Abstract
This multicenter phase 2 trial investigated safety and efficacy of a new immunochemotherapeutic regimen combining rituximab (R) and fludarabine (F) in patients with fludarabine- and anthracycline-naive chronic lymphocytic leukemia (CLL). The rationale for using R + F includes single-agent efficacy of both drugs, in vitro synergism of R and F, and no apparent overlapping toxicity. Of 31 eligible patients with B-CLL enrolled, 20 were previously untreated and 11 relapsed. Treatment consisted of fludarabine administered at standard doses (25 mg/m2/d; days 1-5, 29-33, 57-61, and 85-89) and rituximab (375 mg/m2/d) given on days 57, 85, 113, and 151. Side effects such as fever, chills, and exanthema were generally mild (National Cancer Institute Common Toxicity Criteria [NCI-CTC] grade 1/2 in 48% and grade 3 and/or 4 in 3% of patients). Fever and chills were mainly associated with the first rituximab infusion. Hematologic toxicity included neutropenia (grade 1 and/or 2 in 26%, grade 3 and/or 4 in 42%) and thrombocytopenia (grade 1 and/or 2 in 19%, grade 3 and/or 4 in 9%). One patient died of cerebral bleeding during prolonged thrombocytopenia after the second cycle of fludarabine. There were a total of 32 infections in 16 patients, none of which was fatal. The overall response rate (complete remission [CR] and partial remission [PR]) was 87% (27 of 31 evaluable patients). In 20 previously untreated patients, 17 (85%) responded. Ten of 31 patients achieved CR (5 of 20 untreated; 5 of 11 pretreated; 9 of 21 Binet stage B, 1 of 10 Binet stage C). The median duration of response was 75 weeks. We conclude that the combination of rituximab and fludarabine is feasible and effective in patients with B-CLL.
Introduction
Recent advances in understanding the biology of chronic lymphocytic leukemia (CLL) indicate that there are 2 variants arising at different stages of B-cell differentiation. This variance is reflected by the mutational status of the immunoglobulin variable region (IgV) genes.1 In addition, by using fluorescence in situ hybridization technique, genomic aberrations can be diagnosed in up to 80% of cases. Both the IgV mutation status and the pattern of genomic aberrations have a high predictive value for disease progression and survival in patients with CLL.2 3Therefore, therapeutic approaches are currently being reassessed with emphasis on prognostic factor–directed therapy.
This development is accompanied by promising new treatment options. The most convincing results were reported for a single-agent therapy using the purine analog fludarabine. In pretreated patients the overall response rates range from 50% to 60%. Approximately 80% of untreated patients respond with a complete remission (CR) rate of 35%.4,5 Randomized trials demonstrated that fludarabine induces higher responses and more durable remissions compared with chlorambucil; cyclophosphamide, hydroxydaunomycin, Oncovin (vincristine), and prednisone (CHOP); or cyclophosphamide, Adriamycin (doxorubicin), and prednisone (CAP).6,7 However, the most relevant clinical end point, overall survival, was not substantially different.6 7
Despite encouraging results with fludarabine or fludarabine combinations, all patients ultimately relapse. Relapse is most probably a result of residual tumor cells. Studies using minimal residual disease (MRD) assays with lower sensitivity reported some MRD-negative cases after fludarabine therapy.8,9 In contrast, a more recent study comprising 16 newly diagnosed cases of CLL documented that all patients in CR had polymerase chain reaction (PCR)–detectable residual tumor cells.10 Thus, most likely all patients with CLL treated with conventional chemotherapy have residual tumor cells. Because a true CR is the major therapeutic goal in CLL, there is a need for new therapeutic approaches with different mechanism of action.
Aggressive treatment such as high-dose chemotherapy followed by autologous or allogeneic transplantation for selected younger high-risk patients or combinations of fludarabine with other cytostatic agents are currently under investigation. Different regimens using fludarabine plus anthracylines and/or cyclophosphamide have been evaluated. In untreated patients combining fludarabine (F; 30 mg/m2, days 1-3) and cyclophosphamide (C; 300 mg/m2 daily for 3 days) increases the overall response rate to 88% and the CR rate to 35%.11 However, neutropenic fever or severe infections occurred in up to 40% of patients treated.11-13 Thus, randomized clinical trials are currently being conducted to compare the FC combination to fludarabine alone.
