increased expression of HLA-DR and costimulatory molecules as well as up-regulation of CD83 (mDCs). As shown in Figure 2B, day-7 MD-DCs induced a significant proliferation of allogeneic PBMCs that dramatically increased after induction of maturation (optimal ratio of 1:5 versus 1:25,P < .001). Overall, regardless of the conditioning regimen, neither phenotypic nor functional differences were evidenced between MD-DCs derived from PBSCs or paired PBMC samples (data not shown).
MD-DCs from PBSCs collected in children with NB.
MD-DCs were derived from adherent monocytes and cultured for 7 days in AIM-V medium with 10% FCS supplemented with rhuIL-4 and GM-CSF (1000 IU/mL each). Induction of maturation was performed by adding LPS (20 μg/mL) on day 5 for 48 hours. (A) Immunophenotyping of immature (iDC, LPS−) versus mature (mDC, LPS+) MD-DCs was performed by 3-color flow cytometry by means of the combinations of FITC-, PE-, and PerCP-conjugated mAbs CD40/CD83/HLA-DR, CD86/CD1a/HLA-DR, and CD80/CD14/HLA-DR. One representative experiment is shown. Induction of maturation is evidenced by up-regulation of CD83 (mean fluorescence intensity [MFI], 29.6 versus 3.4); CD86 (MFI, 152.7 versus 60.4); CD80 (MFI, 61.2 versus 18.9); HLA-DR (MFI, 454.1 versus 129.4); and down-regulation of CD1a (MFI, 235.5 versus 187.3). (B) Allostimulatory capacities of immature (LPS−) versus mature (LPS+) MD-DCs. Results are presented as mean ± SEM (n = 9) proliferation of 2 × 105 allogeneic mononuclear cells as a function of decreasing numbers of stimulating MD-DCs. Stars indicate significant statistical differences (P < .01) between values obtained with immature versus mature MD-DCs.
MD-DCs from PBSCs collected in children with NB.
MD-DCs were derived from adherent monocytes and cultured for 7 days in AIM-V medium with 10% FCS supplemented with rhuIL-4 and GM-CSF (1000 IU/mL each). Induction of maturation was performed by adding LPS (20 μg/mL) on day 5 for 48 hours. (A) Immunophenotyping of immature (iDC, LPS−) versus mature (mDC, LPS+) MD-DCs was performed by 3-color flow cytometry by means of the combinations of FITC-, PE-, and PerCP-conjugated mAbs CD40/CD83/HLA-DR, CD86/CD1a/HLA-DR, and CD80/CD14/HLA-DR. One representative experiment is shown. Induction of maturation is evidenced by up-regulation of CD83 (mean fluorescence intensity [MFI], 29.6 versus 3.4); CD86 (MFI, 152.7 versus 60.4); CD80 (MFI, 61.2 versus 18.9); HLA-DR (MFI, 454.1 versus 129.4); and down-regulation of CD1a (MFI, 235.5 versus 187.3). (B) Allostimulatory capacities of immature (LPS−) versus mature (LPS+) MD-DCs. Results are presented as mean ± SEM (n = 9) proliferation of 2 × 105 allogeneic mononuclear cells as a function of decreasing numbers of stimulating MD-DCs. Stars indicate significant statistical differences (P < .01) between values obtained with immature versus mature MD-DCs.
NK/LAK cells from IL-2–activated PBSCs display high cytotoxicity against NB tumor cells
PBSCs and paired PBMCs were cultured in the presence of 1000 IU/mL rIL-2 at a concentration of 1 × 106/mL effectors CD3+/−CD56+ lymphocytes. As shown in Figure 3A, phenotypic analysis displayed a significant and gradual expansion of CD56brightCD3− NK cells between day 0 and 7 (ie, 4- to 9.4-fold), representing 6.1% ± 3.0% to 31.8% ± 12.6% of CD45+cells, respectively (P < .001). The same range of expansion was observed in paired PBMC samples collected before (13.0% ± 5.2% to 65.2% ± 21.5%) and during (10.1% ± 7.3% to 49.0% ± 25.6%) mobilization. All together, starting with PBSCs or PBMCs, the percentage of all NK cells was increased by 5-fold. At day 7, the early and late activation markers, CD69 and HLA-DR, were significantly up-regulated on CD56brightCD3−NK cells in a mean percentage range of 58.6% ± 2.3% to 22.3% ± 10.8% of these cells, respectively (data not shown).
Selective expansion of NK/LAK from IL-2–activated PBSCs.
