Abstract
The B-cell receptor (BCR) for antigen is composed of surface immunoglobulin (sIg), which provides antigen specificity, and a noncovalently associated signaling unit, the CD79a/b heterodimer. Defects in CD79 can influence both BCR expression and signaling and may explain why cells from certain malignancies, such as B-chronic lymphocytic leukemia (B-CLL), often express diminished and inactive BCR. Recently, an alternative transcript of CD79b (ΔCD79b) has been reported that is up-regulated in B-CLL and may explain this diminished BCR expression. Here we assess the expression of ΔCD79b in B-CLL and other lymphoid malignancies and investigate its function. High relative expression of ΔCD79b was confirmed in most cases of B-CLL and found in 6 of 6 cases of splenic lymphomas with villous lymphocytes (SLVLs) and hairy cell leukemia. In a range of Burkitt lymphoma cell lines, expression of ΔCD79b was relatively low but correlated inversely with the ability of the BCR to signal apoptosis when cross-linked by antibody (Ab). Interestingly, when Ramos-EHRB cells, which express low ΔCD79b, were transfected with this transcript, they were transformed from being sensitive to anti-Fcμ–induced apoptosis to being highly resistant. Although ΔCD79b was expressed as protein, its overexpression did not reduce the level of cell surface BCR. Finally, we showed that the inhibitory activity of ΔCD79b depended on an intact leader sequence to ensure endoplasmic reticulum (ER) trafficking and a functional signaling immunoreceptor tyrosine-based activation motif (ITAM) in its cytoplasmic tail. These results point to ΔCD79b being a powerful modulator of BCR signaling that may play an important role in normal and malignant B cells.
Introduction
The CD79a/b heterodimer is critical to the structure and function of the B-cell receptor (BCR), being necessary for both its signaling capacity1-5 to regulate processes such as allelic exclusion, proliferation, differentiation, anergy, and apoptosis6-8 and for the transport of the complete receptor to the cell surface.9 The individual a and b chains of CD79 are coded by the mb-110 andB2911 genes, respectively, and are linked to each other by a disulfide bridge in the extracellular portion of the complex, just adjacent to its immunoglobulin (Ig)–like domains.1 Abnormalities in the BCR have often been associated with cells in certain neoplastic diseases, particularly B-chronic lymphocytic leukemia (B-CLL),12 which is characterized by the progressive accumulation of circulating monoclonal B cells, which tend to be CD5+ and to express low levels of surface BCR.13 Furthermore, recently it has been found that these tumors can be subdivided into 2 distinct groups depending on whether the Ig V regions are mutated or not, with the unmutated group being associated with a more aggressive type of disease.14The low level of surface Ig may explain the reduced ability of cells from many cases of B-CLL to capture, present, and respond to antigen.15 Defective BCR signaling has also been associated with low levels of CD38 on subsets of B-CLL cells.16 Interestingly, defects in the BCR of B-CLL have now been attributed to functional deficiency in the CD79 heterodimer, especially the CD79b, which is expressed at low levels on these tumors.17 Among the mechanisms proposed to explain these observations are reduced expression of CD79b mRNA,18somatic mutation of the B29 gene18 or, most recently, through alternative splicing of CD79b to yield ΔCD79b.19
Alternative gene splicing is emerging as an increasingly important mechanism for regulating gene expression, whereby a single pre-mRNA gives rise to different mature mRNA species by altering which exons are spliced together and in what order. Alternatively spliced forms of both CD79 a and b genes have been described in both normal and malignant B cells.20,21 The mb-1 gene encodes for a variant that is truncated by 110 extracellular base pairs as a result of splicing at cryptic sites in the gene, while the alternative transcript of the B29 gene is spliced at conventional sites, which removes the entire exon 3 and results in a β chain lacking the extracellular, Ig-like, domain.20 In both alternative transcripts, the Cys residues necessary to form the disulfide bridge between ΔCD79a and ΔCD79b are lost. As such, these variants, if translated, would not be expected to form stable heterodimers at the cell surface.
Although described some years ago, the importance or possible function of ΔCD79a and ΔCD79b has not been fully addressed. However, recently, Alfarano et al19 demonstrated that B-CLL cells consistently show elevated levels of the mRNA for ΔCD79b, indicating a possible physiological role. For this reason we set out to explore the role of ΔCD79b more fully and to determine if ΔCD79b influences the activity of the BCR in neoplastic B cells.
Materials and methods
Tumor samples and cell lines
Tumors were classified according to the standard World Health Organization (WHO) clinical criteria and included 16 B-CLL, 3 follicle center cell lymphomas (FCLs), 8 diffuse large cell lymphomas (DLCLs), 3 splenic lymphomas with villous lymphocyte (SLVL) samples, 3 hairy cell leukemias (HCLs), and 5 myelomas. Disease status was not used as a criterion in selecting patients. Peripheral blood lymphocyte (PBL) samples were isolated from the venous blood of patients or healthy volunteers on a Hypaque density gradient (Lymphoprep; Nicomed, Invitrogen Life Technologies, Paisley, United Kingdom) before washing in phosphate-buffered saline (PBS). DLCLs were taken from lymph node sections. Nodes were excised and RNA isolated and converted to cDNA as detailed below. Normal cell contamination, assessed by V region analysis, did not usually exceed 20% in these DLCL samples.
Daudi, Ramos, Ramos-EHRB, Raji, and Namalwa cells were obtained from the European Collection of Cell Cultures (ECACC, Salisbury, United Kingdom). K-562 and COS-7 cells were kind gifts from Dr A. Al-Shamkani (Cancer Sciences Division, University of Southampton, United Kingdom). All cell lines were maintained in supplemented RPMI 1640 (RPMI 1640 medium containing glutamine [2 mM], pyruvate [1 mM], penicillin and streptomycin [100 IU/mL], amphotericin B [2 μg/mL], and 10% fetal calf serum [FCS; Myoclone]; all supplied by Gibco, Paisley, United Kingdom) at 37°C in a 5% CO2 humidified incubator. Cells used for apoptosis studies were maintained in log phase of growth for 24 hours prior to experiments.
Antibody preparation
All hybridoma lines secreting monoclonal antibody (mAb) were expanded in tissue culture and purified from culture supernatant using a protein A column.22 Monoclonal Ab used in the study included CP1/17 (irrelevant, IgG1), M15/8 (anti-μ chain), ZL7/4 (anti-CD79a), ZL9/1 and ZL9/2 (anti-CD79b), and AT80 (anti-CD20), which were all raised in this laboratory and have been reported previously.23 24 AT105/1 is a murine mAb recently raised in this laboratory to a CD79b peptide-KLH construct. CB3-1 and SN8 (anti-CD79b) were purchased from BD Pharmingen (Cowley, United Kingdom) and Ancell (Bayport, MN), respectively. Binding of anti-CD79 mAb to CD79 Fc fusion protein was tested by a standard “capture” enzyme-linked immunosorbent assay (ELISA) on plates (Maxisorb; Nunclon, Invitrogen Life Technologies) coated with rabbit antihuman Fc (2.5 μg/mL) as first layer followed by the fusion proteins as a second layer.
Isolation of RNA and conversion to cDNA
Total RNA and mRNA were isolated using the Puregene (Gentra Systems, Minneapolis, MN) total RNA and Microquickprep mRNA (Amersham Pharmacia Biotech UK, Little Chalfont, United Kingdom) kits, respectively. RNA was converted to cDNA using the first-strand cDNA synthesis kit (Amersham Pharmacia Biotech United Kingdom) according to the manufacturer's instructions.
PCR
Polymerase chain reaction (PCR) was performed in thin-walled PCR tubes with approximately 100 ng cDNA, 100 ng 5′ and 3′ primers, 1 unit DNA polymerase, in the presence of deoxyribonucleoside triphosphates (dNTPs), and 1 × reaction buffer (all from Promega, Southampton, United Kingdom). PCR reactions carried out to generate chimeric CD79b constructs detailed below or to sequence CD79b genes from tumor samples were performed with Pfu polymerase. Reverse transcriptase (RT)–PCR was performed using Taq polymerase. DNA was first denatured at 95°C for 5 minutes, followed by 25 to 30 amplification cycles. Annealing temperatures for each set of primers used are shown in Table1. PCR products were analyzed by electrophoresis on 1.5% to 2% agarose gels and visualized under UV light after staining with ethidium bromide.
Construction of CD79 chimeric molecules
Construction of the CD79b-Fc fusion protein, displaying the extracellular domain of CD79b, was reported previously.23Essentially the same technique was applied to yield an Fc fusion protein with the extracellular region of ΔCD79b. Briefly, the extracellular domain of CD79 or ΔCD79b was PCR amplified from Ramos-EHRB cells using primers c) and d), which incorporate a splice donor site (ACAGGTAAGT) at the 3′ end. The products were cloned into pGEM-T vector (Promega), sequenced, and subcloned into the pIG1 vector, which contains the genomic Fc region (hinge, CH2, and CH3) of human IgG1. These, and all other constructs, with the exception of the yellow fluorescent protein (YFP) chimeras, were further subcloned into pcDNA3 (Invitrogen Life Technologies) for expression.
The full-length ΔCD79b molecule retaining the CD79b leader sequence was amplified from Ramos-EHRB cDNA using primers g) and h). The same primers were used to amplify the full-length CD79b molecule. A ΔCD79b molecule lacking the CD79b leader sequence was constructed using primers i) and h) and subcloned into the pHA-CMV vector (BD Clontech United Kingdom, Basingstoke, United Kingdom). This construct, hereafter referred to as leaderless ΔCD79b, possesses an additional 30-bp sequence encoding for a 10 amino acid–hemagglutinin (HA) tag at the end of the extracellular domain, which facilitates detection of the transfected construct above endogenous levels of ΔCD79b. Rat CD4 leader sequence (CD4L) was added to the leaderless ΔCD79b molecule using a 2-step recombinant PCR strategy. In the first step, leaderless ΔCD79b and rat CD4L constructs with overlapping regions were amplified using primer pairs i) and h) and j) and k); 100 ng of each amplified DNA was then used in the second step. This second reaction was initially carried out for 15 cycles without end primers i) and k), which were then added, and a further 15 cycles were performed. This construct was ligated directly into the PCR-Blunt II-TOPO vector (Invitrogen Life Technologies) before subcloning into pcDNA3. A CD4L-CD79b molecule with Tyr207 of the CD79b immunoreceptor tyrosine-based activation motif (ITAM) mutated to Ala (Tyr207-Ala207) was created in a similar fashion using primers j) and l) and m) and h) in the first step and primers j) and h) in the second. To construct yellow fluorescent protein (YFP) CD79 chimeric molecules, full-length and truncated CD79 transcripts were amplified from Ramos-EHRB cDNA using primers h) and n), prior to subcloning into the pEYFP-N1 vector (BD Clontech United Kingdom).
Transfection
Transfection of Ramos-EHRB and K-562 cell lines was achieved via electroporation using a Gene Pulser (Bio-Rad, Hemel Hempstead, United Kingdom), with voltage and capacitance settings of 0.3 to 0.32 mV and 960 microfarads (μF), respectively. Transfection of COS-7 cells was performed in chamber slides using a standard diethylaminoethyl (DEAE) dextran method.25 For stable expression, cells were seeded onto 96-well plates and subjected to selection with geneticin (1-2 mg/mL) 24 to 48 hours later. Expression of the relevant ΔCD79b transcript was determined by RT-PCR. YFP transfectants were screened by fluorescence-activated cell sorter (FACS) analysis and positive clones sorted using a FACS Vantage cell sorter (BD Pharmingen).
SDS-PAGE and Western blotting
Whole cell lysates were prepared from 5 × 106 to 10 × 106 cells in lysis solution containing 1% Nonidet P-40 (NP-40), 150 mM NaCl, 10 mM Tris (tris(hydroxymethyl)aminomethane) HCl, 2.5 mM EDTA (ethylenediaminetetraacetic acid), 1 mM phenylmethylsulfonyl fluoride (PMSF), 2.5 mM iodoacetic acid, and 1 mg/mL aprotinin. Insoluble material was removed by centrifugation at 15 000 rpm in a Kendro microcentrifuge for 15 minutes at 4°C. Samples were then diluted 1:3 in sample buffer and heated at 100°C for 3 minutes prior to loading. For Western blotting, proteins were transferred immediately onto nitrocellulose paper (Hybond; Amersham Pharmacia Biotech United Kingdom) using a semidry transfer system (TE 22 system; Hoeffer, Amersham Pharmacia Biotech United Kingdom). The blot was blocked overnight with PBS/10% bovine serum albumin (BSA) buffer and then incubated with the desired primary Ab (1-5 μg/mL) in PBS/10% BSA containing 0.1% Tween 20 at room temperature for 1 to 2 hours. Bound mAb was detected using F(ab′)2 rabbit antimouse horseradish peroxidase (HRP) for 60 to 90 minutes and enhanced chemiluminescence (ECL) reagents (Amersham Pharmacia Biotech United Kingdom) before exposure to light-sensitive film (Hyperfilm ECL, Amersham Pharmacia Biotech United Kingdom).
In vitro translation
In vitro translation was performed using the coupled transcription translation TnT rabbit reticulocyte system (Promega). Briefly, the alternative transcripts of CD79 were cloned into pcDNA3 as detailed above and then retranscribed into mRNA by reverse transcriptase and translated into protein using rabbit reticulocytes in the presence of [3H]Leu.[3H]Leu was incorporated as the labeled amino acid due to low numbers of Met and Lys in ΔCD79b. Transcription/translation of luciferase cDNA was used as a positive control. Following translation, the proteins were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Translated protein was detected by fluorography using the Amplify (Amersham Pharmacia Biotech United Kingdom) reagent and exposing the gel to light-sensitive film (Hyperfilm; Amersham Pharmacia Biotech United Kingdom).
Flow cytometry
Flow cytometry was performed on a FACScan cytometer (BD Pharmingen) equipped with a 488-nm argon ion laser. Data were collected using CellQuest or Consort 30 software and analyzed by Lysis II or CellQuest (BD Pharmingen). Cell debris was excluded using the forward scatter (FSC) threshold, and at least 7500 events were collected per sample.
Measurement of surface antigens by flow cytometry has been reported previously.23 Briefly, cells were incubated with the fluorescein isothiocyanate (FITC)–conjugated (direct) or unlabeled (indirect) mAb of choice (50 μg/mL final concentration) and, in the case of indirect immunofluorescence, detected with an appropriate FITC-conjugated secondary Ab before washing and analyzing. Fluorescence intensities were assessed in comparison to that given by an isotype-matched control Ab and expressed as histograms of fluorescence intensity versus cell number.
Detection of apoptosis
Apoptosis was detected and quantified using 3 different methods. Routine assessment of apoptotic cell death was performed by flow cytometry using a method modified from Dive et al26 based on the observation that apoptotic cells have lower FSC and higher side scatter (SSC) properties compared with viable cells. Alternatively, cells were stained with annexin V–FITC (BD Pharmingen) and 10 μg/mL propidium iodide (PI) and assessed by flow cytometry, as detailed by Vermes et al.27 The percentage of annexin V–positive cells were scored as apoptotic. In addition, cell samples were assessed for apoptosis on a basis of DNA fragmentation, essentially according to the method of Nicoletti et al28as detailed previously.29
Results
Expression of alternative transcripts of CD79 in B-CLL and other B-cell malignancies
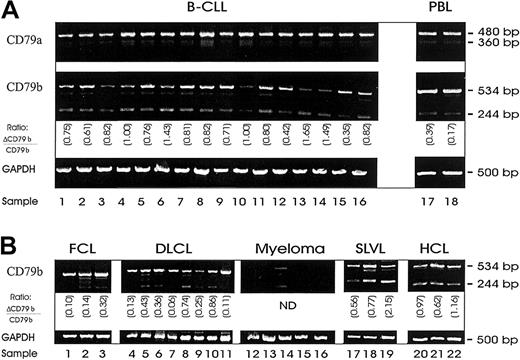
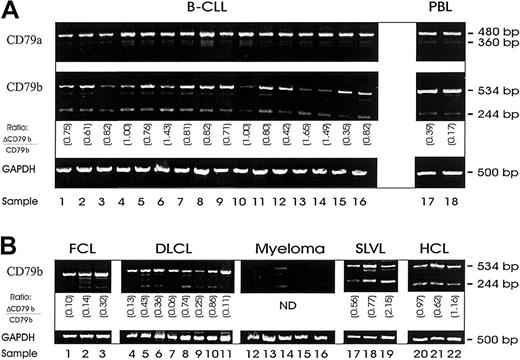
In our initial work we wished to confirm whether the alternative transcripts of CD79a and CD79b (ΔCD79a and ΔCD79b) were present in B-CLL. Using a semiquantitative RT-PCR, the ΔCD79b but not the ΔCD79a was present at high levels in these cells (Figure1A). Full-length CD79a and CD79b transcripts were also present in all samples, although in some cases for CD79b only at low levels (Figure 1A, lanes 3, 10, 13, 14). Although PCR techniques are not quantitative, simultaneous amplification of 2 different transcripts using the same primers, in the same reaction, as was done here, does provide a good estimate of the relative frequency of each species. In most cases of CLL assessed, the level of ΔCD79b was high relative to the full-length CD79b transcript (0.89 ± 0.36) and elevated in comparison with the relative level seen in normal peripheral blood samples (0.20 ± 0.17) as suggested previously.19
Messenger RNA expression of alternative transcripts of CD79, ΔCD79a and ΔCD79b.
Shown are DNA fragments resulting from semiquantitative RT-PCR of RNA isolated from malignant B cells with primers specific for CD79a, CD79b, or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Table 1). Messenger RNA was first isolated from samples and then reverse transcribed to yield cDNA for PCR. Primers for GAPDH were used to verify integrity and quantity of cDNA. (A) RT-PCR from 16 B-CLL samples. (B) RT-PCR from a selection of other B-cell malignancies: follicle center cell lymphoma (FCL) (lanes 1-3); diffuse large B-cell lymphoma (DLCL) (lanes 4-11); myeloma (lanes 12-16); splenic lymphoma with villous lymphocytes (SLVL) (lanes 17-19); and hairy cell leukemia (HCL) (lanes 20-22). The ratios in each panel show the relative level of ΔCD79b/CD79b.
Messenger RNA expression of alternative transcripts of CD79, ΔCD79a and ΔCD79b.
Shown are DNA fragments resulting from semiquantitative RT-PCR of RNA isolated from malignant B cells with primers specific for CD79a, CD79b, or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Table 1). Messenger RNA was first isolated from samples and then reverse transcribed to yield cDNA for PCR. Primers for GAPDH were used to verify integrity and quantity of cDNA. (A) RT-PCR from 16 B-CLL samples. (B) RT-PCR from a selection of other B-cell malignancies: follicle center cell lymphoma (FCL) (lanes 1-3); diffuse large B-cell lymphoma (DLCL) (lanes 4-11); myeloma (lanes 12-16); splenic lymphoma with villous lymphocytes (SLVL) (lanes 17-19); and hairy cell leukemia (HCL) (lanes 20-22). The ratios in each panel show the relative level of ΔCD79b/CD79b.
The level of ΔCD79b in other B-cell malignances was generally lower than that seen in the B-CLL cases (0.19 ± 0.11 for FCL and 0.33 ± 0.3 for DLCL), and in several individuals no alternative transcript could be detected (Figure 1B). For example, lanes 1, 4, 7, and 11 in the FCL and DLCL cases showed no evidence of the ΔCD79b transcript, and in 4 of 5 myeloma samples tested, neither the full nor the truncated transcript of CD79b could be detected. However, in 3 of 3 cases of SLVL and 3 of 3 cases of hairy cell lymphoma (Figure 1B), ΔCD79b was also highly expressed (0.6 ± 0.15 for SLVL and 0.92 ± 0.27 for HCL), demonstrating that up-regulation of the variant transcript is not restricted to B-CLL.
Correlation of ΔCD79b expression and decreased sensitivity to BCR-induced apoptosis
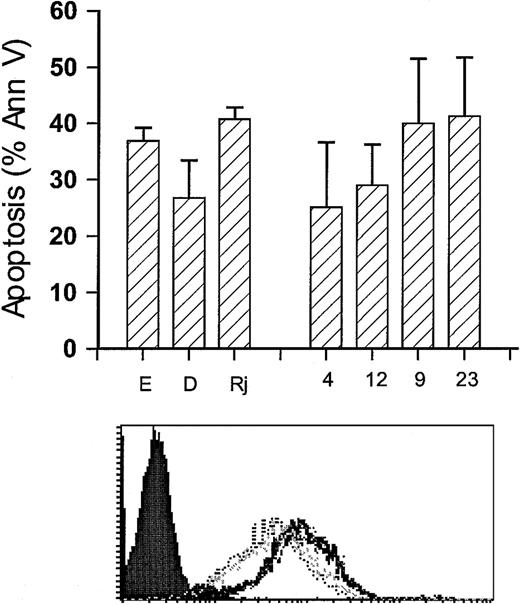
Having confirmed that the ΔCD79b was present in B-CLL and having found high levels in certain other B-cell tumors, we next considered what function this product might have in malignant B cells. In our previous work, we described a range of Burkitt lymphoma cell lines that differed in their susceptibility to growth inhibition and apoptosis after BCR ligation with mAb.29 Therefore, we decided to assess whether the level of the ΔCD79b transcript differed in these lines using the RT-PCR approach (Figure2A). The results showed a spectrum of expression, from very low levels of ΔCD79b in Ramos-EHRB cells to relatively high levels, compared with the full-length transcript, in Raji and Namalwa. The relative levels of the ΔCD79b transcript in these latter 2 lines was analogous to that seen in B-CLL and in all 3 samples from SLVL patients. Figure 2B illustrates the surface expression levels of CD79a, CD79b, and suface Ig (sIg) in EHRB, Daudi, Raji and Namalwa cells, indicating that levels of CD79b message do not directly correlate with surface expression of CD79a, CD79b, and sIg. Intriguingly, those Burkitt cell lines that expressed high levels of the alternative transcript were also the same lines that were most refractive to the apoptosis induced through ligation of their BCR with anti-Fcμ mAb (Figure 2C). Conversely, cell lines that were sensitive to apoptosis displayed low levels of the alternative transcript. Figure2D shows the clear inverse relationship between ΔCD79b expression and insensitivity to anti-μ–induced apoptosis. Such data suggested that ΔCD79b transcript might protect cells against apoptosis signaled via the BCR.
Correlation of expression of ΔCD79b and sensitivity to anti-Fcμ–induced apoptosis of Burkitt lymphoma cell lines.
(A) Level of ΔCD79b transcript in Burkitt cell lines. To assess the level of ΔCD79b in a range of Burkitt lymphoma cell lines, RT-PCR analysis was performed using the specific primers for CD79b as detailed in Figure 1. Samples assessed were Ramos-EHRB (E), Ramos (R), Daudi (D), Raji (Rj), and Namalwa (N). For comparison, the PCR products from an example of a B-CLL (C) and an SLVL (S) are also shown. The ratios in each panel show the relative level of ΔCD79b/CD79b. (B) The levels of surface BCR on EHRB (tightly grouped dotted outline […]), Namalwa (spaced dotted outline [. . .]), Raji (solid histogram), and Daudi (light gray solid outline) cells as measured by flow cytometry. The cell lines were stained with FITC-conjugated mAb to CD79a (ZL7-4), CD79b (ZL9-1), and sIg (M15/8). (C) Induction of apoptosis with anti-Fcμ mAb. Namalwa, Daudi, and EHRB Burkitt cell lines were assessed for their sensitivity to anti-μ–induced apoptosis by exposure to 10 μg/mL anti-μ mAb for 24 hours. Apoptosis was assessed by flow cytometry using annexin V–FITC and PI. (D) Correlation of ΔCD79b expression and sensitivity to anti-Fcμ mAb. The ratio of ΔCD79b/CD79b is taken from panel A, and the sensitivity to apoptosis is taken from the same experiments as those shown in panel C.
Correlation of expression of ΔCD79b and sensitivity to anti-Fcμ–induced apoptosis of Burkitt lymphoma cell lines.
(A) Level of ΔCD79b transcript in Burkitt cell lines. To assess the level of ΔCD79b in a range of Burkitt lymphoma cell lines, RT-PCR analysis was performed using the specific primers for CD79b as detailed in Figure 1. Samples assessed were Ramos-EHRB (E), Ramos (R), Daudi (D), Raji (Rj), and Namalwa (N). For comparison, the PCR products from an example of a B-CLL (C) and an SLVL (S) are also shown. The ratios in each panel show the relative level of ΔCD79b/CD79b. (B) The levels of surface BCR on EHRB (tightly grouped dotted outline […]), Namalwa (spaced dotted outline [. . .]), Raji (solid histogram), and Daudi (light gray solid outline) cells as measured by flow cytometry. The cell lines were stained with FITC-conjugated mAb to CD79a (ZL7-4), CD79b (ZL9-1), and sIg (M15/8). (C) Induction of apoptosis with anti-Fcμ mAb. Namalwa, Daudi, and EHRB Burkitt cell lines were assessed for their sensitivity to anti-μ–induced apoptosis by exposure to 10 μg/mL anti-μ mAb for 24 hours. Apoptosis was assessed by flow cytometry using annexin V–FITC and PI. (D) Correlation of ΔCD79b expression and sensitivity to anti-Fcμ mAb. The ratio of ΔCD79b/CD79b is taken from panel A, and the sensitivity to apoptosis is taken from the same experiments as those shown in panel C.
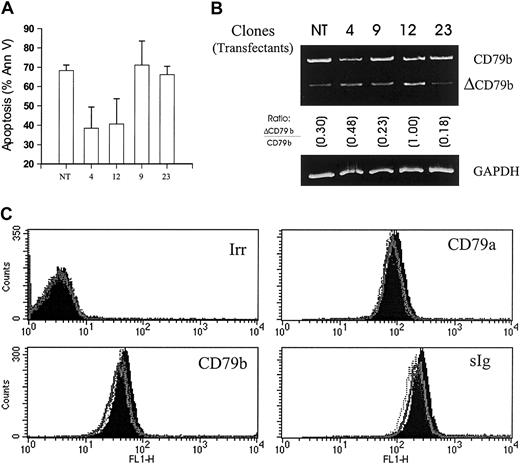
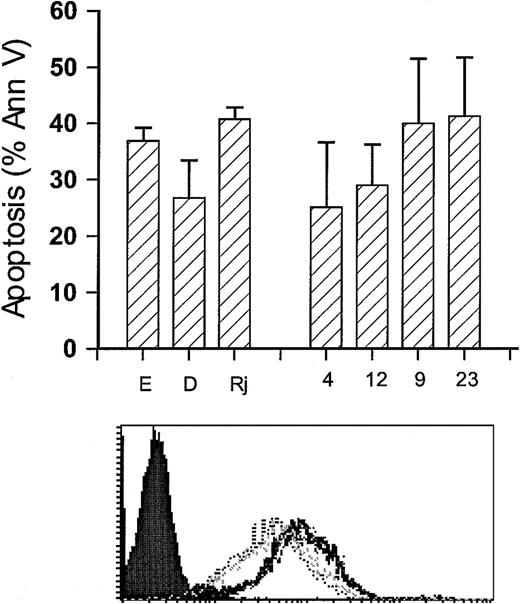
To test this hypothesis, we next cloned and overexpressed ΔCD79b in Ramos-EHRB, the cell line that was most sensitive to anti-Fcμ–induced apoptosis. As can be seen in Figure3A, overexpression of the ΔCD79b gene rendered certain clones—for example, 4 and 12—resistant to this form of apoptosis. When the mRNA was assessed in these clones, inhibition of apoptosis correlated closely with the level of ΔCD79b transcript (Figure 3B). For example, clones 4 and 12, which express relatively high levels of ΔCD79b, were resistant to apoptosis, while clones 9 and 23, which were transfected in the same way but did not show overexpression of the gene, were still sensitive to anti-μ mAb. Resistance to apoptosis induced by anti-μ mAb was not simply due to down-regulation of the BCR, because the levels of surface Ig, CD79a, and CD79b on all the transfected clones remained unchanged (Figure 3C). Similarly, the rate and extent of internalization in these clones when these molecules were cross-linked by the appropriate mAb were indistinguishable from that seen in the wild-type EHRB cells (data not shown). These data are important because they demonstrate that the overexpression of the ΔCD79b transcript relative to the full-length CD79b molecule per se did not cause changes in the surface level of the BCR or alter the way in which it responded to being cross-linked by mAb.
Sensitivity of Ramos-EHRB clones to anti-Fcμ mAb following transfection with ΔCD79b.
(A) Untreated cells (NT) and 4 ΔCD79b-transfected clones of Ramos-EHRB were cultured (5 × 105/mL) in the presence of anti-Fcμ mAb for 24 hours before assessing apoptosis via flow cytometry using the annexin V–FITC and PI as detailed in Figure 2. Apoptosis was also verified using the hypo-PI method to detect fragmented DNA (data not shown). The histogram shows average results, ± SD, for 3 experiments on each clone. (B) The expression of CD79b and ΔCD79b in the nontransfected (NT) and transfected clones, 4, 9, 12, 23, was confirmed by RT-PCR as detailed in Figure 1. Ratios show the relative level of ΔCD79b/CD79b. GAPDH message was also determined as a control to ensure that equal amounts of DNA were present in each sample. (C) The levels of surface BCR on wild-type and ΔCD79b-transfected Ramos-EHRB clones as measured by flow cytometry. The cells were stained with FITC-conjugated mAb to CD79a (ZL7-4), CD79b (ZL9-1), sIg (M15/8), and an irrelevant control mAb (Irr, CP1/17). No difference was seen in the various clones compared with untransfected cells.
Sensitivity of Ramos-EHRB clones to anti-Fcμ mAb following transfection with ΔCD79b.
(A) Untreated cells (NT) and 4 ΔCD79b-transfected clones of Ramos-EHRB were cultured (5 × 105/mL) in the presence of anti-Fcμ mAb for 24 hours before assessing apoptosis via flow cytometry using the annexin V–FITC and PI as detailed in Figure 2. Apoptosis was also verified using the hypo-PI method to detect fragmented DNA (data not shown). The histogram shows average results, ± SD, for 3 experiments on each clone. (B) The expression of CD79b and ΔCD79b in the nontransfected (NT) and transfected clones, 4, 9, 12, 23, was confirmed by RT-PCR as detailed in Figure 1. Ratios show the relative level of ΔCD79b/CD79b. GAPDH message was also determined as a control to ensure that equal amounts of DNA were present in each sample. (C) The levels of surface BCR on wild-type and ΔCD79b-transfected Ramos-EHRB clones as measured by flow cytometry. The cells were stained with FITC-conjugated mAb to CD79a (ZL7-4), CD79b (ZL9-1), sIg (M15/8), and an irrelevant control mAb (Irr, CP1/17). No difference was seen in the various clones compared with untransfected cells.
Protein expression of the alternative transcripts of CD79
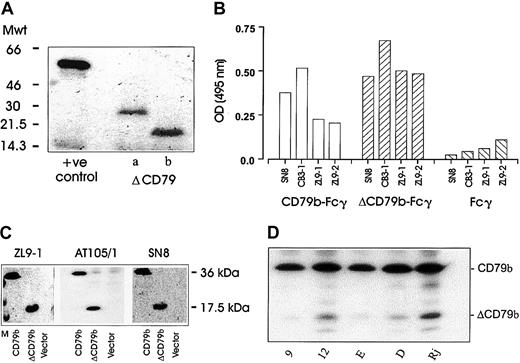
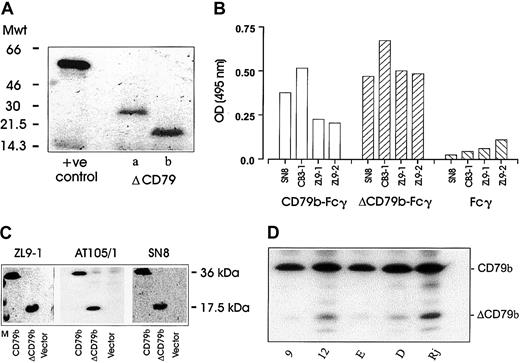
Next, we wished to ascertain whether protein could be translated from the alternative transcripts of CD79. Initially, we performed in vitro coupled transcription-translation assays on both ΔCD79a and ΔCD79b expressed in pcDNA3. The alternative transcripts were both well translated and produced proteins of the correct molecular weight (Figure 4A). A fusion protein approach was also undertaken using YFP as a reporter partner, wherein YFP was joined to the ends of the cytoplasmic domains of both the full-length and the alternative transcripts of CD79b (see “Materials and methods”). The YFP fusion constructs were transfected into various cell lines and expression assessed either by fluorescence microscopy or flow cytometry. Expression of both products was observed in COS-7, J558L, and K562 cells, and also in Ramos-EHRB B cells (data not shown).
Protein expression of alternative transcripts of CD79.
(A) The results obtained when the alternative transcripts of CD79 were cloned into pcDNA3 and translated into protein using a coupled transcription translation system (see “Materials and methods”). Proteins were resolved on a 15% SDS-PAGE gel and detected by fluorography. The positive control used was a vector coding for the luciferase protein. (B) An ELISA using a panel of anti-CD79b mAbs was used to assess binding to the full-length (full-CD79b-Fcγ) or alternately spliced (ΔCD79-Fcγ) extracellular domain of CD79b. (C) COS-7 cells were transiently transfected with full-length or truncated CD79b and then harvested 24 to 48 hours later for blotting with different anti-CD79b mAbs and detection with ECL reagents. All mAbs show specific reactivity with both the full and truncated forms of CD79b, shown as bands of 33 and 17.5 kDa, respectively. “Vector” indicates cells transfected with empty pcDNA3 plasmid, and “M” indicates the molecular weight markers. (D) Expression of ΔCD79b in Raji, Daudi, and EHRB B-cell lines and the EHRB transfectant clones 9 and 12 detailed in Figure 2. Expression was determined following Western blotting with anti-CD79b mAb AT105/1.
Protein expression of alternative transcripts of CD79.
(A) The results obtained when the alternative transcripts of CD79 were cloned into pcDNA3 and translated into protein using a coupled transcription translation system (see “Materials and methods”). Proteins were resolved on a 15% SDS-PAGE gel and detected by fluorography. The positive control used was a vector coding for the luciferase protein. (B) An ELISA using a panel of anti-CD79b mAbs was used to assess binding to the full-length (full-CD79b-Fcγ) or alternately spliced (ΔCD79-Fcγ) extracellular domain of CD79b. (C) COS-7 cells were transiently transfected with full-length or truncated CD79b and then harvested 24 to 48 hours later for blotting with different anti-CD79b mAbs and detection with ECL reagents. All mAbs show specific reactivity with both the full and truncated forms of CD79b, shown as bands of 33 and 17.5 kDa, respectively. “Vector” indicates cells transfected with empty pcDNA3 plasmid, and “M” indicates the molecular weight markers. (D) Expression of ΔCD79b in Raji, Daudi, and EHRB B-cell lines and the EHRB transfectant clones 9 and 12 detailed in Figure 2. Expression was determined following Western blotting with anti-CD79b mAb AT105/1.
In an attempt to address directly whether the native ΔCD79b transcript is expressed as a protein, we considered a Western blot approach. First we analyzed a panel of 4 anti-CD79b mAbs for their ability to bind to fusion proteins of Fcγ linked to the extracellular regions of either the full-length or the truncated CD79b. A “capture” ELISA was used for this investigation (Figure 4B). First a polyclonal anti-Fcγ Ab was used to capture the Fc domain of the fusion proteins and then the various anti-CD79b mAb used to detect either of the products in a standard format. As expected, all anti-CD79b mAbs reacted with the Fc fusion protein displaying the extracellular domain of the full-length CD79b molecule (Figure 4b). Surprisingly, however, these mAbs also bound to the Fcγ-ΔCD79b molecule. Thus, despite having only 25 amino acids remaining as an extracellular domain, ΔCD79b is recognized by all the mAbs tested. We next assessed these anti-CD79b mAbs in Western blots with cell lysates from COS-7 cells transfected with either full-length CD79b, ΔCD79b, or empty vector. The data show (Figure 4C) that all mAbs tested reacted with both the full-length and truncated forms of CD79b and confirm a protein product from the ΔCD79b mRNA transcript. Next, we wished to address whether the alternative transcript was present in our various cell lines and EHRB transfectants. Figure 4D demonstrates that the expression of the ΔCD79b protein corresponds well with the expression of the ΔCD79b mRNA seen in Figure 2A-B, such that Raji cells expressed high levels of ΔCD79b protein and EHRB cells very little, with Daudi cells intermediate. EHRB transfectant clone 12 showed elevated levels of ΔCD79b protein compared with mock transfectant clone 9, as expected. Interestingly, additional bands were seen on the Western blots in these B-cell lines, which may correspond to other, posttranslationally modified, forms of ΔCD79b not produced in COS-7 cells.
Functional importance of the leader sequence and the ITAM of ΔCD79b
We postulated that, because the apoptosis induced via the BCR requires the signaling activity of the CD79 heterodimer,7the ΔCD79b transcript might inhibit this process by interfering with a signaling pathway from the BCR. Furthermore, we reasoned that the ΔCD79b molecule might perform this function by competing for vital signaling adaptor molecules at the plasma membrane. Therefore, we undertook a mutation strategy to probe the ΔCD79b, first to determine if a leader sequence was required to traffic the inhibitory ΔCD79b into the ER and, second, to see if, like the full-length transcript, it utilizes the ITAM.
The results in Figure 5 show again that the alternative transcript inhibits anti-Fcμ–induced apoptosis (compare the first and second pair of bars) and that this inhibitory activity is completely lost once the leader sequence had been removed. Thus, clones transfected with an empty vector (Figure 5; first pair of bars) and cells transfected with a leaderless ΔCD79b (Figure 5; third pair of bars) are equally sensitive to anti-Fcμ mAb despite the transfected gene being readily detectable by RT-PCR (data not shown). Interestingly, when we used a construct in which the leader sequence of rat CD4 had been added back to the leaderless ΔCD79b, the inhibitory activity was fully restored (Figure 5; fourth pair of bars). To assess whether the ITAM of ΔCD79b might be involved in the inhibition, a mutation of the second Tyr in the ITAM (Tyr207-Ala207), known to ablate signaling function,5 was performed. As shown in Figure 5(last pair of bars), loss of ITAM function rendered the construct unable to inhibit apoptosis triggered by anti-Fcμ mAb.
Functional analysis of ΔCD79b.
Constructs encoding the wild-type ΔCD79b, a leaderless ΔCD79b, a ΔCD79b construct in which the endogenous leader was replaced with that from rat CD4 (CD4L), and the CD4LΔCD79b possessing a mutated ITAM (CD4LΔCD79b [Y207A]) were transfected into Ramos-EHRB cells and then positive clones assessed for sensitivity to anti-μ–induced apoptosis after 48 hours by annexin V–FITC/PI analysis. Two clones from each construct are shown. The average of 3 experiments, ± SD, are shown for each clone. Underneath, expression levels of CD79a, CD79b, and sIg are shown for each clone, determined as indicated in Figure 2, illustrating that all clones expressed similar levels of all BCR components at the cell surface.
Functional analysis of ΔCD79b.
Constructs encoding the wild-type ΔCD79b, a leaderless ΔCD79b, a ΔCD79b construct in which the endogenous leader was replaced with that from rat CD4 (CD4L), and the CD4LΔCD79b possessing a mutated ITAM (CD4LΔCD79b [Y207A]) were transfected into Ramos-EHRB cells and then positive clones assessed for sensitivity to anti-μ–induced apoptosis after 48 hours by annexin V–FITC/PI analysis. Two clones from each construct are shown. The average of 3 experiments, ± SD, are shown for each clone. Underneath, expression levels of CD79a, CD79b, and sIg are shown for each clone, determined as indicated in Figure 2, illustrating that all clones expressed similar levels of all BCR components at the cell surface.
Specificity of apoptotic inhibition
Lastly, we wished to address whether the inhibitory properties of ΔCD79b were specific for the BCR apoptosis pathway. Therefore, we stimulated our various cell lines and transfectant EHRB cells with anti-CD20 mAb to investigate whether this pathway was also blocked by overexpression of ΔCD79b. As shown in Figure6, Raji, Daudi, and EHRB cells seemed similarly sensitive to anti-CD20 apoptosis, although they are differently susceptible to BCR apoptosis and express different levels of ΔCD79b. ΔCD79b overexpressing EHRB clones 4 and 12 were slightly less sensitive to anti-CD20 apoptosis compared with the mock transfectant clones 9 and 23. However, when the level of surface expression of CD20 was assessed, it was apparent that the resistant clones expressed less CD20 on their surface, possibly accounting for this difference in sensitivity.
Sensitivity of Ramos-EHRB clones to anti-CD20 mAb following transfection with ΔCD79b.
Four ΔCD79b-transfected clones of Ramos-EHRB were cultured (5 × 105/mL) in the presence of anti-CD20 mAb for 48 hours before assessing apoptosis via flow cytometry using the annexin V–FITC and PI assay as detailed in Figure 2. Underneath, the lower panel shows the levels of surface CD20 on wild-type and ΔCD79b-transfected Ramos-EHRB cells as measured by flow cytometry. The 4 clones, 4, 9, 12, and 23, were stained with FITC-conjugated mAb to CD20 (AT80), washed, and then assessed by flow cytometry. The solid histogram represents cells stained with an irrelevant control mAb (CP1/17). The 2 least intense FACS profiles represent clones 4 and 12.
Sensitivity of Ramos-EHRB clones to anti-CD20 mAb following transfection with ΔCD79b.
Four ΔCD79b-transfected clones of Ramos-EHRB were cultured (5 × 105/mL) in the presence of anti-CD20 mAb for 48 hours before assessing apoptosis via flow cytometry using the annexin V–FITC and PI assay as detailed in Figure 2. Underneath, the lower panel shows the levels of surface CD20 on wild-type and ΔCD79b-transfected Ramos-EHRB cells as measured by flow cytometry. The 4 clones, 4, 9, 12, and 23, were stained with FITC-conjugated mAb to CD20 (AT80), washed, and then assessed by flow cytometry. The solid histogram represents cells stained with an irrelevant control mAb (CP1/17). The 2 least intense FACS profiles represent clones 4 and 12.
Discussion
B-CLL is a malignancy characterized by a low expression of sIg, diminished response to antigen, poor antigen capture and presentation, and defective apoptosis (reviewed by Rozman and Montserrat,12 Hamblin and Oscier,13 and Alfarano et al19). This intriguing series of related aberrations has led workers to speculate that B-CLL possesses deficiencies in BCR function and recently that these may be due to defects in CD79b gene expression. Alfarano et al19 have suggested that increased alternative splicing of the CD79b mRNA is the cause of the diminished surface expression and function of the BCR, while in contrast Thompson et al18 have attributed it to reduced levels of CD79b mRNA or to mutations in CD79b. In contrast to other reports,19-21 the latter authors found no evidence for ΔCD79b in B-CLL or in normal PBLs, but this may reflect the forward primer positioning used in this study.
Here we found relatively high levels of the alternatively spliced variant of CD79b, ΔCD79b, in most B-CLL cells. Only 4 cases (25%) showed somewhat lower levels, which were more typical of the pattern found in PBLs. These results are consistent with those reported by Alfarano et al19 showing that most cases of B-CLL overexpress ΔCD79b. Interestingly, in addition to ΔCD79b, all cases of B-CLL were found to express the full-length CD79b transcripts. This agrees with the results of Alfarano et al,19 Verschuren et al,30 and Rassenti and Kipps31 but disagrees with Thompson et al,18 who found B29 message undetectable in a number of B-CLL samples using Northern blotting or RNase protection assays. We have no clear explanation for such discrepancies, but they may reflect either the different detection techniques used or the fact that in our study mRNA was isolated from fresh cells and immediately converted to cDNA, whereas samples assessed by Thompson were from total RNA isolated from cells stored at −70°C, as we have observed degradation of CD79b message levels in CLL cells stored at −80°C (data not shown). We did not assess the CD79b base sequences of any of the samples to check for mutations. Others have found that, in B-CLL samples with normal or at least detectable surface expression of CD79b, mutations are present within the B29 gene in one or both alleles18 and have demonstrated that some of these mutations could severely affect BCR expression and/or function.32
Three other B-cell malignancies, FCL, DLCL, and myeloma, did not express high levels of ΔCD79b. Four of 5 myeloma samples did not express readily detectable levels of any CD79b transcripts, in agreement with the notion that CD79b gene expression becomes down-regulated in plasma cells.33 Conversely, all SLVL and HCL samples expressed high levels of the ΔCD79b transcript, as did other non-Hodgkin lymphoma samples (data not shown). Clearly, therefore, overexpression is not confined to B-CLL. Because up-regulation of ΔCD79b is known to occur when normal B cells are activated with stimuli such as interleukin-4 (IL-4), lipopolysaccharide (LPS), and anti-IgM,21 it is perhaps not surprising to also find it in a range of malignant B cells. One interpretation of such results is that overexpression of ΔCD79b relates more to the activation status of a given neoplasm than to its causation.
Our most unexpected observation was that the level of ΔCD79b in a range of Burkitt lymphoma lines correlated inversely with the sensitivity of these cells to anti-Fcμ–induced apoptosis. These cells all express the BCR, albeit at variable levels, and are, with the exception of the Ramos cell line, Epstein-Barr virus (EBV) positive29 (data not shown). By way of confirming the importance of ΔCD79b to the apoptosis triggered via the BCR, we transfected a highly sensitive cell line, Ramos-EHRB, with a vector carrying ΔCD79b. A range of clones (2 examples shown in Figure 3) confirmed a clear relationship between expression of this transcript and readiness to undergo apoptosis when engaged by anti-BCR mAb but not anti-CD20 mAb. These results gave a strong indication that the product of the ΔCD79b mRNA interferes specifically with signaling from the BCR and inhibits the readiness of the cells to undergo programmed cell death.
Various assays were undertaken to show that the ΔCD79b transcript was translated into protein. In vitro transcription-translation assays revealed that the alternative transcripts of both CD79a and CD79b produced proteins of about 25 and 17.5 kDa, respectively, in close agreement with that predicted from the gene sequences.20Furthermore, transfection of the YFP-ΔCD79b fusion constructs into COS-7, K-562, and Ramos-EHRB cells demonstrated that protein product is expressed in whole cells (data not shown). Finally, we also used Western blotting experiments with a panel of anti-CD79b mAbs directed to the extracellular domain of CD79b to confirm the existence of truncated CD79b in transfected COS-7 cells, B-cell lines, and transfected EHRB cells. These experiments revealed that protein species are generated from the alternative transcripts of CD79 and confirm the work of Benlagha et al,34 which has recently demonstrated the truncated form of CD79a in normal B cells by Western blotting. Intriguingly, all of the anti-CD79 mAbs available bound to both full-length and truncated forms of the CD79b molecule, despite the fact that the truncated molecule has lost the complete extracellular Ig-like domain and is left with only 25 amino acids to provide the Ab epitope. Presumably, all of these mAbs recognize the same short extracellular peptide region distal to the transmembrane domain, which is present in both the full-length and truncated CD79b molecule, because the proximal peptide would probably be inaccessible in the full-length molecule. This indicates either that there is only one immunodominant epitope in the CD79b protein or that other sites are obscured by the binding orientation of CD79b in the BCR. Importantly, these data also demonstrate that current anti-CD79b mAbs are incapable of differentiating between the full-length and alternative transcripts of CD79b.
It has been suggested by one group,21 based upon L-cell reconstitution studies, that overexpression of ΔCD79b might account for the low levels of surface Ig that are usually found on B-CLL cells. Three lines of evidence presented here indicate that this is not a dominant effect of ΔCD79b: First, forced overexpression of ΔCD79b in transfectant cell lines was shown not to modulate the surface levels of CD79a, CD79b, or Ig; second, Namalwa cells express high levels of endogenous ΔCD79b but still display high levels of surface Ig; and third, SLVL cells express high levels of ΔCD79b but are characterized by their high level of surface Ig expression.35 Therefore, we feel that these data argue that ΔCD79b expression per se does not account for a decrease of Ig at the cell surface. Although decreased expression of full-length CD79b, occurring as a result of elevated splicing to the ΔCD79b form, may account for decreased CD79b protein expression, this was not indicated by our RT-PCR analysis. Rather, it suggests that B-CLL cells may possess a defect in posttranslational pathways of intracellular synthesis and transport or enhanced proteolysis, such as discussed by Payelle-Brogard et al.36Furthermore, these properties may well be a product of an anergic state similar to that detailed by Bell and Goodnow37 for tolerant mouse B cells exposed to endogenous hen egg lysozyme (HEL) self-antigen in the HEL-transgenic mouse system.
It is not clear by what mechanism ΔCD79b could control apoptosis induced via the BCR. Alternative splicing is common in signaling receptors of the immune system. Examples include the coreceptors CD22 and CD45, T-cell receptor (TCR) components, cytokine receptors, Fc receptors of the α and all 3 γ types, as well as Ig itself (reviewed by Hashimoto et al20). The purpose of alternative splicing is not always apparent, but the literature tends to support the notion that alternatively spliced truncated receptors are negative regulators of their full-length counterparts, acting either as inert decoy molecules or as proteins with directly opposing functions.38,39 A good illustration is seen with the Bcl-2 family member Bcl-x, wherein the full-length form of this molecule (Bcl-xL) is a potent inhibitor of apoptosis, while the truncated form (Bcl-xS) is a proapoptotic protein. Furthermore, altering the ratio of splice forms can drastically alter cellular fate (ie, life or death).40
We would speculate that ΔCD79b is a decoy molecule that acts as a negative regulator of the BCR by sequestering critical signaling adaptor molecules away from the functional CD79a:CD79b heterodimer. First, our data clearly show that overexpression of this molecule inhibits apoptosis induced via the BCR. Although overexpression studies are not always reliable exponents of real biologic systems, we would argue that the levels of ΔCD79b induced in the transfectants are within the physiological spectrum of expression seen in normal and malignant B cells. Second, we have found that a leaderless version of the short transcript does not function. This is consistent with the ΔCD79b protein needing to traffic through the ER perhaps for export to the cell surface to deliver its inhibitory activity. Koyama et al21 would argue against this suggestion, because they showed, using reconstitution experiments in murine fibroblast L cells, that transfection of ΔCD79b (along with CD79a and Ig) does not evoke transport of the BCR to the cell surface, indicating that ΔCD79b cannot facilitate exit from the ER. However, this work was done in mouse fibroblasts, which may lack important trafficking molecules expressed in B cells. Third, we have found that the ΔCD79b molecule needs a functional ITAM in order to provide inhibitory function. Mutation of Y207 in the ITAM has previously been shown to ablate the signaling potential of CD79b,7 and in our system this mutation was sufficient to remove the inhibitory capacity of ΔCD79b. A similar dependence on the ITAM motif was recently demonstrated for the inhibitory effect of the IgεR.41 In this instance, the binding and sequestering of important signaling molecules was responsible for the inhibition of productive signaling. We are currently exploring the immediate downstream signaling events that are induced in the absence and presence of ΔCD79b, although preliminary data indicate that global tyrosine phosphorylation and Ca++release are similar in cells irrespective of their ΔCD79b status (data not shown).
Another possible mechanism for how ΔCD79b might function is through a continual signaling or desensitization model. Recent studies by Vilen et al42 have shown that the BCR can become desensitized and dissociated from the CD79 heterodimer when ligated by low- to moderate-affinity antigens and that this destabilization requires only a small proportion of the available BCR to be ligated. In this way, it is conceivable that ΔCD79b might only interact or interfere with a small number of receptors, desensitizing the cell by interacting with, for example, Syk, but not causing mass down-regulation of surface expression. Furthermore, it is a phenomenon not observed with high-affinity antigens but with low- to moderate-affinity antigens such as those feasibly encountered by self-specific BCR of B-CLL. To survive constant antigen stimulation, the cell would require not only down-regulation of surface Ig but also dampening of intracellular signaling pathways. Perhaps ΔCD79b is capable of this function.
Whatever the mechanism, we would postulate that up-regulation of ΔCD79b is a physiological response of B cells that allows control of signaling via the BCR. As such, we would predict it to be present at times of B-cell activation, perhaps preventing cells from being overstimulated and killed via apoptosis, such as might occur in the germinal center. We are currently pursuing this proposal and examining the expression levels of ΔCD79b in apoptosis-sensitive B-CLL cells bearing CD38.
The authors thank Dr Ruth French for help with cell sorting and preparation of the manuscript and Dr Will Howatt for help with fluorescence microscopy. We are indebted to Dr Mike Neuberger and Theresa O'Keefe for reagents and helpful discussion. Thanks to Dr Christian Ottensmeir, Dr Surinder Sahota, Dr Rachel Ibbotson, Dr Stuart Lanham, and Zadie Davis for biopsy cells and cDNA samples.
Supported by the Biotechnology and Biological Sciences Research Council (BBSRC), Tenovus (Cardiff, United Kingdom), the Leukaemia Research Fund, and the Cancer Research Campaign.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mark Cragg, Tenovus Research Laboratory, Cancer Sciences Division, Southampton General Hospital, Tremona Rd, Southampton, SO16 6YD, United Kingdom; e-mail: msc@soton.ac.uk.

![Fig. 2. Correlation of expression of ΔCD79b and sensitivity to anti-Fcμ–induced apoptosis of Burkitt lymphoma cell lines. / (A) Level of ΔCD79b transcript in Burkitt cell lines. To assess the level of ΔCD79b in a range of Burkitt lymphoma cell lines, RT-PCR analysis was performed using the specific primers for CD79b as detailed in Figure 1. Samples assessed were Ramos-EHRB (E), Ramos (R), Daudi (D), Raji (Rj), and Namalwa (N). For comparison, the PCR products from an example of a B-CLL (C) and an SLVL (S) are also shown. The ratios in each panel show the relative level of ΔCD79b/CD79b. (B) The levels of surface BCR on EHRB (tightly grouped dotted outline […]), Namalwa (spaced dotted outline [. . .]), Raji (solid histogram), and Daudi (light gray solid outline) cells as measured by flow cytometry. The cell lines were stained with FITC-conjugated mAb to CD79a (ZL7-4), CD79b (ZL9-1), and sIg (M15/8). (C) Induction of apoptosis with anti-Fcμ mAb. Namalwa, Daudi, and EHRB Burkitt cell lines were assessed for their sensitivity to anti-μ–induced apoptosis by exposure to 10 μg/mL anti-μ mAb for 24 hours. Apoptosis was assessed by flow cytometry using annexin V–FITC and PI. (D) Correlation of ΔCD79b expression and sensitivity to anti-Fcμ mAb. The ratio of ΔCD79b/CD79b is taken from panel A, and the sensitivity to apoptosis is taken from the same experiments as those shown in panel C.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/9/10.1182_blood.v100.9.3068/4/m_h82123348002.jpeg?Expires=1768798868&Signature=38MLbefpY0QrvFyG~RsZx5icYUlG3zCj7QiE~JEp6jNkmzEu8qklGuA1xBq0bW0lTj4N26ZasEbKO4R-mJq3TEzKvOgre6N7jWzqoz-QWs~a6w0iMdK0ZG8hKKzcBdF-8aDxiK3jvfjA5ymMDdMsZyU3EN3kMIXGphli-UwEvdtIbqTQGbRNCIJILMYd0I8SnCRn~B5b2Zbu1Ky763VsMIwg6kH3Gwpq7iy2yPs4uwzxzP6GFyRZidhAsvWkoUjIkesS7RrS~xt8Urx9viyGy0x5KDwIJlRXLJ9be8mQq23Ox69nOldF8TMEw1P4IWX0LZkO3Fjv07NlO3QjmMVNbw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Functional analysis of ΔCD79b. / Constructs encoding the wild-type ΔCD79b, a leaderless ΔCD79b, a ΔCD79b construct in which the endogenous leader was replaced with that from rat CD4 (CD4L), and the CD4LΔCD79b possessing a mutated ITAM (CD4LΔCD79b [Y207A]) were transfected into Ramos-EHRB cells and then positive clones assessed for sensitivity to anti-μ–induced apoptosis after 48 hours by annexin V–FITC/PI analysis. Two clones from each construct are shown. The average of 3 experiments, ± SD, are shown for each clone. Underneath, expression levels of CD79a, CD79b, and sIg are shown for each clone, determined as indicated in Figure 2, illustrating that all clones expressed similar levels of all BCR components at the cell surface.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/9/10.1182_blood.v100.9.3068/4/m_h82123348005.jpeg?Expires=1768798868&Signature=sYxukYx4LLdpGru0C9hg2SuI6BQsY3KhtMkN~5VdinxNVl54mgTw0CIPau~djwtE-Be3Ds8xPF5wqZvR2X2R0k8GmxiH-kBu~JXZVS7Mx83GmxcKoBRJ0UjHxR36ycSCHYQTShg0RogGt8h1CpZlctYE7CqDdpN8LZav4ezrKt8Nf8jFiInV18qQD~WbCfnTIUj6-V~wsGytrLHKzoEnwVHU2L1zBNCoTqHUYfist95yWdYrMCxfIXC-NdBZ1whYH2yagWWrBmoP90qqfU2K9YXyN7-ji-YNLly-1VB9Hqu1tlLMAjF4yrxAaZHe-bxKmHbRm~jQtPPDvEWtyIa~Xw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. Correlation of expression of ΔCD79b and sensitivity to anti-Fcμ–induced apoptosis of Burkitt lymphoma cell lines. / (A) Level of ΔCD79b transcript in Burkitt cell lines. To assess the level of ΔCD79b in a range of Burkitt lymphoma cell lines, RT-PCR analysis was performed using the specific primers for CD79b as detailed in Figure 1. Samples assessed were Ramos-EHRB (E), Ramos (R), Daudi (D), Raji (Rj), and Namalwa (N). For comparison, the PCR products from an example of a B-CLL (C) and an SLVL (S) are also shown. The ratios in each panel show the relative level of ΔCD79b/CD79b. (B) The levels of surface BCR on EHRB (tightly grouped dotted outline […]), Namalwa (spaced dotted outline [. . .]), Raji (solid histogram), and Daudi (light gray solid outline) cells as measured by flow cytometry. The cell lines were stained with FITC-conjugated mAb to CD79a (ZL7-4), CD79b (ZL9-1), and sIg (M15/8). (C) Induction of apoptosis with anti-Fcμ mAb. Namalwa, Daudi, and EHRB Burkitt cell lines were assessed for their sensitivity to anti-μ–induced apoptosis by exposure to 10 μg/mL anti-μ mAb for 24 hours. Apoptosis was assessed by flow cytometry using annexin V–FITC and PI. (D) Correlation of ΔCD79b expression and sensitivity to anti-Fcμ mAb. The ratio of ΔCD79b/CD79b is taken from panel A, and the sensitivity to apoptosis is taken from the same experiments as those shown in panel C.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/9/10.1182_blood.v100.9.3068/4/m_h82123348002.jpeg?Expires=1768871648&Signature=hL8BaHFxZhhnc5bDOs5aSrcgW3NUx~JkQzVNw0-4yPtdKa7NytLHilmayCTxOJllMLrCq2hz5Z3zCsHT0Pqq0dO4JIQ~hErj7~l3pMd5VMvhd7-O1v9mPnK-KMfQhM6MmgcHuWGZj-rXQGxQV8haB29n3vr5YW5z6Yiz0ew6aPSAmdiXYkxZKY7JD4EzGj4AIizg4iJonRVhAZ18tX89-cT8V~m~rSHI8FETTKREcllmtd9V4HI8Mcp3HjkOWR8Kv9aEK1AsuJ4Qi4SfftXeSEJPpnLLi07OkMi72EcICx6u4iUzSxPwaucCfVdQW1HvQXH2REgUO6yZyXrF~NW1tw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 5. Functional analysis of ΔCD79b. / Constructs encoding the wild-type ΔCD79b, a leaderless ΔCD79b, a ΔCD79b construct in which the endogenous leader was replaced with that from rat CD4 (CD4L), and the CD4LΔCD79b possessing a mutated ITAM (CD4LΔCD79b [Y207A]) were transfected into Ramos-EHRB cells and then positive clones assessed for sensitivity to anti-μ–induced apoptosis after 48 hours by annexin V–FITC/PI analysis. Two clones from each construct are shown. The average of 3 experiments, ± SD, are shown for each clone. Underneath, expression levels of CD79a, CD79b, and sIg are shown for each clone, determined as indicated in Figure 2, illustrating that all clones expressed similar levels of all BCR components at the cell surface.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/9/10.1182_blood.v100.9.3068/4/m_h82123348005.jpeg?Expires=1768871648&Signature=CeblLSVXHPXeJ-Ou646oQyWuNBArLAt~2mWJgRDaKH5ROcBVvse6MFtvBSRW40H3xkMqKmtN3-ufNUBpCD-psqz87nHzIpRRHLI7lwUfwJi8GTyt40ntBBya7LjnjG-dKix~bSR-Zoj5rTmOCNBlj7IpzFbZ14-2ZPNcX7vV-ahtMjq7rm6Svik-aLCD5z1JJASeWZp78r1FozMNR7teUNKTu~IHmVQcvOaSjdl24k9am~mVwf7Ex9ERfm2nARXUGaFEAmEP3wn8YGU1iLzljYreB1NKJr9t2iiizzGoCAShkoN8i1plQVQ2qpe1GX9oUsc4AVIh~gw1AERlpMqYpA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)