Monoclonal antibodies such as rituximab (anti-CD20) have attracted substantial interest as a new class of effective reagents in the treatment of malignant lymphoma.14 Rituximab is a chimeric-humanized monoclonal antibody that has given response rates of 50% in relapsed or refractory low-grade non-Hodgkin lymphoma (NHL). Treatment results with single-agent rituximab in patients with CLL using conventional doses were inferior compared with follicular lymphoma.15 This result might, at least in part, be due to the lower density of CD20 antigen expression on CLL cells.16,17 Pharmacokinetic studies revealed a substantially lower pretreatment plasma level of rituximab in patients with CLL compared with those with other low-grade lymphoma.18 Patients with higher numbers of circulating CD20+ tumor cells were more likely to experience severe side effects related to a massive release of cytokines.19Severe acute reactions were generally more common during initial infusion.19,20 These side effects can be controlled by a “stepped-up” dosing of rituximab or the addition of steroids.21
Experimental data indicate a potential superadditive effect of combining rituximab with fludarabine because of modified BcL-2 expression.22 In addition rituximab can enhance the sensitivity of tumor cells to chemotherapy-induced apoptosis and might thus help to functionally overcome clinical drug resistance. A recent preclinical study demonstrated synergy between rituximab and fludarabine.23 This synergistic effect might be due to the down-regulation of Bcl-2 through inhibition of interleukin 10. Down-regulation of the anticomplement proteins CD55, CD46, and CD 59 might also contribute to this synergy.23-25
On the basis of the rationale of synergistic and potential superadditive effects of fludarabine and rituximab, we conducted a multicenter phase 2 trial, investigating the safety and efficacy of a sequential application schedule in patients with fludarabine- and anthracycline-naive CLL. Here, we report the clinical results of this combined immunochemotherapy.
Patients, materials, and methods
This prospective multicenter, open-label, phase 2 study was conducted between August 1999 and February 2001 and involved 6 centers in Germany. The study was carried out in accordance to the Declaration of Helsinki (Hong Kong Amendment, 1989). The protocol met all requirements of the European Good Clinical Practice Guidelines (July 1990) and received approval from the ethics committees of all participating centers. All eligible patients gave written informed consent. The first objective of this study was to determine whether the combination of fludarabine and rituximab is feasible and well tolerated. The second objective was to test the efficacy of this combination treatment in CLL patients. The response rate (CR + PR) was chosen as primary efficacy end point.
Patients with CLL as defined by the National Cancer Institute (NCI)–sponsored working group, aged 18 to 75 years, with stages Binet B or C, at first diagnosis or after treatment with chlorambucil, prednisone, or a combination of both, were enrolled into the study. More than 30% of all peripheral lymphocytic cells had to be CD20+. An Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 3 was required with a life expectancy of at least 3 months. Patients were excluded if they had received prior treatment with fludarabine or anthracycline-containing regimens or had a positive Coombs test. Other exclusion criteria were Richter syndrome, previous treatment with murine antibodies, active opportunistic infections, any other severe infection not controlled by medical or surgical therapy, or major organ dysfunction. Patients who were pregnant or lactating or those who had participated in other trials during the past 12 weeks were also excluded.