PBSCs were incubated in 24-well plates at 1 × 106 T/NK (CD3+/CD56+CD3−) lymphocytes per milliliter and cultured for 7 days in RPMI 1640 with 10% human AB serum supplemented with rIL-2 (1000 IU/mL). (A) Immunophenotyping of PBSC cultures was performed at day 0 (left vertical panel), day 4 (center vertical panel), and day 7 (right vertical panel) by 4-color flow cytometry with the use of the combinations of FITC-, PE-, PerCP-, and APC-conjugated mAbs CD4/CD8/CD3/CD45, CD25/CD56/CD3/CD45, and HLA-DR/CD69/CD3/CD45. One representative experiment showing the expansion of CD56brightCD3− NK/LAK cells is presented. (B,C) Cytolytic activity of cultures at days 2 (white bars), 4 (gray bars), and 7 (black bars), depicted as the percentage of lysis at a 6.25:1 E/T ratio against tumor targets. In panel B, results are presented as mean ± SEM (n = 10) percentage of lysis against K562, Daudi, and the 2 NB cell lines IGR-NB1 and IGR-NB2. Panel C shows a representative experiment displaying the cytolytic activity of immunopurified CD3−CD56+ NK/LAK cells on autologous NB tumor cells freshly isolated from invaded bone marrow (AUTO-NB) compared with lysis of allogeneic targets (NB1 and DAUDI).
Selective expansion of NK/LAK from IL-2–activated PBSCs.
PBSCs were incubated in 24-well plates at 1 × 106 T/NK (CD3+/CD56+CD3−) lymphocytes per milliliter and cultured for 7 days in RPMI 1640 with 10% human AB serum supplemented with rIL-2 (1000 IU/mL). (A) Immunophenotyping of PBSC cultures was performed at day 0 (left vertical panel), day 4 (center vertical panel), and day 7 (right vertical panel) by 4-color flow cytometry with the use of the combinations of FITC-, PE-, PerCP-, and APC-conjugated mAbs CD4/CD8/CD3/CD45, CD25/CD56/CD3/CD45, and HLA-DR/CD69/CD3/CD45. One representative experiment showing the expansion of CD56brightCD3− NK/LAK cells is presented. (B,C) Cytolytic activity of cultures at days 2 (white bars), 4 (gray bars), and 7 (black bars), depicted as the percentage of lysis at a 6.25:1 E/T ratio against tumor targets. In panel B, results are presented as mean ± SEM (n = 10) percentage of lysis against K562, Daudi, and the 2 NB cell lines IGR-NB1 and IGR-NB2. Panel C shows a representative experiment displaying the cytolytic activity of immunopurified CD3−CD56+ NK/LAK cells on autologous NB tumor cells freshly isolated from invaded bone marrow (AUTO-NB) compared with lysis of allogeneic targets (NB1 and DAUDI).
Lytic activity of cultures was tested at days 2, 4, and 7 against the K562 and Daudi cell lines, as well as against the 2 NB cell lines. As early as day 2, rIL-2–treated PBSCs displayed a strong cytotoxicity against K562 and Daudi (42.3% ± 14.7% and 34 % ± 15.6%, respectively, at a 25:1 E/T ratio). This NK/LAK activity progressively increased with culture duration with up to 50.2 % ± 15.3% and 49.8 % ± 14.2% lysis, respectively, at day 7, and remained high, even at a low E/T ratio (30.2 % ± 9.8% and 35.8 % ± 13.2% against K562 and Daudi, respectively, at a 1.5:1 E/T ratio). Interestingly, the 2 NB cell lines were also sensitive to lysis by activated effectors. Although lower at day 2 and 4, cytotoxicity against NB-1 and NB-2 was comparable to that achieved against K562 and Daudi at day 7 (Figure 3B). Interestingly, we confirmed the susceptibility of freshly isolated NB cells to autologous NK/LAK killing. As shown in Figure 3C, day-7 immunopurified CD3−CD56+ NK/LAK cells lysed autologous freshly isolated NB cells from invaded bone marrow as efficiently as allogeneic targets (NB1 and DAUDI cell lines).
Taken together, PBSCs displayed a significant cytotoxicity increase on the 4 target cells lines between day 2 and day 7, (P = .005 and P = .007, respectively, with the use of a paired t test), but not between day 2 and day 4. In addition, we did not observe major significant differences related to mobilization (data not shown), except against the Daudi target at day 4 (mean ± SD percentage lysis at 6.25:1 ratio): PBMCs before mobilization were 64.5% ± 17.0% versus PBMCs during mobilization (40.7% ± 17.7%) versus PBSCs (35.0 % ± 12.0%) (PBMCs before mobilization versus PBMCs during mobilization,P = .010; PBMCs before mobilization versus PBSCs,P < .001; PBMCs during mobilization versus PBSCs; nonsignificant); this suggests a potential impact of G-CSF on NK functions, but this impact was not evidenced at day 7 or on other cell targets.
Overall, these results suggest that potent non–MHC-restricted cytolytic activity (against autologous and allogeneic NB tumor cells) is optimally displayed by PBSCs after 7 days of rIL-2 stimulation that is mediated mostly by activated CD56brightCD3−NK/LAK cells.
