CXC chemokines play a central role in regulation of neutrophil activation and chemotaxis. Because the chemotactic responses of neutrophils are impaired after phagocytosis, we explored the effect of phagocytic stimuli on the expression of interleukin-8 (IL-8) receptors, CXCR1 and CXCR2, in human neutrophils. After phagocytosis of opsonized yeast, the expression of CXCR1 and CXCR2 was substantially down-regulated and was accompanied by reduced Ca++responses to corresponding ligands, IL-8 and neutrophil-activating peptide–2 (NAP-2). The levels of CXCR1 and CXCR2 mRNA were constant during phagocytic stimulation of neutrophils. Confocal microscopy revealed that CXCR reduction was not via internalization. Metalloproteinase inhibitor, 1,10-phenantroline, prevented the reduction of CXCRs induced by phagocytosis, indicating that proteolytic degradation may be responsible for down-regulation. These observations suggest that down-regulation of CXCR expression may substantially reduce the responsiveness of phagocytosing neutrophils to CXC chemokines.
Introduction
Two major responses are central to neutrophil leukocyte (polymorphonuclear neutrophil [PMN]) functioning: chemotaxis and phagocytosis. The migration of leukocytes is governed by chemotactic cytokines called chemokines. Chemokines are a large family of small chemotactic proteins divided into 4 subfamilies according to the positioning of cysteines in their primary sequences. Those whose first 2 cysteines are separated by one amino acid belong to the CXC (or α) subfamily and regulate the responses of PMNs.1,2Human CXC chemokines interact with the specific G-protein–coupled receptors CXCR1 and CXCR2. CXCR1 has high affinity for interleukin-8 (IL-8) and low affinity for other α-chemokines, neutrophil-activating peptide–2 (NAP-2), melanoma growth-stimulatory activity (growth-regulated oncogene), and others, whereas CXCR2 shows a high affinity for all CXC chemokines that contain an N-terminal Glu-Leu-Arg motif.3 Because it has long been shown that after phagocytosis the chemotactic responses of neutrophils are impaired,4 we investigated the effect of phagocytic stimulation of human neutrophils on the expression of IL-8 receptors CXCR1 and CXCR2.
Study design
Neutrophils
Neutrophils were isolated from heparinized blood of healthy volunteers by dextran sedimentation of erythrocytes followed by centrifugation on Lymphoprep (Nycomed, Oslo, Norway) and lysis of residual erythrocytes with distilled water.5,6 Approval was obtained from the institutional review board at the Institute of Hematology and Blood Transfusion, Minsk, Belarus, for these studies. Informed volunteer consent was obtained according to the Declaration of Helsinki. Neutrophils were 95% to 96% pure and more than 98% viable. Purified neutrophils were incubated at 37°C in RPMI containing 2% fetal calf serum (Hyclone, Logan, UT) at 2 to 3 × 106 cells per milliliter in polypropylene tubes (Corning, NY) as described.6 The cells were stimulated with heat-killed and opsonized7Saccharomyces cerevisiae (OSC; OSC/PMN ratio, 2:1), opsonized sheep red blood cells (SRBCs; 5:1), or opsonized zymosan particles (30 mg/mL; Sigma, St Louis, MO). All reagents and solutions were controlled for endotoxin contamination by means of Limulus amebocyte assay (E-toxate; Sigma). Phagocytic activity of PMNs was determined as described previously8 in cell smears by means of Wright stain (Accustain; Sigma).
Flow cytometry analysis
Neutrophils were stained as described6 with the use of our mouse monoclonal antibody (mAb)6 E3 recognizing N-terminal amino acids 10 through 12 (Trp-Asp-Phe) of human CXCR1, mAb R115 to CXCR2,9 kindly provided by Dr Ernst Brandt (Forschungszentrum, Borstel, Germany), mAb to receptor for complement fragment C5a (C5aR, CD8810; all mAbs were immunoglobulin G1 [IgG1]), kindly donated by Prof Otto Goetze (Georg-August-University, Goettingen, Germany), or isotype control mAb and were analyzed by FACScan (Becton Dickinson, Mountain View, CA) by means of Lysis II software (Becton Dickinson).
Confocal microscopy
Smears were fixed in acetone, permeabilized with 0.1% Triton X-100 and stained with mAbs against CXCR1 and CXCR2 or IgG isotype control mAb at 20 μg/mL followed by incubation with rhodamine-conjugated bovine antimouse IgG antibody (Santa Cruz Biotechnology, CA). The stained cells were observed in confocal microscope (LSM 310; Zeiss, Oberkochen, Germany).