Treatment plan
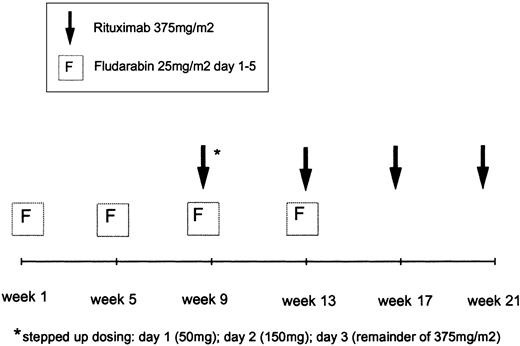
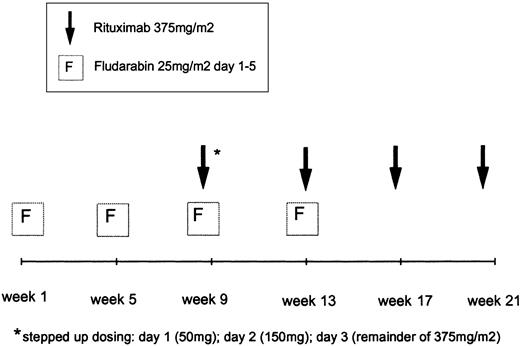
Patients received a total of 4 cycles of fludarabine and 4 cycles of rituximab. Fludarabine was administered intravenously at doses of 25 mg/m2/d (days 1-5) in 28-day intervals. Fludarabine cycles 3 and 4 were given in combination with rituximab (Figure 1). The anti-CD20 monoclonal antibody rituximab (MabThera) was supplied by Hoffmann-LaRoche AG (Grenzach-Wyhlen, Germany). The first infusion of rituximab was fractionated (50 mg on day 1; 150 mg on day 2; remainder of 375 mg/m2 on day 3) during the third cycle of fludarabine (days 57-59). The second infusion of rituximab (375 mg/m2) was given on day 1 of the fourth fludarabine cycle (day 87). The fludarabine infusion was always applied prior to the rituximab infusion. The 2 final infusions of rituximab were administered on days 113 and 151, respectively, without concomitant application of fludarabine. When grade 3 or 4 adverse events such as chills, fever, or bronchospasm were encountered during rituximab infusion, the application of the antibody was interrupted. After the symptoms had ceased, the infusion at half the initial infusion rate was continued. Thirty minutes prior to the first rituximab infusion 1000 mg acetaminophen was administered. Antibiotic prophylaxis with 960 mg co-trimoxazole (160 mg trimethoprim/800 mg sulfamethoxazole) 3 times a week was recommended during therapy and up to 3 months after the last application of rituximab. Concomitant corticosteroids as prophylaxis or treatment of infusion-related side effects were allowed.
Treatment schema of fludarabine in combination with rituximab.
Patients received 4 cycles of fludarabine and 4 rituximab infusions, starting with 2 cycles of fludarabine 25 mg/m2/d, days 1 to 5 every 28 days. The first rituximab infusion was administered on days 1 to 3 as a stepped-up dosing.
Treatment schema of fludarabine in combination with rituximab.
Patients received 4 cycles of fludarabine and 4 rituximab infusions, starting with 2 cycles of fludarabine 25 mg/m2/d, days 1 to 5 every 28 days. The first rituximab infusion was administered on days 1 to 3 as a stepped-up dosing.
Patient monitoring
Baseline assessment included disease history, details of previous and concomitant medication, current stage of disease, and B symptoms, as well as measurement of lesions by physical examination and radiography (chest x-ray, computed tomography, ultrasound). The histologic examination of representative material (bone marrow biopsy or aspiration, immunophenotyping of peripheral blood, lymph nodes) had to be performed in the year prior to enrollment and had to include CD20-antigen detection. Laboratory testing involved complete and differential blood counts, flow cytometric phenotyping of mononuclear cells of the peripheral blood, and clinical chemistry, including immunoglobulins, β2-microglobulin, serum thymidinkinase, urine analysis, and Coombs test. Results of physical examination, laboratory tests, and adverse events were recorded every 2 weeks throughout the treatment period.
Definition of end points
Patients were assessed for response 3 to 5 weeks after the last rituximab infusion. This investigation included physical examination with measurement of lymph node, liver, and spleen, as well as differential blood counts. Two months later, an additional detailed clinical examination was performed. Response criteria were those previously defined by the (NCI) Working Group.26 Response criteria in all patients as well as the time point of subsequent progression were evaluated by an expert panel in an extramural review session.
Complete remission.