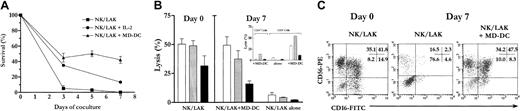
MD-DCs sustain survival and activation of NK/LAK cells
Because IL-2 was associated with toxicity in children but was required to maintain NK/LAK cell survival in vivo, we tested whether cocultures of MD-DCs with NK/LAK cells in the absence of exogenous rIL-2 would maintain and/or enhance LAK activity in autologous systems. Therefore, day-7 rIL-2–deprived NK/LAK cells were cocultured with day-7 immature MD-DCs derived from autologous PBSCs in 10 independent experiments from 7 NB patient samples. These cocultures, as well as control cultures of NK/LAK alone with or without IL-2, were evaluated for cell viability, immunophenotype, and cytolytic activity. Survival of activated CD56brightCD3− NK/LAK cells cocultured with MD-DCs was dramatically enhanced compared with control cultures (NK/LAK cells with or without IL-2) as assessed by trypan blue exclusion and flow cytometry analysis counting (Figure4A). In addition to survival, NK/LAK cell cytotoxic activity was also significantly maintained until day 7 of coculture in the absence of IL-2. Very high levels of cytotoxicity were obtained with median percentages of lysis at a 6.25:1 E/T ratio of 56% (range, 52%-99%); 56% (range, 53%-72%); 12% (range, 9%-26%); 3% (range, 1%-23%) against K562, Daudi, NB1, and NB2 respectively (Figure 4B). Cytolytic activity was not detected when the cells were separated by a porous membrane (transwells), arguing in favor of a direct cell-to-cell contact for the DC-mediated NK cell survival and activation (data not shown). The MD-DC–mediated NK cell activation could not be accounted for by the T/LAK component. Indeed, coculture of MD-DCs with CD3− and CD3+ cell subsets immunopurified from NK/LAK bulk suspensions demonstrated that the responding effectors were indeed CD56+CD3− and not CD3+ cells (Figure 4B, inset). Therefore, MD-DCs selectively activate NK/LAK cells in vitro in a cell-to-cell contact– dependent manner.
Effect of autologous MD-DCs on survival and activation of rIL-2–deprived NK/LAK cells.
Autologous MD-DCs sustain survival and activation of rIL-2–deprived NK/LAK cells. NK/LAK cells with or without MD-DCs were cultured at a 1:1 E/T ratio for 7 days in RPMI 1640 with 10% human AB serum without IL-2 at 1 × 106 cells per milliliter. (A) At days 3, 5, and 7, viable lymphocytes were counted after trypan blue exclusion of dead cells, and mean ± SEM (n = 7) percentages of CD56+CD3− NK/LAK cells were determined by flow cytometry. Survival values were determined as the percentages of live CD56+CD3− total numbers at days 3, 5, and 7 compared with day 0. In comparison, mean ± SEM (n = 3) survival percentages of NK/LAK cells alone left in culture with IL-2 (1000 IU/mL) is reported. (B) Cytotytic activity of day-7 cocultures of bulk NK/LAK cells (n = 10) or CD3− and CD3+ immunopurified subsets (n = 3, inset) with or without MD-DCs. Results are presented as mean ± SEM percentages of lysis at a 6.25:1 E/T ratio against K562 (white bars), Daudi (gray bars), and NB1 (black bars). (C) Immunophenotyping of cocultures was performed by 4-color flow cytometry with the use of the combination of FITC-, PE-, PerCP-, and APC-conjugated mAbs CD16/CD56/CD3/CD45. One representative experiment showing the persistence of CD3−CD56brightCD16+ NK/LAK cells in a day-7 coculture with MD-DCs is presented.
Effect of autologous MD-DCs on survival and activation of rIL-2–deprived NK/LAK cells.
Autologous MD-DCs sustain survival and activation of rIL-2–deprived NK/LAK cells. NK/LAK cells with or without MD-DCs were cultured at a 1:1 E/T ratio for 7 days in RPMI 1640 with 10% human AB serum without IL-2 at 1 × 106 cells per milliliter. (A) At days 3, 5, and 7, viable lymphocytes were counted after trypan blue exclusion of dead cells, and mean ± SEM (n = 7) percentages of CD56+CD3− NK/LAK cells were determined by flow cytometry. Survival values were determined as the percentages of live CD56+CD3− total numbers at days 3, 5, and 7 compared with day 0. In comparison, mean ± SEM (n = 3) survival percentages of NK/LAK cells alone left in culture with IL-2 (1000 IU/mL) is reported. (B) Cytotytic activity of day-7 cocultures of bulk NK/LAK cells (n = 10) or CD3− and CD3+ immunopurified subsets (n = 3, inset) with or without MD-DCs. Results are presented as mean ± SEM percentages of lysis at a 6.25:1 E/T ratio against K562 (white bars), Daudi (gray bars), and NB1 (black bars). (C) Immunophenotyping of cocultures was performed by 4-color flow cytometry with the use of the combination of FITC-, PE-, PerCP-, and APC-conjugated mAbs CD16/CD56/CD3/CD45. One representative experiment showing the persistence of CD3−CD56brightCD16+ NK/LAK cells in a day-7 coculture with MD-DCs is presented.
The phenotypic characterization of NK/LAK cells cocultured with MD-DCs included CD16/CD56/CD3/CD45 immunostainings. Before coculture, on the basis of CD16 density, 3 CD56bright NK/LAK subsets were identified, CD56brightCD16−, CD56brightCD16dim, and CD56brightCD16bright cells, while most CD56dim cells expressed CD16 at high density. Interestingly, at day 7, the percentage of CD56brightCD16+ NK/LAK cells surviving in coculture with DCs was similar to that observed prior to IL-2 deprivation, while residual viable NK/LAK from control cultures deprived of IL-2 were mostly CD56brightCD16−with a dramatic clearance of mature CD56dim cells (Figure4C). Overall, this phenotype was associated with high levels of cytotoxicity displayed by the cocultures. However, such CD56bright/CD16+ cells did not up-regulate NKRP1- or NKp46 (BAB281)–activating receptors nor did they down-regulate CD158a/p58.1, CD158b/p58.2, CD94/NKG2A, KIR2DS4/p58.3, or KIR3DL2/p140 inhibitory receptors following interaction with MD-DCs. All together, autologous immature MD-DCs maintain survival and effector functions of CD56bright/CD16+ NK/LAK cells in the absence of exogenous IL-2.
LPS-activated MD-DCs are the most potent antigen-presenting cells (APCs) for NK/LAK activation
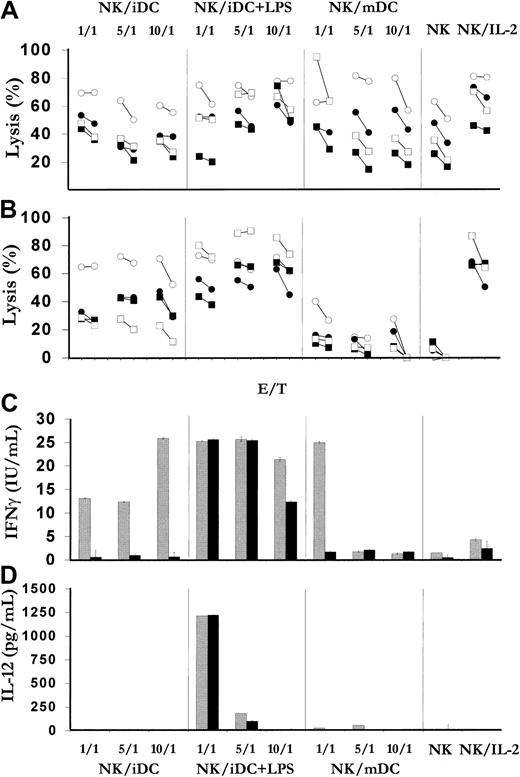
To further dissect the dynamic of the DC/NK cell cross-talk, cocultures of CD56+CD3− immunopurified day-7 NK/LAK cells were performed with MD-DCs at various stages of maturation, that is, immature MD-DCs (iDCs; CD86dim/CD83−), maturing DCs (iDCs with LPS stimulation), or mature MD-DCs (mDCs; CD86bright/CD83+) after 2 days of LPS stimulation, at various NK/DC ratios (1:1, 5:1, 10:1). NK cell functions were assessed (cytotoxicity, IFN-γ production) following 3- to 7-day cocultures. Interestingly, at all differentiation stages, DCs can maintain (day 3; Figure 5A) and promote (day 7; Figure 5B) NK cell activation. This DC-mediated NK cell activation even occurred at a 10:1 NK/DC ratio and, most strikingly, for maturing DCs. Importantly, at late time points (day 7), maturing DCs were more potent than rhuIL-2 at triggering IFN-γ production from NK cells (Figure 5C). Immature DCs could promote NK cell activation only at early time points (day 3 compared with NK alone) for both killing and IFN-γ secretion. In contrast, mature DCs promoted only cytolytic activity but not IFN-γ production, an activation pattern resembling IL-2 (Figure 5C). As mentioned above, DC-mediated NK cell cytolytic activity required cell-to-cell contact (not shown). However, at a ratio of 1 DC to 5 or 10 NK cells, the capacity of maturing DCs to trigger IFN-γ production was not dependent on IL-12 (Figure 5D). Overall, immature DCs stressed with an inflammatory stimulus electively induced and/or maintained NK/LAK effector functions.
Effect of LPS-activated MD-DCs on NK/LAK activation.
LPS-activated MD-DCs are the most potent APCs for NK/LAK cell activation. Cocultures of day-7 immature MD-DCs (iDCs) with or without LPS or mature MD-DCs (mDCs) with 1 × 106 immunopurified day-7 NK/LAK cells per milliliter were performed at 3 NK/MD-DC ratios (1:1, 5:1, and 10:1). Control cultures included NK/LAK alone with or without IL-2 (1000 IU/mL). At days 3 (panel A) and 7 (panel B), NK/LAK cells from the different conditions were counted and assayed for cytotoxicity at 2 E/T ratios (10:1 and 5:1) against K562 (○), Daudi (■), IGR-NB1 (●), and IGR-NB2 (▪). ELISA assay for IFN-γ (panel C) and IL-12–p70 heterodimer production (panel D) were performed on supernatants of day-3 (gray bars) and day-7 (black bars) cocultures. This figure shows a representative of 2 experiments performed on 1 patient.
Effect of LPS-activated MD-DCs on NK/LAK activation.
LPS-activated MD-DCs are the most potent APCs for NK/LAK cell activation. Cocultures of day-7 immature MD-DCs (iDCs) with or without LPS or mature MD-DCs (mDCs) with 1 × 106 immunopurified day-7 NK/LAK cells per milliliter were performed at 3 NK/MD-DC ratios (1:1, 5:1, and 10:1). Control cultures included NK/LAK alone with or without IL-2 (1000 IU/mL). At days 3 (panel A) and 7 (panel B), NK/LAK cells from the different conditions were counted and assayed for cytotoxicity at 2 E/T ratios (10:1 and 5:1) against K562 (○), Daudi (■), IGR-NB1 (●), and IGR-NB2 (▪). ELISA assay for IFN-γ (panel C) and IL-12–p70 heterodimer production (panel D) were performed on supernatants of day-3 (gray bars) and day-7 (black bars) cocultures. This figure shows a representative of 2 experiments performed on 1 patient.
Reciprocal cross-talk between MD-DCs and activated NK/LAK cells
Because immature DCs were also capable of maintaining long-term activation of NK/LAK cells, we addressed the question of whether NK/LAK cells could activate such iDCs. Indeed, a significant up-regulation of CD86 and CD83 molecules was observed in the whole CD45+HLA-DR+ population (Figure6A, center panels), as compared with iDCs without NK cells (Figure 2A). Up to 33% of iDCs became CD83/CD86bright in the presence of NK/LAK cells, a percentage that was further enhanced (56%) in the maturing DCs (iDCs with LPS in the coculture). As already reported, it is likely that iDCs represented NK cell targets, since we observed 26%, 27.8%, and 41.8% of CD45+HLA-DR+ cells representing iDCs, iDCs with LPS, and mDCs, respectively (DC1 + DC2 in left panels, Figure 5A) alive at day 3 of cocultures. Recruitment of multiple NK/LAK cells onto single iDCs could be observed at day 7 (Figure 7A).
Reciprocal cross-talk between MD-DCs and activated NK/LAK cells.
Cocultures were performed as described briefly in Figure 5. Immunophenotyping of 1:1 ratio cocultures of NK cells/iDCs, NK cells/iDCs with LPS, and NK cells/mDCs were performed by 4-color flow cytometry with the combinations of FITC-, PE-, PerCP-, and APC-conjugated mAbs CD86/CD83/HLA-DR/CD45 and CD16/CD3/CD8/CD56. Left panels show the cellular morphology according to FSC/SSC criteria, allowing the identification of 3 cell subpopulations: DC1 cells, DC2 cells, and CD56+CD3− NK/LAK cells. Center panels show a dot plot analysis of CD86/CD83 staining after gating on CD45+HLA-DR+ cells (not shown). Percentages of DC1 CD86bright/CD83bright and DC2 CD86dim/CD83dim are reported; these 2 subsets of DCs are identified by color backgating on the FSC/SSC dot blots. Right panels show a dot plot analysis of CD56/CD16 staining after gating on CD56+CD3− cells (not shown), whose percentages are reported on the corresponding FSC/SSC dot plots. Percentages of CD56+CD16+ are reported. This shows a representative experiment performed in 1 patient out of 2. (A) Results of immunophenotyping at day 3. At this early time point, NK/LAK cells turn iDCs into maturing DCs exhibiting a CD86+/CD83+ phenotype, which is further enhanced with addition of LPS. (B) Results of immunophenotyping at day 7. At this late time point, survival of CD56+CD3− is significantly increased in the NK cells/iDCs with LPS.
Reciprocal cross-talk between MD-DCs and activated NK/LAK cells.
Cocultures were performed as described briefly in Figure 5. Immunophenotyping of 1:1 ratio cocultures of NK cells/iDCs, NK cells/iDCs with LPS, and NK cells/mDCs were performed by 4-color flow cytometry with the combinations of FITC-, PE-, PerCP-, and APC-conjugated mAbs CD86/CD83/HLA-DR/CD45 and CD16/CD3/CD8/CD56. Left panels show the cellular morphology according to FSC/SSC criteria, allowing the identification of 3 cell subpopulations: DC1 cells, DC2 cells, and CD56+CD3− NK/LAK cells. Center panels show a dot plot analysis of CD86/CD83 staining after gating on CD45+HLA-DR+ cells (not shown). Percentages of DC1 CD86bright/CD83bright and DC2 CD86dim/CD83dim are reported; these 2 subsets of DCs are identified by color backgating on the FSC/SSC dot blots. Right panels show a dot plot analysis of CD56/CD16 staining after gating on CD56+CD3− cells (not shown), whose percentages are reported on the corresponding FSC/SSC dot plots. Percentages of CD56+CD16+ are reported. This shows a representative experiment performed in 1 patient out of 2. (A) Results of immunophenotyping at day 3. At this early time point, NK/LAK cells turn iDCs into maturing DCs exhibiting a CD86+/CD83+ phenotype, which is further enhanced with addition of LPS. (B) Results of immunophenotyping at day 7. At this late time point, survival of CD56+CD3− is significantly increased in the NK cells/iDCs with LPS.