Cytosolic-free Ca++ measurements
Neutrophils at 107/mL were loaded with 2 μM Fura-2am (Molecular Probes, Eugene, OR) for 30 minutes at 37°C, washed, and resuspended in 20 mM HEPES buffer/1% bovine serum albumin/1 mM CaCl2 to a final concentration of 106/mL. To induce intracellular Ca++accumulation via CXCR-mediated pathway, IL-8 (Peprotech, Rocky Hill, NJ) or NAP-2 (kindly provided by Dr Ernst Brandt, Forschungszentrum, Borstel, Germany), both at 75 ng/mL, were added to PMNs and monitored as described.11 The capacity of phagocytosing PMNs to induce Ca++ influx was tested with the use of recombinant complement fragment C5a at 100 ng/mL (kindly donated by Prof Otto Goetze, Goettingen, Germany) or 1 μM Ca-ionophore A23187 (Serva, Heidelberg, Germany).
Reverse transcriptase–polymerase chain reaction analysis of purified neutrophil mRNA
Total RNA from neutrophils was extracted by acidic guanidinum thiocyanate and reverse-transcribed with Moloney murine leukemia virus reverse transcriptase (Perkin Elmer, San Jose, CA) under the recommended conditions and amplified by PCR with the use of the CXCR1-, CXCR2-, or β2-microglobulin–specific oligo primer pairs as described.6 A total of 10 μL each final PCR product was fractionated in 1.5% agarose gel in the presence of ethidium bromide. Quantitation of fluorescence of cellular product bands was performed with Scion Image Software (Frederick, MD). To correct for any variation in RNA content and cDNA synthesis in the different preparations, each sample was normalized on the basis of its β2-microglobulin content. Results were expressed as the calculated ratio of CXCR mRNAs to β2-microglobulin mRNA.6
Results and discussion
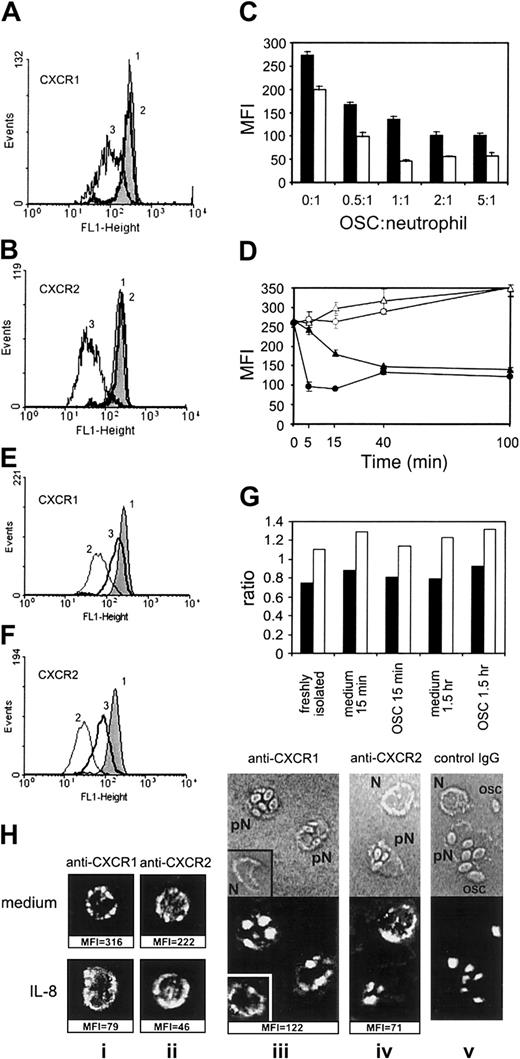
Figure 1A-B shows the effect of opsonized S cerevisiae (OSC) on the expression of CXCR1 and CXCR2, compared with nonopsonized S cerevisiae. PMNs showed no phagocytosis of nonopsonized S cerevisiae, while about 80% of PMNs contained intracellular OSC by 15 minutes of incubation (not shown). The expression of CXCR1 in PMNs (mean fluorescence intensity [MFI]) was reduced by 46% ± 8% (P < .01; n = 16; data from 16 independent donors), and the expression of CXCR2 was reduced by 75% ± 10% (P < .01; n = 16) after 15 minutes of phagocytic stimulation with OSC (mean ± SD of percentage of CXCR down-regulation in each experiment). The kinetics of CXCR2 reduction was different from that of CXCR1: CXCR2 expression was decreased after 5 minutes of phagocytosis maximally (Figure 1D), whereas the expression of CXCR1 gradually decreased during 40 minutes of observation. In agreement with our data, it was previously shown that phagocytosis of OSC by PMNs peaked at 5 minutes.8 Notably, the reduced levels of expression of both receptors were maintained during 6 hours of PMN incubation with OSC (not shown). A 2:1 ratio of OSC to PMN was sufficient for maximal down-regulation of CXCRs (Figure 1C). Similar down-regulation of CXCR1 and CXCR2 expression was observed after phagocytic stimulation with opsonized SRBCs or zymosan particles (not shown).
Effect of phagocytic stimulation on the expression of CXCR1 and CXCR2 in human neutrophils.
(A,B) PMNs were incubated alone for 15 minutes at 37°C (1) or treated with nonopsonized (2) or opsonized S cerevisiae(OSC) (3) at an OSC/PMN ratio of 2:1. (C) Dose dependence of the effect of phagocytic stimulation with OSC on the expression of CXCR1 (closed bars) and CXCR2 (open bars, mean of triplicates ± SD). (D) Time dependence of the expression of CXCR1 (▴) and CXCR2 (●) in phagocytosing neutrophils (closed symbols) and in control cells (open symbols; mean ± SD, n = 3). (E,F) Effect of phenantroline at 2 mM (line 3) on the modulation of CXCR1 (E) and CXCR2 (F) expression after OSC (2), compared with untreated PMNs (1). The expression levels in panels A, B, E, F, and H are displayed as mean fluorescence intensity (MFI) of duplicate samples; the difference between double FACScan estimates was in the range of 2% to 10%. Each PMN sample was stained with irrelevant monoclonal antibody of IgG1 isotype, and the resulting MFI was subtracted from MFI with specific mAb. To test a possible effect of the OSCs themselves on the PMN-staining procedure, OSC suspension was added to control PMNs at the end of incubation, along with sodium azide, following by a full staining procedure: no effect of OSC was detected (not shown). Results similar to those of panels A and B were obtained in 16 separate experiments; dose dependence similar to that of panel C was obtained in 3 experiments; time dependence similar to that of panel D, in 5 experiments; and the effect of phenantroline was reproduced in 4 separate experiments. (G) CXCR1 (closed bars) and CXCR2 (open bars) mRNA expression in human neutrophils after treatment of PMNs with OSC. Results are expressed as a ratio of CXCR mRNA to β2-microglobulin mRNA as external control. (H) Confocal images showing the distribution of CXCR1 and CXCR2 in PMNs incubated alone (Hi,ii; upper section), treated with IL-8 at 500 ng/mL for 15 minutes (i,ii; lower section) or in PMNs treated with OSC (iii-v); corresponding MFI data are shown below the pictures (Hiii-v) Fluorescence is shown in the lower part of the pictures, and transmission light microscopy of the same cells is shown in the upper part (pN indicates PMN phagocytosing OSC; N, nonphagocytosing PMN; OSC, nonspecific fluorescence of OSC at 543 nm).
Effect of phagocytic stimulation on the expression of CXCR1 and CXCR2 in human neutrophils.
(A,B) PMNs were incubated alone for 15 minutes at 37°C (1) or treated with nonopsonized (2) or opsonized S cerevisiae(OSC) (3) at an OSC/PMN ratio of 2:1. (C) Dose dependence of the effect of phagocytic stimulation with OSC on the expression of CXCR1 (closed bars) and CXCR2 (open bars, mean of triplicates ± SD). (D) Time dependence of the expression of CXCR1 (▴) and CXCR2 (●) in phagocytosing neutrophils (closed symbols) and in control cells (open symbols; mean ± SD, n = 3). (E,F) Effect of phenantroline at 2 mM (line 3) on the modulation of CXCR1 (E) and CXCR2 (F) expression after OSC (2), compared with untreated PMNs (1). The expression levels in panels A, B, E, F, and H are displayed as mean fluorescence intensity (MFI) of duplicate samples; the difference between double FACScan estimates was in the range of 2% to 10%. Each PMN sample was stained with irrelevant monoclonal antibody of IgG1 isotype, and the resulting MFI was subtracted from MFI with specific mAb. To test a possible effect of the OSCs themselves on the PMN-staining procedure, OSC suspension was added to control PMNs at the end of incubation, along with sodium azide, following by a full staining procedure: no effect of OSC was detected (not shown). Results similar to those of panels A and B were obtained in 16 separate experiments; dose dependence similar to that of panel C was obtained in 3 experiments; time dependence similar to that of panel D, in 5 experiments; and the effect of phenantroline was reproduced in 4 separate experiments. (G) CXCR1 (closed bars) and CXCR2 (open bars) mRNA expression in human neutrophils after treatment of PMNs with OSC. Results are expressed as a ratio of CXCR mRNA to β2-microglobulin mRNA as external control. (H) Confocal images showing the distribution of CXCR1 and CXCR2 in PMNs incubated alone (Hi,ii; upper section), treated with IL-8 at 500 ng/mL for 15 minutes (i,ii; lower section) or in PMNs treated with OSC (iii-v); corresponding MFI data are shown below the pictures (Hiii-v) Fluorescence is shown in the lower part of the pictures, and transmission light microscopy of the same cells is shown in the upper part (pN indicates PMN phagocytosing OSC; N, nonphagocytosing PMN; OSC, nonspecific fluorescence of OSC at 543 nm).
Proteolytic cleavage by metalloproteinases represents the main mechanism of reducing CXCR expression induced by tumor necrosis factor (TNF) or lipopolysaccharide.12 13 To investigate the mechanism of CXCR down-regulation by phagocytic stimuli, we explored the effect of the metalloproteinase inhibitor 1,10-phenantroline. Figure 1E-F shows that phenantroline at 2 mM prevented reduction of CXCRs. The levels of protection varied from 75% to 82% for CXCR1 and from 60% to 83% for CXCR2 (data of 4 separate experiments). It should be mentioned that 1,10-phenantroline itself did not reduce the phagocytic activity of PMNs (a reduction of less than 10%) and exerted no effect on expression of CXCRs (not shown).
To explore mechanisms of down-regulation of the expression of CXCRs by phagocytosis, we assessed the levels of CXCRs mRNA in PMNs during incubation with OSC. Figure 1G demonstrates no mRNA down-regulation after 15 minutes or 1.5 hour of phagocytosis.
Exogenous and endogenous cytokines, mainly IL-8 and TNF, were shown to reduce the expression of CXCRs on PMNs.12-14 Because phagocytosis induces the synthesis of IL-8 and TNF in PMNs,7,15,16 we tested the effect of anti-TNF antibody 5N and anti–IL-8 antibody WS-4 at a neutralizing concentration of 20 μg/mL.6 However, the antibodies did not prevent phagocytosis-induced reduction of CXCR expression (not shown). Besides, when supernatants aspirated from neutrophils phagocytosing OSC for 60 minutes were immediately added to a fresh portion of the same PMNs for 60 minutes at 37°C, no reduction of CXCR1 and CXCR2 expression was detected (not shown). These data imply that down-regulation of CXCRs in the phagocytosing neutrophils is not mediated by soluble factors.
Internalization represents a second important mechanism of membrane-receptor regulation.17,18 Because inhibitors of internalization, cytochalasin B and vinblastine,18 also arrested the phagocytosis (not shown), we explored the distribution of CXCRs in phagocytosing PMNs using confocal microscopy. Figure 1H (i,ii; upper section) demonstrates that PMNs incubated alone expressed high levels of the membrane CXCRs as revealed by FACScan analysis and confocal microscopy. Exogenous IL-8 caused a dramatic drop of the CXCR expression on the surface of PMNs revealed by FACScan analysis, whereas the CXCR fluorescence on confocal microscopy was not reduced and was redistributed in the area of cell membrane and cytoplasm (panels Hi,ii; lower section), representing a picture of IL-8–induced CXCR internalization.12 Both FACScan analysis and confocal microscopy showed a substantial reduction of CXCR expression in phagocytosing PMNs (Figure 1Hiii-iv). On the confocal microscopy, there was no redistribution but there was significant reduction of fluorescence of the CXCRs in the phagocytosing PMNs containing intracellular OSC (pN), thus indicating that the mechanism of down-regulation of CXCRs by phagocytosis is not via receptor internalization. Notably, in the same cell suspension, nonphagocytosing PMNs that contained no intracellular OSC (N) showed normal CXCR fluorescence pattern (Figure 1Hiii-iv), confirming that soluble factors play no role in the down-regulation of CXCRs induced by phagocytosis.
To reveal functional consequences of CXCR1 and CXCR2 reduction caused by phagocytosis, we explored the effect of corresponding ligands, IL-8 and NAP-2, on intracellular Ca++ signaling in phagocytosing PMNs. Figure 2A,B,D demonstrates that the Ca++ accumulation induced by IL-8 and NAP-2 (CXCR2 ligand)3 was suppressed in PMNs after 20 minutes of incubation with OSC, compared with PMNs incubated alone. Similar reduction of Ca++ accumulation in phagocytosing PMNs was observed at various IL-8 concentrations, ranging from 10 ng/mL to 1000 ng/mL (not shown). Nonopsonized S cerevisiae did not affect Ca++ signaling (not shown). In spite of transitory elevation of intracellular Ca++ by phagocytosis itself (Figure 2A-C), the responsiveness of OSC-treated PMNs to complement fragment C5a19 at 100 ng/mL (Figure 2C-D) and Ca-ionophore20 at 1 μM (not shown) was not suppressed, indicating that the capacity of phagocytosing neutrophils to increase cytoplasmic calcium was not impaired by phagocytosis itself. Interestingly, the expression of C5a receptors (CD88)10was reduced in phagocytosing PMNs by 38% ± 17% (not shown, data of 7 experiments).
Effect of phagocytic stimulation with opsonizedSaccharomyces cerevisiae (OSC) on Ca++mobilization induced by IL-8, NAP-2, or C5a in human neutrophils.
(A-C) PMNs were treated with Fura-2 am and incubated alone (line 1) or treated with OSC (line 2) for 20 minutes, followed by the addition of IL-8 at 75 ng/mL (panel A), NAP-2 at 75 ng/mL (panel B), or C5a at 100 ng/mL (panel C). Levels of Ca++ are presented as fluorescence ratio determined from background-corrected fluorescence exited alternately at 340 and 380 nm (F340/F380). Panels A, B, and C are based on data of one representative experiment. (D) Mean ± SD of percentages of Ca++influx in response to IL-8 (n = 11), NAP-2 (n = 7), or C5a (n = 7) in phagocytosing neutrophils compared with control cells. The statistical differences between groups were determined by the Student t test, with P < .05 considered significant. *P < .05 versus untreated incubated control.
Effect of phagocytic stimulation with opsonizedSaccharomyces cerevisiae (OSC) on Ca++mobilization induced by IL-8, NAP-2, or C5a in human neutrophils.
(A-C) PMNs were treated with Fura-2 am and incubated alone (line 1) or treated with OSC (line 2) for 20 minutes, followed by the addition of IL-8 at 75 ng/mL (panel A), NAP-2 at 75 ng/mL (panel B), or C5a at 100 ng/mL (panel C). Levels of Ca++ are presented as fluorescence ratio determined from background-corrected fluorescence exited alternately at 340 and 380 nm (F340/F380). Panels A, B, and C are based on data of one representative experiment. (D) Mean ± SD of percentages of Ca++influx in response to IL-8 (n = 11), NAP-2 (n = 7), or C5a (n = 7) in phagocytosing neutrophils compared with control cells. The statistical differences between groups were determined by the Student t test, with P < .05 considered significant. *P < .05 versus untreated incubated control.
It has long been known that the chemotactic responses of neutrophils are impaired after phagocytosis.4,21 The data presented here demonstrate phagocytosis-induced down-regulation of the expression of chemokine receptors CXCR1 and CXCR2 and reduced Ca++mobilization in response to corresponding CXCR ligands. Since CXC chemokines regulate various functions of PMNs via Ca-mediated pathway, the data suggest that phagocytosis may inhibit the capacity of CXC chemokines to modulate these functions. The phenomenon described may be relevant to a recent demonstration of the substantial reduction of CXCR expression on PMNs in septic conditions,22 since phagocytosis of micro-organisms by circulating PMNs is a common event in bacteremia.
The authors are grateful to Dr Joost Oppenheim, Laboratory of Molecular Immunoregulation, National Cancer Institute at Frederick, National Institutes of Health, Frederick, MD, for his support of a present research project and review of the manuscript; to Svetlana Akalovich, Institute of Hematology and Blood Transfusion, Minsk, Belarus, for kind help with leukocyte FACScan staining; to Prof Otto Goetze, Georg-August-University, Goettingen, Germany, for the gift of recombinant human C5a and monoclonal antibody to CD88; and to Dr Ernst Brandt, Forschungszentrum, Borstel, Germany, for kind donation of NAP-2 and monoclonal antibody R115 to human CXCR2.
Supported by research funding from the Office of International Affairs, Department of Health and Human Services, National Cancer Institute, National Institutes of Health, Bethesda, MD; grants from INTAS, Brussels, Belgium; research funding from the Belarussian Ministry of Health; and grant B00-147 from the Belarussian Foundation for Fundamental Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nikolai N. Voitenok, Institute of Hematology and Blood Transfusion, Dolginovski Tract 160, Minsk, 223059, Republic of Belarus; e-mail: nvoitenok@infonet.by.