CR criteria included no evidence of disease; absence of lymphadenopathy, hepatomegaly, splenomegaly or constitutional symptoms; normal blood count (neutrophils > 1.5 × 109/L, platelets > 100 × 109/L, hemoglobin > 11 g/dL, lymphocytes < 4.0 × 109/L); bone marrow biopsy with normal cellularity; and lymphocytes less than 30%. A CR not confirmed by bone marrow biopsy was designated as CRu. CR had to be maintained for at least 8 weeks.
Partial remission.
A PR was defined as change from stage C to stage A or B, change from stage B to A (Binet), or at least 50% reduction in blood lymphocytes and 50% reduction in lymphadenopathy and/or 50% reduction in splenomegaly and/or hepatomegaly, plus at least one of the following features: neutrophils more than 1.5 × 109/L or 50% improvement over baseline, platelets more than 100 × 109/L or 50% improvement over baseline, or hemoglobin more than 11.0 g/dL (not supported by transfusion) or 50% improvement over baseline. A PR had to be maintained for at least 8 weeks.
Stable disease.
Stable disease (SD) was defined as no change in stage (Binet) and no CR, PR, or progression.
Progressive disease.
Progressive disease (PD) was defined as change from stage A disease to stage B or C, or from stage B to C. At least one of the following also needed to be present: more than 50% increase in the size of at least 2 lymph nodes or new palpable lymph nodes; more than 50% increase of splenomegaly or hepatomegaly, transformation to a more aggressive histology, Richter syndrome, or prolymphocytic leukemia; and at least more than 50% increase in the absolute number of circulating lymphocytes.
Event-related criteria.
Progression-free survival was defined as the time from initiation of treatment until last follow-up, the time at which progression occurred according to the modified NCI criteria26 or further therapy for symptomatic CLL was required or death occurring from any cause. Duration of response is defined as progression-free survival in the subgroup of patients who achieved a complete or partial remission.
Sample size determination
A single-stage design according to the method of Fleming27 was selected, based on the following assumptions: (1) The experimental treatment under study in this trial would be considered as not sufficiently effective, if the true response rate (CR + PR) was lower than 60%; (2) the combination treatment with fludarabine/rituximab would be regarded as very promising for further investigation (eg, in a phase 3 comparative trial), if the true response rate exceeded 90%; (3) the probability of erroneously declaring the drug as sufficiently active for further investigation despite a true response rate of less than 60% (type I error) is set at 5%; (4) the probability of erroneously rejecting the therapy as not sufficiently active (< 60%) in case of a true promising response rate (> 90%) is 20% (type II error; power = 80%).
Concerning feasibility and tolerability, a failure rate of up to 10% was considered to be acceptable. On the basis of 30 patients, an intolerability rate of more than 10% is ruled out with a probability of 80% (one-sided 80% confidence limit) if the combination treatment is found to be not feasible in all patients or all but one patient.
Statistical analysis
For the efficacy analysis, patients had to have received at least one cycle of therapy. All patients having received at least one application of study therapy were also evaluable for toxicity. Rate comparisons between patient subgroups were performed by using the Fisher exact test. Event-related data (survival and progression-free survival, response duration) were estimated by the product limit method.28 Prognostic subgroups were compared by using the log-rank test.29
Results
A total of 34 patients were enrolled. One patient was found to have no B-CLL and was therefore withdrawn by the participating center before having received any trial medication. Two patients had been pretreated with fludarabine or other purine analogues and were thus excluded from the study. The remaining 31 patients were evaluable for toxicity and response. One patient developed a prolonged thrombocytopenia after the second cycle of fludarabine and was refractory to platelet transfusion. This patient died as a result of cerebral bleeding on day 69, without receiving further study medication.
Demographic characteristics
Table 1 shows the demographic characteristics of the 31 evaluable patients with B-CLL. Patients had a median age of 59 years (range, 30-70 years). More than half of the patients (51%) had a reduced performance status (ECOG Index > 0). The time from first diagnosis ranged from 1 to 10 years. At the time of study entry 21 patients had Binet stage B and 10 had Binet stage C. Fifty-two percent of the patients exhibited B symptoms. The median number of prior treatments in 11 pretreated patients was 1 (range, 0-2 treatments), consisting mainly of chlorambucil alone or in combination with prednisone. Cytologic and/or histologic examination of bone marrow samples was provided in the majority (87%) of cases. The infiltration pattern was predominantly diffuse (85%).
Adverse events
Adverse events were graded according to the NCI Common Toxicity Criteria Grading System (version 2.0, April 1999). Table2 shows the hematologic toxicity during treatment. Severe anemia (grade 3 and/or 4) was observed in 10% of patients, severe leukopenia in 25%, neutropenia in 42%, and thrombopenia in 9%, respectively. The overall figures for leucocytes, lymphocytes, and platelets during treatment are shown in Figure2. The most frequent nonhematologic side effects were infections (52%) and infusion-related symptoms such as chills (39%), fever (32%), and erythema (26%) (Table3). Importantly, only one grade 3 fever was encountered. A total of 32 infections were observed in 16 patients (Table 4). Most of the infections were respiratory (10 of 32) and herpes virus infections (7 of 32). However, only 4 infections were fever of unknown origin (grade 3 and/or 4). More than 60% of the 167 adverse events reported were judged as nontreatment-related by the treating physician (data not shown).
Blood counts during treatment.
Median lymphocyte, leukocyte, and platelet counts before, during, and after the end of treatment (week 25).
Blood counts during treatment.
Median lymphocyte, leukocyte, and platelet counts before, during, and after the end of treatment (week 25).
Response
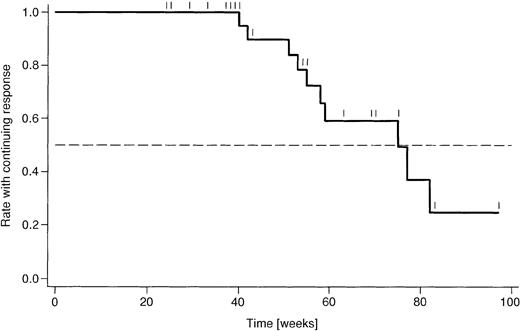
Responses are listed in Table 5. The overall response rate was 87%, based on 31 evaluable patients. The combined CR/CRu rate was 33%. In addition, 55% of patients achieved a PR. One patient had SD, and 2 (7%) had refractory disease. There were no statistically significant differences in the subgroup analysis between untreated and pretreated patients. In addition, there were no substantial differences between Binet stage B and C patients, respectively, although there were fewer CRs in Binet C patients (11%) compared with Binet B patients (43%). The analysis of duration of response (Figure 3) is rather preliminary, due to a median follow-up duration of only 54 weeks. Currently, the median duration of response amounts to 75 weeks.
Kaplan-Meier analysis of duration of response.
Shown are the proportions of 27 patients who had a response to treatment and remained in complete or partial remission. Censoring points are indicated like Tic marks. Median duration of response amounts to 75 weeks.
Kaplan-Meier analysis of duration of response.
Shown are the proportions of 27 patients who had a response to treatment and remained in complete or partial remission. Censoring points are indicated like Tic marks. Median duration of response amounts to 75 weeks.
Discussion
The following findings emerge from the present study:
(1) The combined chemoimmunotherapy consisting of 4 cycles of fludarabine and 4 cycles of rituximab is very effective in patients with B-CLL. The overall response rate in the present phase 2 study involving 31 patients was 87% with a CR rate of 33% (CR and CRu) and a PR rate of 55%. The overall response rate was similar in previously treated (90%) and untreated patients (85%). There was no substantial difference in the overall response between patients in stage Binet B or C, although there were more CRs in Binet B patients (43%) compared with Binet C patients (11%).
(2) This new treatment regimen is feasible and can be administered safely on an outpatient basis. Toxicity was moderate with no increased risk of severe infection. There were only 4 patients (13%) with grade 3 or 4 (World Health Organization [WHO]) infections, none of which was fatal. Grade 3 and/or 4 hematologic toxicity was observed for neutropenia (42% of patients), anemia (10%), and thrombocytopenia (9%), respectively. Other side effects included chills (39%), fever (32%), and erythema (26%).
The overall response rate of 87% in our study compares favorably to those reported from the most effective chemotherapy combinations in patients with CLL.11-13 However, the response rates observed in the present trial were achieved with 4 courses of fludarabine, whereas 6 cycles were generally used in single-agent fludarabine or fludarabine/cyclophosphamide trials. Thus, the total fludarabine dose is lower in the present trial compared with most trials in which fludarabine is involved. This low dose might, at least in part, explain the mild side effects observed in the present trial. In addition, only very moderate dose reduction or deviation from the planned time schedule was necessary (data not shown). There were only minor acute side effects and also no event of hemolytic anemia in the present trial. The incidence of autoimmune hemolytic anemia in patients with CLL treated with fludarabine alone ranges between 11% and 21%.30-32 The temporary B-cell depletion caused by rituximab might prevent the development of fludarabine-associated autoimmune hemolytic anemia. This hypothesis is supported by the findings of others who observed effective treatment of acute immune hemolytic anemia with rituximab.33 34 Thus, the combined chemoimmunotherapy might actually reduce treatment-related side effects because of the nonoverlapping toxicity profile.
Promising results of combination regimens with rituximab and cytotoxic drugs have been reported earlier in patients with advanced-stage low-grade B-cell NHL. In a nonrandomized study, the response rate was 95% with CR in 55%.35 In addition, 7 of 7 patients with follicular histology and complete remission were rendered bcl-2 negative. A more recent prospective phase 3 study randomly assigned 399 elderly patients with diffuse large B-cell lymphoma.36Patients received either 8 cycles of CHOP or 8 cycles of CHOP plus rituximab (R-CHOP). The CR rate was significantly higher in the R-CHOP group (76% versus 63%, P = .005). With a median follow-up of 2 years, event-free and overall survival times were also substantially higher in the R-CHOP group: at 2 years, 70% of patients treated with R-CHOP were alive as compared with 57% of those treated with CHOP alone. Disease progression during treatment was observed in 22% of patients in the CHOP group and 9% of patients in the R-CHOP group.
Recent trials with rituximab in patients with CLL have suggested a dose-response relationship whereby most cases responded at higher doses.37 These trials used up to 4 times the conventional dose of rituximab. However, the costs associated with rituximab at very high doses are substantial. In addition, patients with CLL with high peripheral tumor cell load are at risk of developing severe infusion-related side effects and thrombocytopenia when treated with rituximab alone.15,19 20
The promising results reported from this phase 2 trial are in line with those of Byrd et al,38 who conducted a randomized phase 2 trial of sequential versus concurrent rituximab in patients receiving fludarabine.38 Patients received either 6 cycles of fludarabine (25 mg/m2 days 1-5) followed 2 months later by rituximab (375 mg/m2 × 4) or concurrent rituximab at standard dose (375 mg/m2 at days 1 and 4 of fludarabine cycle 1 and on day 1 of fludarabine cycles 2-6). CR rate and overall response of 104 patients randomized were superior in the concurrent application arm. Investigators at the M.D. Anderson Center performed a retrospective analysis in patients with CLL that compared fludarabine (82 patients); fludarabine plus cyclophosphamide (53 patients); or fludarabine, cyclophosphamide, and rituximab (135 patients).39 Response rates were 85%, 91%, and 95%, respectively, with more CRs in the FCR combination (35%, 43%, and 63%). They also observed complete molecular remission as measured by PCR for immunoglobulin heavy-chain rearrangement in 56% of those patients who achieved CR. By using a more sensitive newly developed real-time ASO PCR technique with individual primers, we quantitatively analyzed 7 patients. Interestingly, one pretreated patient achieved complete molecular remission after rituximab and fludarabine.40
In summary, the results of a combined immunochemotherapy of rituximab and fludarabine demonstrate low toxicity and good efficacy in both pretreated and untreated patients with CLL. A randomized clinical trial in which this combination is being compared with fludarabine alone is warranted for patients with previously untreated CLL.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-03-0972.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Holger Schulz, Department I of Internal Medicine, University of Cologne, Cologne, 50924, Germany; e-mail:a.engert@uni-koeln.de.