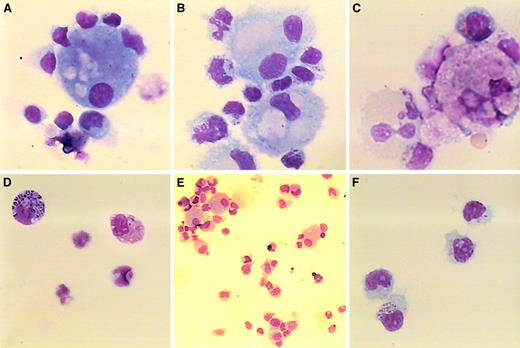
Morphologic analysis of NK/DC clusters from NB patients.
Cytospins were carried out with cells harvested in the different cocultures described in the Figure 5 legend (1:1 ratio, day 7), stained with May-Grünwald-Giemsa coloration and observed under optical microscopy: NK cells/iDCs (panel A); NK cells/iDCs with LPS (panels B and E); NK cells/mDCs (panel C); NK cells alone without IL-2 (panel D); and NK cells alone with IL-2 (1000 IU/mL) (panel F). Original magnification was × 1000 for all panels, except that it was × 400 for panel E. These observations are representative of 2 experiments.
Morphologic analysis of NK/DC clusters from NB patients.
Cytospins were carried out with cells harvested in the different cocultures described in the Figure 5 legend (1:1 ratio, day 7), stained with May-Grünwald-Giemsa coloration and observed under optical microscopy: NK cells/iDCs (panel A); NK cells/iDCs with LPS (panels B and E); NK cells/mDCs (panel C); NK cells alone without IL-2 (panel D); and NK cells alone with IL-2 (1000 IU/mL) (panel F). Original magnification was × 1000 for all panels, except that it was × 400 for panel E. These observations are representative of 2 experiments.
Interestingly, the most activating DCs for NK/LAK cells, that is, maturing DCs (iDCs with LPS), were also those that significantly promoted NK cell long-term survival without exogenous IL-2 (up to 25%; Figure 6B, left panels). Morphologic analyses highlighted clustering of DCs with NK/LAK cells, mostly in the maturing DCs (Figure 7B,E). In contrast, mature DCs did not promote NK cell survival (27% and 2% survival at days 3 and 7, respectively). As expected, mDCs seemed to be engaged toward apoptotic process (Figure 7C). It is noteworthy that most NK cells alive at day 7 exhibited a mature phenotype, CD56brightCD16bright, that is associated with effector functions.
All together, NK/LAK cells can directly activate immature DCs with or without additional inflammatory stimulus, and such activated DCs, in return, are empowered to maintain not only NK cell effector functions but also long-term survival.
Discussion
The purpose of this study was to investigate the potential therapeutic relevance of the newly described DC/NK cell cross-talk on the basis of our previous results demonstrating that mouse DCs enhance NK cell functions in vitro and in vivo,9 and on recent similar reports in human in vitro model systems demonstrating that DCs can act on the priming phase of NK cell activation.10-14The dynamics of such DC/NK cell cross-talk was dissected in a feasibility study aimed at generating autologous DC-NK/LAK cell cocultures designed from G-CSF–mobilized PBSCs in a series of 12 children with metastatic NB. In this poor-prognosis tumor model, high therapeutic numbers of functional MD-DCs and NK/LAK cells could be propagated and further cocultured without exogenous IL-2 to allow expansion of CD56bright/CD16+ NK cells with enhanced survival and effector functions (cytotoxicity, IFN-γ production).
G-CSF is currently used in ASCT to mobilize hematopoietic precursors, and several studies in adults have suggested that this growth factor may have a negative impact on NK cell functions and recovery15-17 and, more recently, on the derivation of fully competent MD-DCs,18 but little is known about this in children. Impaired NK activity of freshly isolated G-CSF–mobilized PBSCs has been documented mainly in series of healthy donors and, moreover, in short-term IL-2 culture (24 hours), while NK cells in prolonged culture with or without feeder cells restored normal cytolytic function.17,19 In accordance with these results, we previously observed that the generation of cytotoxic effectors from PBSCs obtained in short-term cultures in IL-2 (fewer than 4 days) was significantly less efficient in children with tumors compared with adults.20 In the present study, in which we compared expansion and cytolytic functions of NK/LAK from samples collected before (PBMCs) or during (PBMCs and PBSCs) mobilization, we did not, owing to interpatient variations, observe major significant differences imputable to the G-CSF conditoning, except against the Daudi target at day 4 (but not at day 7). One can postulate that children with advanced NB, frequently heavily pretreated with chemotherapy, indeed displayed impaired NK functions compared with healthy children (whom we have not assessed), but, in this situation, it seems difficult to find evidence for an additional impact of G-SCF.
Here we show that day-7 LAK cytolytic activity was potent not only against K562 and Daudi but also against relevant targets, that is, human autologous short-term neuroblastoma cells derived from invaded bone marrow as well as allogeneic neuroblastoma cell lines. It is noteworthy that the NB tumor cells used in this study as target cells for NK/LAK killing do not express detectable levels of MHC class I molecules (eg, with the use of W6.32 mAbs; data not shown), a common feature of NB tumors as reported by others.21 Therefore, the lack of MHC class I molecules, described as major KIR ligands,22 on these tumor cells may account for the high sensitivity of neuroblastoma cells to NK/LAK lysis and constitute a strong rationale for implementing LAK cell–based therapy in this refractory tumor model. Overall, we conclude that a day-7 culture duration is required for optimal expansion of cytotoxic NK/LAK cells.
Although it was shown that in adults G-CSF–mobilized stem cells are a more productive source of DCs than bone marrow–derived CD34+ progenitors,23 few studies have reported the feasibility of generating MD-DC cultures from PBSCs in children. Indeed, in this series of 12 children with NB, up to 10.1% ± 2.1% of total leukocytes from PBSCs could be differentiated into MD-DCs with the use of IL-4 and GM-CSF. The percentage of CD14+ cells contained in the PBSCs was 49.6% ± 25.3%, and this proportion was found to be enriched when G-CSF, rather than chemotherapy alone, was used for mobilization. With respect to the starting monocytic population, one can extrapolate the yield of MD-DC recovery to 89.7% if one considers the starting CD14+CD15− cell subset (but only 23.4% of total CD14+ cells, including a large proportion of CD14+CD15+ cells). Such MD-DCs exhibited an immature phenotype in that they were CD83− and strongly responded to inflammatory stimuli for up-regulation of costimulatory molecules and alloreactivity. In respect to our end point, the proof of the principle of consistantly propagating functional DCs for NK/LAK activation, there was no significant difference in rates or functions with the use of steady-state versus mobilized PBSCs for DC propagation by means of the process of adherence.
IL-2 has been administered in vivo to expand and/or sustain LAK activity of NK/LAK cells propagated at high numbers ex vivo. However, the selective expansion of human NK cells during continuous in vivo infusion of low-dose IL-2 probably results from enhanced NK cell differentiation from CD34+ hematopoietic progenitors combined with an IL-2–dependent delay in NK cell death, rather than from proliferation of mature NK cells in the periphery.24 A similar observation has been recently reported with the newly identified IL-21 cytokine.25 Other NK cell–stimulatory factors, IL-12, tumor necrosis factor–α (TNF-α), IL-1β, IL-10, or cytokines known to use –IL-2 receptor-γ (IL-2Rγ) common chain for signaling (IL-4, IL-7, IL-9, IL-13), were not shown to significantly sustain NK cell survival in vitro. Only IL-15, much more abundant in normal tissues than IL-2, was demonstrated to prolong NK cell survival at picomolar concentrations in vitro, but it is not available for clinical grade infusions.26 We previously demonstrated in a mouse model that BM-DCs promote resting NK cell effector functions in vitro and mediate NK cell–dependent tumor rejection in Rag−/−mice.9 Recent studies10-14 have shown that human MD-DCs enhance proliferation and effector functions of both resting and IL-2–activated NK cells. The DC-mediated NK cell activation relies on cell-to-cell contacts with no substantial contribution of IL-12, IL-15, or IL-18 for the NK cell effector functions. Now, we implement these findings to autologous sytems and demonstrate that MD-DCs are able to maintain the survival of 7-day NK/LAK cells for a week in the absence of exogenous cytokines. Importantly, cytolytic activity of NK/LAK cells in coculture with DCs remained potent against K562, Daudi, and NB cell lines. Such IL-2–deprived 7-day NK/LAK cells in coculture with DCs were mostly CD56bright/CD16+ or CD16− with loss of the mature CD56dim counterparts but not T cells.
We further dissected the dynamics of the DC/NK cell cross-talk in these autologous settings by studying the effects of DC-differentiation stage on NK cell effector functions and survival. Strikingly, immature DCs maturing under inflammatory stimulation or NK/LAK signaling were the most potent antigen-presenting cells triggering cytolysis, IFN-γ production, and survival of NK/LAK cells. In contrast, mature DCs were unable to trigger IFN-γ production or sustained NK cell survival in such conditions. Importantly, cell-to-cell contact was critical for DC-mediated NK cell activation and was even more potent than rhuIL-2. These findings could be relevant in the early steps of innate immune response. Tissue-resident DCs encountering pathogens or tumor cells might undergo an activation process that will concomitantly recruit blood-resting NK cells, favoring maturing DC/NK cell interaction. The DC/NK cell cross-talk that is initiated will likely lead to NK cell triggering and have the result that activated NK cells will favor clustering and activation of newly recruited DCs at the site of inflammation; in addition, when the NK/DC ratio flares up as inflammation goes on, DCs become NK cell targets to shut off the process.
Regulatory pathways of recruitment and migration of DCs and NK cells remain largely unknown. In mice infused with tumor cells, it has been recently shown that tumor cells lacking appropriate MHC class I expression induced NK cell infiltration, cytotoxic activation, and induction of transcription of γ-IFN in KIR-bearing NK cells.27 It is conceivable that reinfused NK/LAK cells could traffick to NB tumor deposits because such tumor cells are often MHC class I–negative. Interestingly, DCs and NK cells have been shown to be responsive to similar chemokines, such as macrophage-inflammatory protein-3α in inflammed sites28 and virus-infected mice,29 where NK cells have been found in close contact with splenic APCs. This interaction could be pivotal for a chemokine-to-cytokine-to-chemokine cascade leading to local control of the infection. In fact, L-selectin adhesion molecule initiates the extravasation of immune effectors specifically into tissues across high endothelial venules. L-selectin has been shown to be uniquely expressed on the CD56brightsubset of NK cells30 and also on plasmacytoid DC.31 Therefore, it is conceivable that DCs as well as NK/LAK cells, when coinfused, might home to inflammatory tumor deposits, contributing to amplification of innate immune responses.
These results support the use of PBSCs not only as a hematopoietic support but also as a source of effector cells with additive or synergistic functions. The ability to generate both NK/LAK effectors and MD-DCs in short-term cultures dramatically extends the therapeutic field of autologous PBSCs. The administration of pluripotent grafts might improve antitumor activity in vivo and lessen IL-2–related morbidity. NB constitutes an ideal tumor model to demonstrate the efficacy of such a combined multicellular adoptive therapy.
We are endebted to Dr Chantal Le Forestier from ETS Créteil for providing PBSC samples and to Gwenaëlle Leroux from Institut Gustave Roussy for expert technical assistance in recovery and culture of neuroblastoma cells. We also thank Dr Eric Vivier for helpful discussions.
Supported by a PHRC96 grant from the French Health Ministry; K.M. was a recipient of grant from Ligue Française contre le Cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eric Angevin, Unité d'Immunologie, Département de Biologie Clinique, Institut Gustave Roussy, 39 Rue Camille Desmoulins, 94805 Villejuif Cedex, France; e-mail:angevin@igr.fr.


![Fig. 2. MD-DCs from PBSCs collected in children with NB. / MD-DCs were derived from adherent monocytes and cultured for 7 days in AIM-V medium with 10% FCS supplemented with rhuIL-4 and GM-CSF (1000 IU/mL each). Induction of maturation was performed by adding LPS (20 μg/mL) on day 5 for 48 hours. (A) Immunophenotyping of immature (iDC, LPS−) versus mature (mDC, LPS+) MD-DCs was performed by 3-color flow cytometry by means of the combinations of FITC-, PE-, and PerCP-conjugated mAbs CD40/CD83/HLA-DR, CD86/CD1a/HLA-DR, and CD80/CD14/HLA-DR. One representative experiment is shown. Induction of maturation is evidenced by up-regulation of CD83 (mean fluorescence intensity [MFI], 29.6 versus 3.4); CD86 (MFI, 152.7 versus 60.4); CD80 (MFI, 61.2 versus 18.9); HLA-DR (MFI, 454.1 versus 129.4); and down-regulation of CD1a (MFI, 235.5 versus 187.3). (B) Allostimulatory capacities of immature (LPS−) versus mature (LPS+) MD-DCs. Results are presented as mean ± SEM (n = 9) proliferation of 2 × 105 allogeneic mononuclear cells as a function of decreasing numbers of stimulating MD-DCs. Stars indicate significant statistical differences (P < .01) between values obtained with immature versus mature MD-DCs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/7/10.1182_blood.v100.7.2554/3/m_h81923173002.jpeg?Expires=1765892066&Signature=EAbdzD4SMak0GBqa83wXVdXi~hNLeJTe6uI9ZOtObKg9Qh1yYPKvwSbbeM15HFU52-PB1C462tJcyXnx5mCGQj9PKNCaT6bpnsGpgZ4he-2xMjVzPWXLjT3RVe4yEhf7eIZWUs~hNwayFdbx8ur2aAoq5DUe5YwqfgxV~JGtbu2WoebPNE6wIlLTmZ~GuWFaF6zfIgcX-Rul-SyKa8CWYDS6TZ29P~lR3asv6usJgP8hZnP0aVtyo-Qf7RUa1naEZx~q5fI6bwG8gaLEe8r9JMXCz36FEWlKdJJLZnhVo4YrczYTnwHcyaDRUSwJ9Ii5AI-ZEv0pl18hRjCwvMHmxQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal