Abstract
Thalidomide (Thal) can overcome drug resistance in multiple myeloma (MM) but is associated with somnolence, constipation, and neuropathy. In previous in vitro studies, we have shown that the potent immunomodulatory derivative of thalidomide (IMiD) CC-5013 induces apoptosis or growth arrest even in resistant MM cell lines and patient cells, decreases binding of MM cells to bone marrow stromal cells (BMSCs), inhibits the production in the BM milieu of cytokines (interleukin-6 [IL-6], vascular endothelial growth factor [VEGF], tumor necrosis factor-α [TNF-α]) mediating growth and survival of MM cells, blocks angiogenesis, and stimulates host anti-MM natural killer (NK) cell immunity. Moreover, CC-5013 also inhibits tumor growth, decreases angiogenesis, and prolongs host survival in a human plasmacytoma mouse model. In the present study, we carried out a phase 1 CC-5013 dose-escalation (5 mg/d, 10 mg/d, 25 mg/d, and 50 mg/d) study in 27 patients (median age 57 years; range, 40-71 years) with relapsed and refractory relapsed MM. They received a median of 3 prior regimens (range, 2-6 regimens), including autologous stem cell transplantation and Thal in 15 and 16 patients, respectively. In 24 evaluable patients, no dose-limiting toxicity (DLT) was observed in patients treated at any dose level within the first 28 days; however, grade 3 myelosuppression developed after day 28 in all 13 patients treated with 50 mg/d CC-5013. In 12 patients, dose reduction to 25 mg/d was well tolerated and therefore considered the maximal tolerated dose (MTD). Importantly, no significant somnolence, constipation, or neuropathy has been seen in any cohort. Best responses of at least 25% reduction in paraprotein occurred in 17 (71%) of 24 patients (90% confidence interval [CI], 52%-85%), including 11 (46%) patients who had received prior Thal. Stable disease (less than 25% reduction in paraprotein) was observed in an additional 2 (8%) patients. Therefore, 17 (71%) of 24 patients (90% CI, 52%-85%) demonstrated benefit from treatment. Our study therefore provides the basis for the evaluation of CC-5013, either alone or in combination, to treat patients with MM at earlier stages of disease.
Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy, affecting 14 400 new and 50 000 total patients in the United States in 2001,1 and remains incurable despite conventional and high-dose chemotherapy. To overcome resistance to current therapies and improve patient outcome, novel biologically based treatment approaches are needed that target mechanisms whereby MM cells grow and survive in the bone marrow (BM). Thalidomide (Thal), used empirically to treat MM based upon its antiangiogenic activity and the increased angiogenesis observed in MM BM, achieves responses even in refractory, relapsed disease.2 Importantly, our in vitro and in vivo preclinical studies both define a role for MM-host interactions in regulating MM cell growth, survival, drug resistance, and migration in the BM3-12 and demonstrate that Thal targets the MM cell in its BM milieu to overcome classical drug resistance both in vitro and in vivo in animal models.13-16 However, Thal has significant and dose-limiting side effects, including somnolence, constipation, and neuropathy, which has prompted the search for more potent and less toxic Thal derivatives.
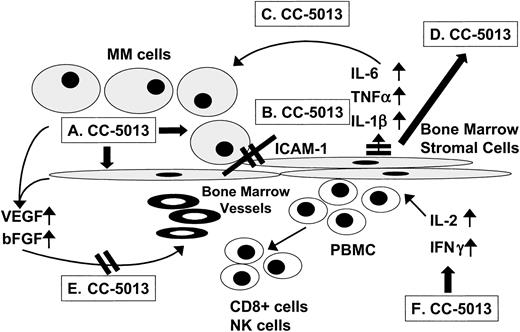
Immunomodulatory drugs (IMiDs) are potent Thal derivatives that markedly stimulate T-cell proliferation, as well as interleukin-2 (IL-2) and interferon-γ (IFN-γ) production, but do not inhibit phosphodiesterase-4 (PDE-4).17 CC-5013 (REVIMID) is 50 to 2000 times more potent than Thal in stimulating T-cell proliferation triggered via the T-cell receptor (TCR) and 50 to 100 times more potent than Thal in augmenting IL-2 and IFN-γ production. In addition, CC-5013 triggers dose-dependent decreased secretion of tumor necrosis factor-α (TNF-α), IL-1β, and IL-6 and triggers increased secretion of IL-10. The CC-5013 concentration for 50% inhibition (IC50) of lipopolysaccharide (LPS)–induced TNF-α secretion by peripheral blood mononuclear cells (PBMCs) is about 100 nM (25.9 ng/mL), whereas Thal has an IC50 of about 194 μM (50.2 μg/mL).18 Based upon these more potent effects of IMiDs than Thal on normal cells, we compared their relative anti-MM activities. Our in vitro studies show an IC50 of 0.4 μM (103.6 ng/mL) for CC-5013 against MM cell lines and patient cells that are resistant to conventional therapy; in contrast, even at concentrations up to 100 μM (25.8 μg/mL), Thal decreases MM cell proliferation by only 15% or 20%.13 Our studies further demonstrate that CC-5013 decreases binding of MM cells to bone marrow stromal cells (BMSCs), inhibits the production in the BM milieu of cytokines (IL-6, vascular endothelial growth factor [VEGF], TNF-α) mediating growth and survival of MM cells, blocks angiogenesis, and stimulates host anti-MM natural killer (NK) cell immunity13-15(Figure 1). In addition, we determined that CC-5013 inhibits tumor growth, decreases angiogenesis, and prolongs host survival in a human plasmacytoma mouse model.16 These preclinical studies suggest that CC-5013 may overcome drug resistance, even to Thal, in MM cells.
Mechanisms of action of CC-5013 targeting MM cells and the BM microenvironment.
(A) MM cell G1 growth arrest and apoptosis. (B) Decreased MM cell to BMSC binding. (C) Decreased cytokine activity. (D) Decreased cytokine production in BM. (E) Decreased angiogenesis. (F) Induced host anti-MM immune response.
Mechanisms of action of CC-5013 targeting MM cells and the BM microenvironment.
(A) MM cell G1 growth arrest and apoptosis. (B) Decreased MM cell to BMSC binding. (C) Decreased cytokine activity. (D) Decreased cytokine production in BM. (E) Decreased angiogenesis. (F) Induced host anti-MM immune response.
The remarkable in vitro and in vivo activity of CC-5013 against resistant MM cells in preclinical studies provided the framework for this phase 1 dose-escalation trial of CC-5013 in patients with relapsed and refractory MM. Importantly, CC-5013 achieved either response or stabilization of disease in 17 (71%) of 24 evaluable patients (90% confidence interval [CI], 52%-85%) and demonstrated a favorable toxicity profile. Our study therefore provides the basis for the evaluation of CC-5013, either alone or in combination, to treat patients with MM at earlier stages of disease.
Patients, materials, and methods
Study objectives
The primary objectives of this study were to identify the maximum tolerated dose (MTD) and to evaluate the safety of CC-5013 when given at doses of 5 mg/d to 50 mg/d in patients with refractory and/or relapsed MM. A secondary objective was to evaluate the response to CC-5013.
Study design
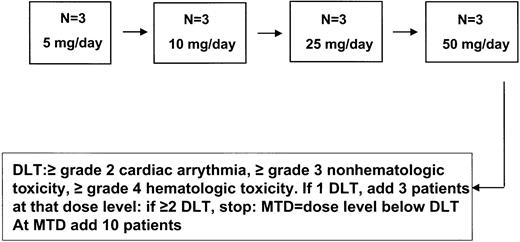
A standard dose escalation of CC-5013 (5, 10, 25, and 50 mg/d) was performed to identify the MTD under the auspices of an institutional review board (IRB) protocol and after obtaining informed consent. Three patients were enrolled at each dose level, with up to 6 patients assigned to each dose level, depending on dose-limiting toxicity (DLT). DLT is defined to be at least grade 2 cardiac arrhythmia, at least grade 3 nonhematologic toxicity, or grade 4 hematologic toxicity using National Cancer Institute (NCI) common toxicity criteria during the first 4 weeks of treatment. Following the standard design, if no DLTs were observed among the first cohort of 3 patients at each dose level, the next set of 3 patients was entered on the next highest dose. If 2 or more DLTs were observed, the previous dose level was identified as the MTD. If 1 DLT was observed among the initial 3 patients, an additional 3 patients would be entered. If none of the additional 3 patients experienced a DLT, the dose was escalated; otherwise, the lower dose was identified as the MTD. Ten additional patients were enrolled at the MTD to better define the toxicity rate (Figure 2).
Patients were assessed before and weekly for the first month after treatment. Patients were hospitalized in the clinical research unit for at least 72 hours after the first dose of CC-5013 and overnight on day 28. Patients who did not have either disease progression or DLT at day 28 continued on treatment until disease relapse or progression, with monthly follow-up. Safety and efficacy assessments were performed at each visit, with blood and urine samples collected for pharmacokinetics on days 1 to 4 and day 28.
Patient selection
Patients with relapsed and/or refractory MM were eligible and must have failed to respond to at least 2 prior regimens of treatment. Patients were considered refractory if they had progressive disease by Southwest Oncology Group (SWOG) criteria (an increase by more than 100% of the lowest level of protein production). Relapse following a remission was defined by SWOG criteria as any of the following: (1) more than 25% increase in M protein from baseline levels, (2) reappearance of M paraprotein, or (3) a definite increase in the size and number of lytic bone lesions recognized on radiographs (compression fractures per se did not constitute a relapse). Prior therapy with Thal was allowed, provided that it was tolerated. Exclusion criteria included a prolonged QT interval (> 430 ms); predisposition to cardiac arrythmias; concomitant medications known to prolong the QT segment; at least grade 3 peripheral neuropathy; inadequate renal function (serum creatinine > 1.5 mg/dL); evidence of mucosal or internal bleeding; thrombocytopenia (platelets < 50 × 109/L [50 000/μL]); neutropenia (absolute neutrophil count < 1 × 109/L [1000/μL]); pregnant or lactating women; and women of childbearing potential who were not using adequate contraception.
Treatment
Each patient received CC-5013 as a single daily oral dose. Depending on the order of study entry and the tolerability of prior dose levels, patients received CC-5013 at 5, 10, 25, or 50 mg/d for the first 4 weeks. Accrual to the next higher dose level did not occur until the safety and tolerability of CC-5013 at prior dose level(s), when given for at least 4 weeks, had been established. Patients experiencing DLT during the first 28 days had therapy discontinued. After the first 4 weeks of treatment, dose reduction was permitted to manage any toxicity.
Safety parameters
Prior to enrollment, at each weekly and monthly visit, and either at the completion of the study or at premature discontinuation, evaluation of each patient included medical history and physical examination (to include assessment of peripheral neuropathy and measurement of vital signs), query for adverse events and concomitant medication use, and clinical laboratory testing (blood chemistry, hematology, urinalysis, and thyroid function testing [every 3 months]). Electrocardiograms (ECGs) were recorded at the first 4 weekly visits and monthly thereafter for the first 12 months of treatment. During the 72-hour inpatient period following the first dose administration, vital signs, ECGs, and adverse events were monitored and recorded at 1, 2, 4, and 8 hours. Adverse events and vital sign measurements were recorded every 8 hours thereafter, and ECGs were performed on days 2, 3, and 4. Severity of adverse experiences, including any clinical laboratory and vital sign abnormalities, were evaluated using common toxicity criteria. No drug-related renal compromise was observed.
Response
Response to treatment was assessed by using M-protein quantification (by protein electrophoresis) in serum and a 24-hour urine collection at screening, start of therapy, day 14 and day 28, as well as monthly thereafter (or upon early termination). Bone marrow aspirations and biopsies were performed at baseline, 3, 6, and 12 months, and/or at completion of therapy. A skeletal survey was also performed in patients who had abnormal pretreatment studies or as clinically indicated.
Pharmacokinetics
Blood and urine samples were collected for analysis of pharmacokinetic parameters on days 1 and 28. Specifically, blood samples were collected before dose as well as at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 18, and 24 hours after dose. In addition, blood samples were collected weekly for CC-5013 level determinations. Total urine was collected and assayed at 0 to 4, 4 to 8, 8 to 12, and 12 to 24 hours after drug administration. Appropriate noncompartmental pharmacokinetic parameters were estimated using the actual blood sampling times for each patient on day 1 and day 28. Values for noncompartmental pharmacokinetic parameters were calculated based on CC-5013 plasma concentration–time data obtained during each dosing period.
Biostatistical analysis
The MTD was identified using a standard phase 1 design (Figure2). With this design, the probability of dose escalation was 0.97 if the true DLT rate was 5%. The 90% confidence intervals are reported on the percentage of patients who experience toxicity at the MTD and the percentage of patients who achieved reduction in their paraprotein.
Results
Patients treated
Twenty-seven patients with a median age of 57 years (range, 40-71 years) were enrolled. Two patients were removed from study on the first day of treatment due to rapid disease progression resulting in renal dysfunction, a known complication of MM, which rendered them ineligible. Of the 25 patients who received therapy, 19 were men and 6 were women. Immunoglobulin G (IgG) MM was present in 15 (60%), IgA in 7 (28%), and light-chain-only disease in 3 (12%) patients. Fifteen (60%) patients had undergone prior autologous stem cell transplantation (SCT), and 16 (64%) had received prior Thal. Patients received a median of 3 prior regimens (range, 2-6 regimens); all patients had relapsed MM, and 18 (72%) were refractory to salvage therapy.
Toxicity profile
The first 3 patients were treated for 28 days with CC-5013 5 mg/d without DLT. In the second cohort of 3 patients treated at 10 mg/d, 1 patient had DLT characterized by grade 3 leukopenia and neutropenia, resulting in her removal from study before day 28; 2 other patients tolerated the drug without complication, and an additional 3 patients treated at 10 mg/d also demonstrated no DLT. The patient who had the DLT previously experienced a similar reaction to Thal and to dexamethasone (Dex). CC-5013 was well tolerated within the first 28 days in all 3 patients treated at 25 mg/d; however, grade 3 thrombocytopenia and grade 4 neutropenia developed during the second month, resulting in 2 patients being removed from study. All 3 patients treated with CC-5013 at 50 mg/d tolerated therapy without DLT in the first 28 days, and an additional 10 patients were treated at 50 mg/d to better define toxicity and outcome. None of the patients (0 of 13) treated at 50 mg/d had DLT in the first 28 days (90% CI, 0%-21%).
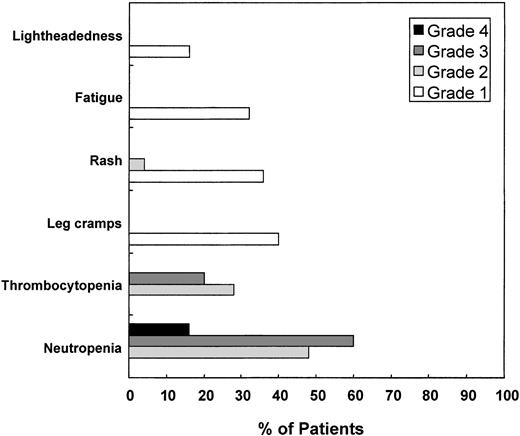
Overall, the median duration of CC-5013 therapy was 6 months (range, 2 weeks to 16 months), and 10 patients continued on treatment (4 patients at 25 mg/d, 3 patients at 10 mg/d, and 3 patients at 5 mg/d). Importantly, no significant somnolence, constipation, or neuropathy has been seen in any cohort. Grade 3 and 4 myelosuppression developed in 12 of 13 patients treated with 50 mg/d CC-5013 beyond 28 days, eventually prompting dose reduction and granulocyte colony-stimulating factor (G-CSF) support in all 12 patients, with a median duration of 3 months (range, 2-4 months) of CC-5013 therapy at 50 mg/d. All 12 patients who were reduced to 25 mg/d of CC-5013 tolerated the lower dose, and in aggregate 13 patients remained on therapy at this dose for a median of 4 months (range, 2-8 months). Based on greater exposures to CC-5013 for more than 28 days, we therefore conclude that 25 mg/d is the MTD. The most common adverse events for treated patients (n = 25), including those observed after day 28, are presented in Figure 3. Grade 3 neutropenia occurred in 15 (60%) of 25 patients (90% CI, 42%-76%) and grade 4 neutropenia in 4 (16%) of 25 patients (90% CI, 6%-33%). Grade 3 thrombocytopenia occurred in 5 (20%) of 25 patients (90% CI, 8%-38%).
Profile of adverse events.
Severity of adverse events was assessed using common toxicity criteria for the entire duration of therapy (n = 25 patients).
Profile of adverse events.
Severity of adverse events was assessed using common toxicity criteria for the entire duration of therapy (n = 25 patients).
Response
Maximal paraprotein reductions observed in 24 patients who received at least 28 days of treatment are summarized in Table1. Best responses of at least 25% reduction in paraprotein occurred in 17 (71%) of 24 evaluable patients (90% CI, 52%-85%), including at least 50% reduction in paraprotein in 7 (29%) of 24 patients (90% CI, 15%-48%). Stable disease (25% or less reduction in paraprotein) was noted in an additional 2 (8%) patients. Therefore, 17 (71%) of 24 patients (90% CI, 52%-85%) demonstrated benefit from treatment, including 11 (46%) patients who had received prior Thal. Eleven (85%) of 13 patients treated at 50 mg/d had at least 25% paraprotein reductions (90% CI, 59%-97%), and 5 (38%) of 13 patients (90% CI, 17%-65%) had at least 50% declines. Most responses have been at 25 mg/d (2 of 3 patients) and 50 mg/d CC-5013 (12 of 13 patients), although clinical activity was also seen in patients treated at both 5 mg/d (3 of 3 patients) and 10 mg/d (1 of 5 patients) dose levels. Median time to best response was 2 months (range, 1-11 months), and median duration of response was 6 months (range, 2-18 months).
Maximal changes in paraprotein after CC-5013 treatment
| Dose, mg/d . | No. of patients . | Decrease . | Increase . | |||
|---|---|---|---|---|---|---|
| < 25% . | ≥ 25% < 50% . | ≥ 50% < 75% . | ≥ 75% < 99% . | ≥ 25% . | ||
| 5 | 3 | 0 | 2 | 1 | 0 | 0 |
| 10 | 5 | 0 | 0 | 1 | 0 | 4 |
| 25 | 3 | 1 | 2 | 0 | 0 | 0 |
| 50 | 13 | 1 | 6 | 2 | 3 | 1 |
| Subtotal | 24 | 2 (8%) | 10 (42%) | 4 (17%) | 3 (13%) | 5 (21%) |
| Dose, mg/d . | No. of patients . | Decrease . | Increase . | |||
|---|---|---|---|---|---|---|
| < 25% . | ≥ 25% < 50% . | ≥ 50% < 75% . | ≥ 75% < 99% . | ≥ 25% . | ||
| 5 | 3 | 0 | 2 | 1 | 0 | 0 |
| 10 | 5 | 0 | 0 | 1 | 0 | 4 |
| 25 | 3 | 1 | 2 | 0 | 0 | 0 |
| 50 | 13 | 1 | 6 | 2 | 3 | 1 |
| Subtotal | 24 | 2 (8%) | 10 (42%) | 4 (17%) | 3 (13%) | 5 (21%) |
Pharmacokinetic analysis
CC-5013 was rapidly absorbed, with maximum plasma concentrations at a median Tmax (time of maximum concentration) of 1 hour or 1.5 hours on day 1 and day 28 in patients treated at each dose level. No trend in Tmax was noted with either increasing dose level or multiple dosings. Following Cmax (maximum concentration), plasma concentrations of CC-5013 declined in a predominantly monophasic manner, with elimination phase starting at 1 to 8 hours after dose on day 1 and day 28. The mean terminal elimination half-lives were 3.1 to 4.2 hours on both day 1 and day 28. There was little or no accumulation of CC-5013: The mean accumulation ratio of CC-5013 in plasma was 0.7 to 1.0, with a Cmax and area under the curve (AUC) (0-) of 0.8 to 1.2 on day 28 compared with day 1. Intersubject variability was generally low to moderate for AUC and Cmax, with values ranging from 10.6% to 51.8% and 3% to 33% on day 1 and day 28, respectively.
Bone marrow examination
Of the 13 patients treated with CC-5013 at 50 mg/d, 12 patients and a single patient developed grades 3 and 4 neutropenia, respectively; BM hemopoiesis was normal or improved in 10 and reduced in just 2 of these patients. In the 5 patients treated with 50 mg/d CC-5013 who developed grade 2 or 3 thrombocytopenia, megakaryocyte numbers were normal in 3 and mildly reduced in 2 cases. In aggregate, the BM findings were therefore most remarkable for the absence of hypoplasia despite low circulating peripheral counts.
Discussion
Our long-term studies, both in vitro and in animal models, have shown the importance of host BM-MM cell interactions in promoting MM cell growth, survival, drug resistance, and migration in the BM microenvironment.3-12 Importantly, our studies have also shown that CC-5013 can target the MM cell in its BM milieu to overcome drug resistance in vitro as well as decrease tumor growth and extend survival in vivo in a murine model.13-16 The present clinical study now demonstrates both the safety and efficacy of CC-5013 in patients with refractory and relapsed MM. In aggregate, these studies therefore provide the framework for development of a new treatment paradigm using CC-5013 to target both the MM cell and its microenvironment, overcome drug resistance, and improve patient outcome.
The choice of dose levels in this phase 1 clinical trial (5-50 mg/d) was targeted to achieve CC-5013 concentrations of approximately 25.9 to 259 ng/mL, levels that modulate the production of cytokines and inhibit MM cell proliferation in vitro. Importantly, except for an idiosyncratic reaction to CC-5013 that had also occurred to Thal, no DLT occurred within the first 28 days in any dose cohort; remarkably, no sedation, constipation, or neuropathy was seen. Twenty-five percent or greater reductions in paraprotein were observed in 17 (71%) of 24 evaluable patients. This anti-MM activity was remarkable given that 15 patients (60%) had undergone prior SCT and 16 patients (64%) had progressive disease despite Thal treatment. In addition, less than 25% paraprotein reductions were observed in an additional 2 patients. These data, coupled with the absence of somnolence, constipation, and neuropathy, support future phase 2 studies of CC-5013 for MM patients earlier in the course of their disease.
Pharmacokinetic studies completed in 24 subjects reveal rapid absorption (Tmax 1-1.5 hours), monophasic elimination (half-life [T1/2] 3.1-4.2 hours), and low to moderate intersubject variability for AUC (11%-52%) and Cmax (3%-33%). In addition, there was no significant accumulation by day 28. The myelosuppression after day 28 seen in the patients treated with 50 mg/d CC-5013 is considered to be DLT. Furthermore, 13 of 15 patients treated at 25 mg/d remained on therapy at this dose for a median of 4 months (range, 2-8 months); therefore, 25 mg/d is considered MTD. The absence of significant marrow hypoplasia observed in most patients with cytopenias suggests etiologies other than BM suppression, which will be evaluated in future studies.
Based upon the current study showing efficacy and tolerability of CC-5013, several future clinical phase 2 trials are planned in patients with refractory relapsed disease. First, because some patients treated with CC-5013 at 25 mg/d developed granulocytopenia and thrombocytopenia, a dosing regimen that cycles drug exposure, with time for granulocyte and platelet recovery, will attempt to achieve clinical benefit while avoiding myelosuppression. Second, given the activity of the drug seen at lower dose levels and the T1/2 of 3 to 4 hours, a comparative trial of 2 cycling regimens will be undertaken: 30 mg/d as a single daily dose versus 30 mg/d in divided doses. Third, because laboratory and clinical data have suggested at least additive effects of dexamethasone in combination with Thal,13,19,20and our preclinical data suggest that Dex also enhances anti-MM activity of CC-5013,13 21 clinical trials will also determine whether the addition of dexamethasone to CC-5013 enhances its clinical efficacy in MM.
Thal has achieved response rates of 30% in patients with refractory, relapsed MM and, when combined with dexamethasone, response rates of 64% in patients with newly diagnosed disease.2 20 However, somnolence, constipation, and neuropathy preclude its use in some patients. Because our study now shows that oral CC-5013 has antitumor activity, favorable pharmacokinetics, and acceptable toxicity in patients with relapsed and refractory MM, we will carry out clinical protocols of CC-5013, alone and in combination with dexamethasone, for the treatment of MM earlier in the disease course, including as initial treatment, therapy for first relapse, and maintenance therapy.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-03-0996.
Supported by National Institutes of Health grants RO-1 50947 and PO-78378, the Multiple Myeloma Research Foundation (T.H., C.M., R.L.), the Myeloma Research Fund (K.C.A.), and the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth C. Anderson, Dana-Farber Cancer Institute, Mayer 557, 44 Binney St, Boston, MA 02115; e-mail:kenneth_anderson@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal