Abstract
Postnatal CD34+ cells expressing vascular endothelial growth factor receptor 2 (KDR) generate hematopoietic or endothelial progeny in different in vitro and in vivo assays. Hypothetically, CD34+KDR+ cells may comprise hemangioblasts bipotent for both lineages. This hypothesis is consistent with 2 series of experiments. In the first series, in clonogenic culture permissive for hematopoietic and endothelial cell growth, CD34+KDR+ cells generate large hemato-endothelial (Hem-End) colonies (5% of seeded cells), whereas CD34+KDR− cells do not. Limiting-dilution analysis indicates that Hem-End colonies are clonally generated by single hemangioblasts. Sibling cells generated by a hemangioblast, replated in unicellular culture, produce either hematopoietic or Hem-End colonies, depending on the specific culture conditions. Identification of endothelial cells was based on the expression of VE-cadherin and endothelial markers and with lack of CD45 and hematopoietic molecules, as evaluated by immunofluorescence, immunocytochemistry, and reverse transcription–polymerase chain reaction. Furthermore, endothelial cells were functionally identified using low-density lipoprotein (LDL) uptake and tube-formation assays. In the second series, to evaluate the self-renewal capacity of hemangioblasts, single CD34+KDR+ cells were grown in 3-month extended long-term culture (ELTC) through 3 serial culture rounds—that is, blast cells generated in unicellular ELTC were reseeded for a subsequent round of unicellular ELTC. After 9 months, 10% blasts from tertiary ELTC functioned as hemangioblasts and generated macroscopic Hem-End colonies in clonogenic culture. These studies identified postnatal hemangioblasts in a CD34+KDR+ cell subset, endowed with long-term proliferative potential and bilineage differentiation capacity. Although exceedingly rare, hemangioblasts may represent the lifetime source/reservoir for primitive hematopoietic and endothelial progenitors.
Introduction
The ontogenic development of hematopoietic and endothelial lineages is linked to vascular endothelial growth factor receptor 2 (KDR)/Flk1. Knockout of theFlk1 gene causes a combined defect of hematopoiesis and endothelial cell (EC) growth,1,2 possibly mediated by a defect of the embryonic hemangioblast—an Flk1+ precursor of both lineages. Consistent with this hypothesis, Flk1+cells isolated from differentiating embryonic stem cells3and the aorta-gonad-mesonephros (AGM) region4 generate in unicellular culture mixed endothelial–hematopoietic colonies.
In human postnatal life, CD34+ cells comprise primitive hematopoietic5 and endothelial6,7 precursors. Specifically, the minuscule KDR+ subset of CD34+ cells is enriched for hematopoietic stem cells (HSCs)8 and endothelial progenitors9; conversely, the CD34+KDR− fraction comprises hematopoietic progenitor cells (HPCs).8 Similarly, murine HSC-enriched cell fractions generate hematopoietic and endothelial cells.10 11
We observed that in liquid culture, CD34+KDR+cells generate hematopoietic and endothelial cells and some cells expressing both types of markers, suggesting their bilineage differentiation potential. On this basis, we hypothesized that postnatal CD34+KDR+ cells comprise hemangioblasts, which feed into hematopoietic and endothelial precursors.
Materials and methods
Cell purification
Bone marrow (BM) cells were obtained from consenting healthy donors. Cord blood (CB) was obtained from healthy, full-term placentas according to institutional guidelines. Low-density mononuclear cells (MNCs) (less than 1.077 g/mL) were isolated by Ficoll-Hypaque density-gradient centrifugation, and CD34+ cells were purified by MACS column (Miltenyi, Bergisch Gladbach, Germany). Purified CD34+ cells were incubated with anti-CD34 monoclonal antibody (mAb) fluorescein isothiocyanate (FITC; clone HPCA-2; Becton Dickinson, San Jose, CA) and biotinylated anti-KDR mAb (KDR1 or KDR2 mAb [Sigma, St Louis, MO], biotinylated at high level [Hölzel Diagnostika, Cologne, Germany]) and then labeled with streptavidin–phycoerythrin (PE) (Becton Dickinson). CD34+KDR+ and CD34+KDR− subpopulations were sorted on FACSVantage or FACSVantage-SE (Becton Dickinson).8 KDR antibody specificity was indicated by the presence of KDR mRNA in the KDR+ but not in the KDR− cell subset8 (and results not presented) and by the interaction of the mAb with exogenous KDR transduced into leukemic cell lines (eg, MV4-11, TF1, HL60, U937) (not shown).
Cell culture
Liquid culture.
CD34+KDR+ and CD34+KDR− cells were seeded in U-shaped wells containing serum-free medium supplemented with vascular endothelial growth factor (VEGF) at saturating levels.8
Clonogenic culture.
Three different culture conditions were applied: condition a, HPCs, including primitive ones (high proliferative potential colony-forming cell [HPP-CFC]); condition b, EC precursors; condition c, progenitors of hematopoietic cells and ECs. In condition a, hematopoietic (Hem) culture involved serum-free methylcellulose medium supplemented with a multilineage hematopoietic growth factor (HGF) cocktail8—that is, methylcellulose in Iscove modified Dulbecco medium (IMDM) containing bovine serum albumin (BSA), saturated human transferrin (Tf), and human LDL, supplemented with HGF (early-acting GFs [kit ligand, flt3 ligand], multilineage GFs [interleukin 3 (IL-3), granulocyte macrophage–colony-stimulating factor (GM-CSF)] and unilineage GFs [G-CSF, M-CSF; erythropoietin (EPO) and thrombopoietin (TPO) were not added, unless indicated]), VEGF, and basic fibroblast bFGF at saturating dosages. In condition b, endothelial (end) culture conditions described in culture a were modified as follows: (1) HGFs were excluded, (2) wells were coated with collagen (Vitrogen-100; Cohesion, Palo Alto, CA) and fibronectin (Sigma),13 and (3) medium was supplemented with fetal calf serum/horse serum (FCS/HS, 12.5% each, which replaced BSA, Tf, and LDL) and, in some experiments, heparin (10 U/mL, Sigma), insulinlike growth factor (IGF1) (2 ng), and EC growth supplement (ECGS) (100 μg; Collaborative Biomedical, Bedford, MA). In condition 3, Hem-Ed culture, we combined the end culture conditions (detailed in culture b) with the addition of HGFs as in Hem culture (detailed in culture a); EPO and TPO were never added. The colonies were scored at day 30 to 40. Identification of Hem-End mixed colonies was based on the colony pattern described below and were further validated by morphological, immunofluorescence (IF), immunocytochemistry (ICC), and reverse transcription–polymerase chain reaction (RT-PCR) analysis.
Secondary culture of split subcolonies.
Colonies were picked up and usually divided in 3 aliquots. The first one was used for RT-PCR analysis; the second one for selective hematopoietic cell growth in clonogenic Hem culture in IMDM and FCS/HS, 12.5% each, supplemented with HGFs as in condition a, including EPO/TPO at saturating concentrations8; the third one for selective EC growth in medium composed of M199 (Gibco, Gaithersburg, MD), 100 μg/mL ECGS, 10 U heparin, 5 ng VEGF and bFGF, 18% FCS, and 2% human serum9 in fibronectin–collagen-coated wells.13 Hematopoietic cells and ECs were collected after 4 weeks of subcolony culture.
Sibling cells.
Single CB CD34+KDR+ cells were seeded in flat wells containing liquid Hem medium (see above) to generate 4-cell clusters. The 4 siblings were seeded in unicellular semisolid Hem, End, or Hem-End culture.
Stroma-based 3-month ELTC: assay of ELTC initiating cells and hemangioblasts.
Primary ELTCs: The ELTC wells were seeded at limiting dilution (1-50 cells/well). After 3 months they were individually harvested, and adherent cells were depleted and seeded in clonogenic culture to evaluate the ELTC-initiating cell (ELTC-IC) frequency.8,14Secondary and tertiary ELTCs: Within the primary ELTCs, a subset of unicellular wells was scored for cobblestone-area–forming cells (CAFCs).15 Wells positive for hematopoietic colony formation (cobblestone area) were harvested, and adherent cells were depleted by overnight incubation in IMDM containing FCS and HS, 12.5% each; small aliquots were then diluted in 0.4% trypan blue to differentially count viable small blastlike cells. Finally, the blasts were reseeded in secondary limiting-dilution ELTC. Tertiary ELTC was performed according to the same procedure. At the end of secondary and tertiary ELTC, we assayed the frequency of ELTC-ICs.8Blasts from a separate subset of unicellular ELTC wells were seeded in Hem-End clonogenic culture to evaluate the generation of hemangioblast colonies.
Cell analysis
IF analysis.
Double and triple labeling immunofluorescence was performed to detect CD45, von Willebrand factor (VWF), and VE-cadherin/CD144. Briefly, cells were fixed and permeabilized with Cytofix/Cytoperm solution (Becton Dickinson/PharMingen, San Diego, CA), then rinsed with phosphate-buffered saline (PBS) spotted on a positively charged glass slide (SuperFrost Plus; Menzel-Glaser, Braunschuring, Germany) and air dried. Cells were incubated for 20 minutes at room temperature in PBS containing 10% normal goat serum and then overnight at 4°C with the appropriate primary antibody or antisera. After washing in 0.2% BSA in PBS, cells were incubated for 1 hour at room temperature with the appropriate secondary antibodies and washed. For triple-labeling immunofluorescence, cells were incubated with anti-VWF and anti-VE–cadherin antibody. Cells were then incubated with antimouse FITC-conjugated and antirabbit tetramethyl rhodamine isothiocyanate (TRITC)–conjugated secondary antibodies. After washing, cells were incubated with anti-CD45 allophycocyanin-conjugated antibodies (Becton Dickinson/PharMingen). After washing, slides were mounted on a glass coverslip with Slow fade mounting medium (Molecular Probes) and analyzed using a Leica TCS 4D confocal microscope. No labeling was detected in the absence of primary antibodies and antisera; cross-reactivity was not observed on control cell lines (HUVEC, U937). In each sample, the number of immunoreactive cells was counted in 8 or more nonoverlapping fields, for a total of more than 300 cells. The total number of cells in each field was determined by transmitted light analysis. Primary antibodies and antisera: 3 μg/mL rabbit immunoglobulin G (IgG) antihuman VWF (DAKO, Glostrup, Denmark); 5 μg mouse antihuman CD45 (DAKO); 5 μg mouse IgG antihuman VE-cadherin (Immunotech, Marseilles, France). Secondary antibodies: 4 μg FITC-conjugated goat antimouse IgG (Caltag Laboratories, Burlingame, CA) and 5 μg TRITC-conjugated swine antirabbit IgG (DAKO).
LDL uptake assay.
Cells from Hem-End colonies were resuspended in fresh medium and seeded onto collagen-fibronectin–coated chamber slides.6 After 3 days of culture, cells were incubated for 4 hours with 2.5 μg Dil Ac-LDL (CellSystems Biotechnologie; Vertrieb GmbH, Katharinen, Germany). Noninternalized Dil Ac-LDL was washed by PBS. Cells were then counterstained to detect CD45 or VE-cadherin expression. Samples were finally fixed with 4% PFA and washed in PBS. Slides were mounted and analyzed as indicated above. Tube formation assay was performed as previously described.17
Immunocytochemistry analysis.
Cells were cytospun and fixed with cold 80% ethanol for 10 minutes. After pretreatment with endogenous peroxidase blocking reagent (Laboratory Vision, Fremont, CA) and protein blocking solution (DAKO) for 10 minutes and 30 minutes, respectively, cells were simultaneously incubated with rabbit antihuman VWF polyclonal antibody (DAKO) and mouse antihuman CD45 mAb (DAKO) for 60 minutes at room temperature, followed by incubation with peroxidase-conjugated goat antirabbit (DAKO Envision System) and alkaline-phosphatase–conjugated goat antimouse (DAKO) for 30 minutes at room temperature. The reaction was first developed with fast red (DAKO) and then with 3,3-diamino-benzidine (DAB) chromogen substrate (Vector Laboratories). Cells were counterstained with Mayer hematoxylin, and slides were mounted with a coverslip by aqueous mounting medium (DAKO).
Semiquantitative RT-PCR analysis.
RNA was isolated using the Dynabeads mRNA direct kit (Dynal, Oslo, Norway) and reverse transcribed by Moloney murine leukemia virus reverse transcriptase (Gibco BRL) with oligo(dT) as primer. RT-PCR was normalized for β2 microglobulin. Oligonucleotide primer sequences were: β2-microglobulin, 5′-GGGTTT CATCCATCCGACAT-3′ and 5′-GATGCTGCTTACATGTCTCGA-3′; CD45, 5′-TTCAACTTATACCC TTCGTGTC-3′ and 5′-CCTGCTTTACTTTGTCCACTTC-3′ or 5′-CAGGCATGGTTTCCACATTC-3′ and 5′-CTACAAATATTGGTTCGCTGC-3′; CD14, 5′-ATGCATGTGGTCCAGCGCCC-3′ and 5′-CCA CCGACAGGGTCGAACG-3′; CD41, 5′-GACTGTGAATGGTCTTCACCTC-3′ and 5′-ACACGTTGAA CCATGCGTGCGA-3; CD1a, 5′-GGTGACAGGAGGCTGTGAGCTGCAC-3′ and 5′-TGTGCTTCA CCCGACAGGACAGGTC-3′; VE-cadherin/CD144, 5′-ACATCCACATAACCCTGTCACC-3′ and 5′-AGAGAATCCTGCCCCACAAGTC-3′; ECSM2, 5′-TGGGAGAAGCAGGCAGTATT-3′ and 5′-CAG CTGCCCTGTGACTACAA-3′; VCAM1/CD106, 5′-GAAGAAAAAAGCGGAGACAGGAG-3′ and 5′-GGAGGATGCAAAATAGAGCACGAG-3′; VWF, 5′-TCACATCTTCACATTCACTCCAC-3′ and 5′-ATCACCTCACACACTTGCTCC-3′ or 5′-ACATCACTGCCAGGCTGCAGTA-3′ and 5′-CACAAGA GCAGAACATGCAGAG-3′; Tie2, 5′-CACCATCCAAACATCATCAATCTC-3′ and 5′-CAATCTCCC ATAGTAACACACC-3′; CD31, 5′-CAAATGACCTCAAATGCCACC-3′ and 5′-TGTCTCTGTGTCC TTCTTTCC-3′; CD34, 5′-ATGGCTTCCTCCTCCCTCCT-3′ and 5′-ATCCCTGCTCAACCCCTCTG-3′. Furthermore, most samples were electrophoresed in 1.5% agarose gel, transferred to a nitrocellulose filter, and hybridized with an internal oligomer probe.
Results
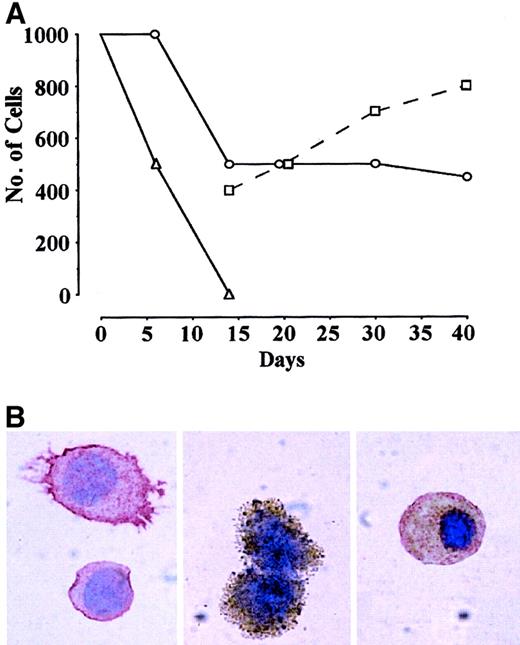
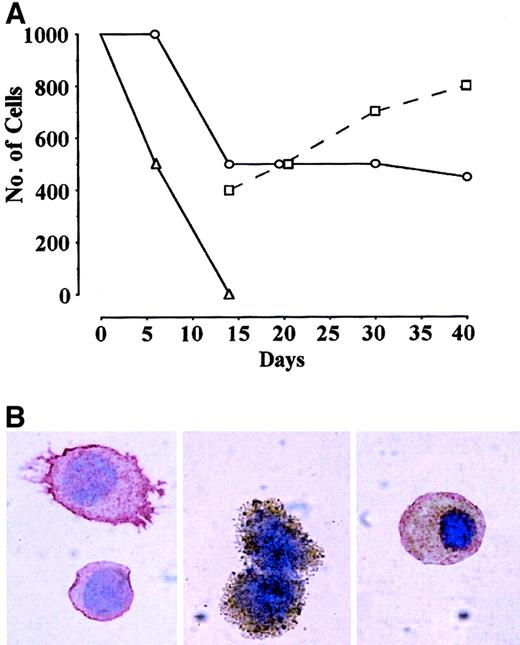
In preliminary studies, we confirmed the capacity of CD34+KDR+ to generate hematopoietic8 and endothelial9 progeny. Thus, CB CD34+ cells labeled by anti-KDR mAb (KDR1 or KDR2) were separated into KDR+ (approximately 1%) and KDR− (approximately 99%) fractions by FACS. KDR+ vs KDR− cells were assayed in sublethally irradiated NOD-SCID mice.8 Several thousand KDR+ cells showed hematopoietic engrafting capacity, whereas their KDR− counterparts did not (results not shown). In serum-free liquid culture supplemented with VEGF, CD34+KDR− cells die within 1 to 2 weeks (Figure 1A), whereas CD34+KDR+ blasts in part survive and generate hematopoietic and endothelial progeny (Figure 1B, left and middle panels). Interestingly, some cells express endothelial and hematopoietic markers, suggesting that they are endowed with bilineage hemangioblast potential (Figure 1B, right). On this basis, we hypothesized that postnatal CD34+KDR+ cells comprise hemangioblasts, which may feed into primitive hematopoietic and endothelial precursors.
Liquid suspension culture of CD34+KDR+ cells generating hematopoietic and endothelial progeny.
(A) Number of cells in FCS− liquid suspension culture of CB CD34+KDR+ (○,■) vs CD34+KDR− (▵) cells supplemented with VEGF. Starting from day 14, the number of small blastlike (○) and larger (■) cells in KDR+ culture is indicated. A representative experiment is shown. (B) Immunocytochemistry analysis of cells in CD34+KDR+ culture at day 14 by combined staining with CD45 (red) and VWF (brown). Right panel shows a double-stained cell (original magnification, × 1000).
Liquid suspension culture of CD34+KDR+ cells generating hematopoietic and endothelial progeny.
(A) Number of cells in FCS− liquid suspension culture of CB CD34+KDR+ (○,■) vs CD34+KDR− (▵) cells supplemented with VEGF. Starting from day 14, the number of small blastlike (○) and larger (■) cells in KDR+ culture is indicated. A representative experiment is shown. (B) Immunocytochemistry analysis of cells in CD34+KDR+ culture at day 14 by combined staining with CD45 (red) and VWF (brown). Right panel shows a double-stained cell (original magnification, × 1000).
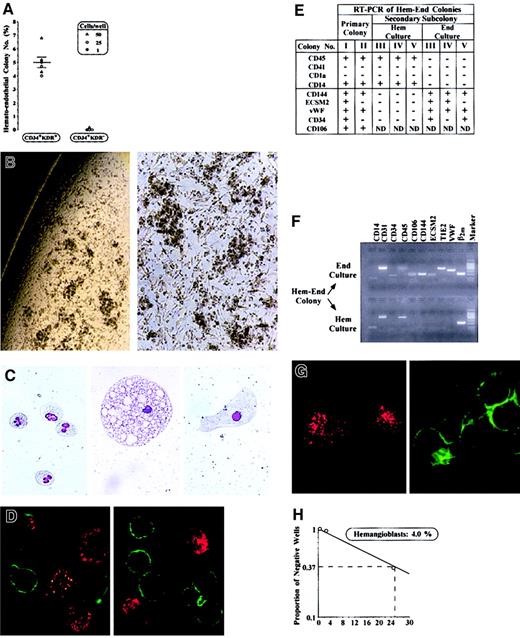
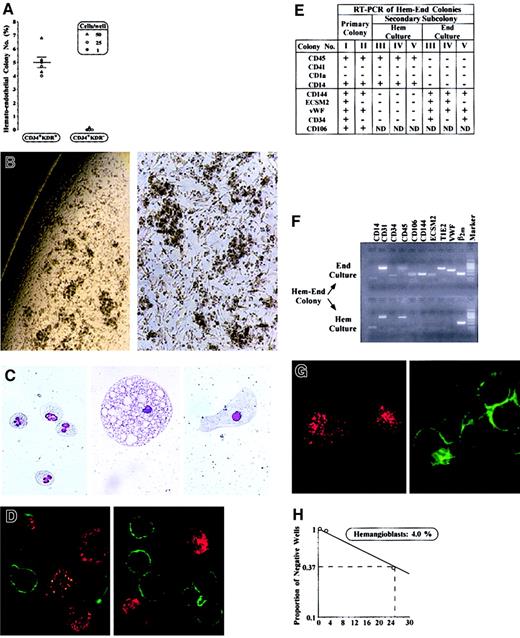
To test this hypothesis, CD34+KDR+ cells from CB and BM were cloned in semisolid medium permissive for growth of hematopoietic (Hem), endothelial (End), or mixed hemato-endothelial (Hem-End) progeny. CB and BM cells, seeded immediately after sorting or after 3 to 14 days of serum-free liquid culture (Figure 1), yielded similar results. In Hem and Hem-End cultures, results were independent of seeding cell level (1-50 cells/well). In End culture, however, colony formation was absent when seeding fewer than 250 cells/well, indicating the requirement of cell–cell interactions in these specific conditions, as reported.18 We specifically observed that in Hem culture CD34+KDR+ cells generated only GM colonies (11.1% ± 1.2%), as reported.8 We also observed that End culture, seeded with 250 cells/well, generated less than 10% colonies, composed of either ECs (3.9% ± 0.3%) or myeloid (4.7% ± 0.6%) progeny. In mixed Hem-End culture, we scored not only GM clones (12.9% ± 2.5%) but also Hem-End colonies (5.0% ± 0.4%; Figure 2A). These mixed colonies are large (5-10 × 103 cells/colony) and are characterized by clusters of round hematopoietic cells combined with spindlelike cells of apparent EC morphology, which are interspersed between and interconnected with the myeloid clusters (Figure 2B). Extensive morphology, immunocytochemistry, immunofluorescence, and RT-PCR analysis, as well as LDL uptake and tube-formation functional assays, indicated that the Hem-End colonies comprise myeloid cells and ECs (Figure 2C-G and results not shown). Myeloid cells, clearly recognized at the morphologic level (Figure 2C), expressed CD45 and other myeloid markers, but not the EC-specific and EC-associated markers detailed below (Figure 2D-F). The ECs, often large (Figure 2C), were identified on the basis of their immunophenotype, RT-PCR profile, and functional tests (Figure 2D-G). Specifically, ECs were negative for CD45 and other hematopoietic markers (CD14, CD1a, and CD41, thus excluding their monocytic, dendritic, and megakaryocytic nature) while positive for endothelial markers. These included EC-specific proteins (VE-cadherin/CD144, ECSM2)9,19 and EC-associated markers shared by a subset of hematopoietic cells (ie, VCAM1/CD106 and VWF, also expressed on dendritic and megakaryocytic cells, respectively; TIE2, CD31, and CD105, also expressed on early hematopoietic precursors).9Furthermore, to confirm the presence of hematopoietic and endothelial progeny, Hem-End colonies were divided in 2 portions, which were replated in 2 separate cultures selective for either endothelial or hematopoietic growth. As expected, the cells generated in secondary culture were essentially either endothelial or hematopoietic (Figure 2E-F). Finally, functional studies revealed that the ECs actively incorporate LDL (Figure 2G) and undergo tube formation on Matrigel (not shown).
Characterization of mixed Hem-End colonies.
(A) Number of Hem-End colonies (% of plated cells) generated by CD34+KDR+ versus CD34+KDR− cells from CB (▵,○) or BM (●) in 6 independent experiments (mean ± SEM values; cell number/well is indicated). See “Results.” (B) Microscopy analysis of a representative Hem-End colony (original magnifications, left × 50; right × 100). (C) Morphology of granulocytes, a large macrophage, and a putative endothelial cell in Hem-End colony (original magnification, × 400). (D) Immunofluorescence analysis of cells in Hem-End colony by double labeling with anti-CD45 (green) and anti-VWF (red) mAb (original magnification, × 600). (E-H) Characterization of mixed Hem-End colonies (representative results). (E,F) RT-PCR analysis of Hem-End colonies from primary Hem-End culture or secondary hematopoietic (Hem) and endothelial (End) culture—that is, a single Hem-End colony was divided in 2, and each half was grown in secondary Hem or End specific medium. (G) Hem-End colonies at day 40 were split into adherent (left) and suspension (right) fractions and analyzed for LDL uptake (red) and CD45 expression (green; original magnification, × 600). (H) Limiting-dilution analysis of Hem-End colony frequency by Poisson single-hit statistics.8
Characterization of mixed Hem-End colonies.
(A) Number of Hem-End colonies (% of plated cells) generated by CD34+KDR+ versus CD34+KDR− cells from CB (▵,○) or BM (●) in 6 independent experiments (mean ± SEM values; cell number/well is indicated). See “Results.” (B) Microscopy analysis of a representative Hem-End colony (original magnifications, left × 50; right × 100). (C) Morphology of granulocytes, a large macrophage, and a putative endothelial cell in Hem-End colony (original magnification, × 400). (D) Immunofluorescence analysis of cells in Hem-End colony by double labeling with anti-CD45 (green) and anti-VWF (red) mAb (original magnification, × 600). (E-H) Characterization of mixed Hem-End colonies (representative results). (E,F) RT-PCR analysis of Hem-End colonies from primary Hem-End culture or secondary hematopoietic (Hem) and endothelial (End) culture—that is, a single Hem-End colony was divided in 2, and each half was grown in secondary Hem or End specific medium. (G) Hem-End colonies at day 40 were split into adherent (left) and suspension (right) fractions and analyzed for LDL uptake (red) and CD45 expression (green; original magnification, × 600). (H) Limiting-dilution analysis of Hem-End colony frequency by Poisson single-hit statistics.8
Negative controls were represented by CD34+KDR− cells grown in the Hem, End, Hem-End culture conditions described above. As expected,8 these cells consistently generated an elevated number of myeloid colonies (eg, 67.3% ± 10.4% in Hem-End culture), but essentially no mixed or endothelial colonies in Hem-End or End cultures, respectively (Figure 2A and data not shown). Together these studies strongly suggested that CD34+KDR+ cells from CB and BM comprise approximately 5% progenitors endowed with hemangioblast capacity to generate endothelial and hematopoietic progeny.
To assess the clonality of mixed colonies, we performed a second series of experiments at limiting dilution (25, 2.5, and 0.25 CB or BM cells/well). Here again, CD34+KDR+ cells seeded in Hem-End culture generate not only 10% to 15% or less myeloid colonies but also 4% mixed colonies, as evaluated by limiting-dilution Poisson statistics (representative results in Figure 2H). Mixed colonies contained hematopoietic and endothelial cells, based on morphology immunofluorescence, immunocytochemistry, and RT-PCR analysis (not shown). These experiments formally demonstrated the clonality of mixed colonies.
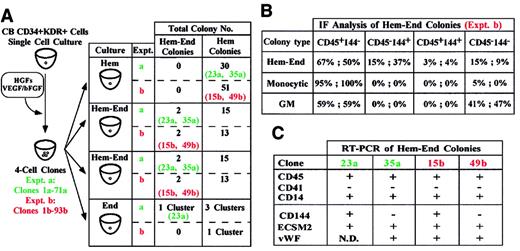
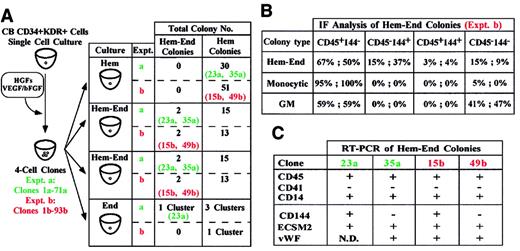
A third series of experiments was performed on sibling cells. Single CD34+KDR+ cells were grown in liquid Hem medium to generate 4 daughter cells; each sibling was then seeded in a secondary well containing either Hem-End, Hem, or End semisolid medium (Figure 3A, left). In 2 representative experiments, 2% and 3% of the 4-cell clones were composed of hemangioblast siblings. Specifically, these siblings consistently gave rise to large hemangioblast colonies in Hem-End medium, whereas they generated small hematopoietic colonies if seeded in Hem culture (Figure3A, right). In End medium, one of the hemangioblast siblings generated a small Hem-End cell cluster (Figure 3A, right). Here again, colony identification was validated by immunofluorescence and RT-PCR analysis (Figure 3B-C and data not shown). These results indicate that the bilineage differentiation gene program of hemangioblasts is flexible and is modulated by specific culture conditions.
Growth of hemangioblast siblings in different culture conditions.
(A) Single CB CD34+KDR+ cells generated 4-cell clones. Sibling cells from each clone were seeded in Hem, Hem-End, or End medium (left). In 2 representative experiments (a, clones 1-71; b, clones 1-93) we show the total number of Hem and Hem-End colonies and specify the clones (23a, 35a; 15b, 49b) generating Hem-End or Hem colonies in Hem-End or Hem medium, respectively. One of these clones (23a) generated a small Hem-End cell cluster in End medium. (B,C) Immunofluorescence and RT-PCR analysis of Hem-End colonies generated by a and b clones.
Growth of hemangioblast siblings in different culture conditions.
(A) Single CB CD34+KDR+ cells generated 4-cell clones. Sibling cells from each clone were seeded in Hem, Hem-End, or End medium (left). In 2 representative experiments (a, clones 1-71; b, clones 1-93) we show the total number of Hem and Hem-End colonies and specify the clones (23a, 35a; 15b, 49b) generating Hem-End or Hem colonies in Hem-End or Hem medium, respectively. One of these clones (23a) generated a small Hem-End cell cluster in End medium. (B,C) Immunofluorescence and RT-PCR analysis of Hem-End colonies generated by a and b clones.
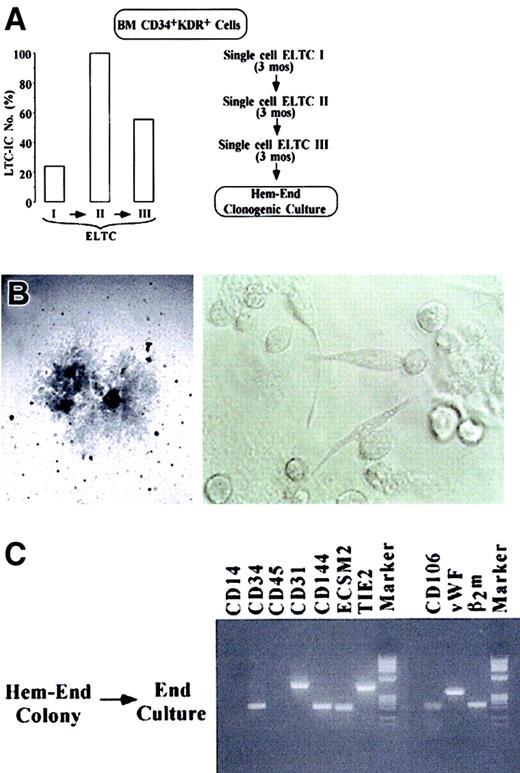
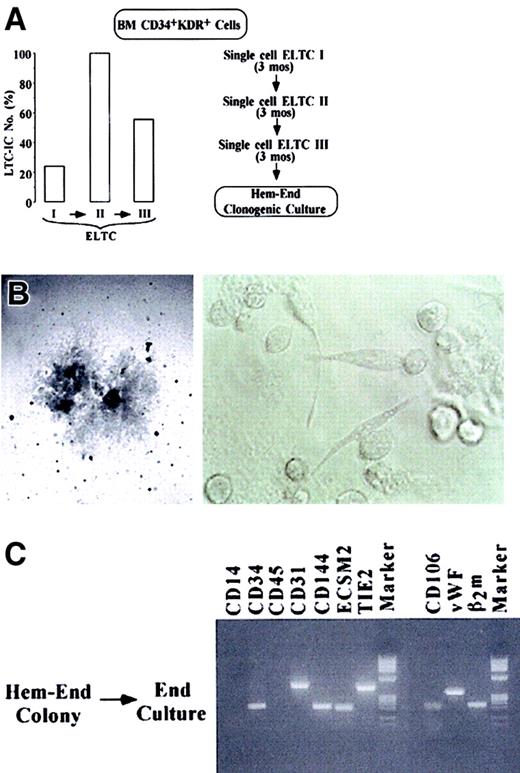
It is apparent that 5% to 6% or fewer CD34+KDR+ cells function as hemangioblast progenitors. However, the above results do not imply that the hemangioblasts have long-term proliferative and self-renewal capacity and, hence, stem cell activity. To address this issue, we used stroma-based ELTCs. This assay identifies primitive hematopoietic cells—ELTC-ICs giving rise to hematopoietic colonies in secondary culture14 and CAFCs generating hematopoietic colonies beneath the ELTC stroma.15 We further developed a culture system involving 2 to 3 rounds of ELTCs (Figure4A) to assay long-term, self-renewing ELTC-ICs (stroma-repopulating putative HSCs, as evaluated by serial-limiting dilutions) (left panel) and self-replicating hemangioblasts by serial unicellular ELTCs (right panel). Specifically, CD34+KDR+ cells were seeded in ELTCs at limiting dilution. After 3 months, the unicellular wells that scored positively for CAFC were harvested; the small blastlike cells were counted and reseeded for a second limiting-dilution ELTC. A third round of ELTC was similarly performed. As expected, serial ELTCs generated a progeny composed of CD45+ hematopoietic cells, as evaluated by FACS analysis (not shown). In a representative experiment, ELTC-IC frequency in primary, secondary, and tertiary ELTCs was 24%, 100%, and 56% (left panel). Because each unicellular ELTC well generated an average of 25 to 30 blasts, these results indicate extensive proliferation and self-renewal of HSCs through serial 9-month ELTC. At the end of the third ELTC, 50 unicellular wells were analyzed (right panel). Twenty-one wells positive for CAFC were harvested and reseeded in Hem-End clonogenic medium. Two of 21 (9.5%) wells contained macroscopic Hem-End colonies, showing at their border the typical mixed populations of round and spindlelike cells (Figure 4B). Here again, the colonies were split in 2 subcolonies, which were reseeded in hematopoietic or endothelial medium. As expected, the final progeny was hematopoietic or endothelial, respectively, based on morphology and RT-PCR analysis (Figure 4C and results not shown). Similar results were obtained in other experiments involving 2 rounds of unicellular ELTCs (data not shown). The results hence indicate that extensive self-replication of HSCs through 9-month ELTCs was coupled with long-term hemangioblast self-renewal, though the ELTC conditions were not permissive for significant EC differentiation.
Extensive self-renewal of hemangioblasts in serial single-cell ELTC.
(A) At the left, LTC-IC frequency in primary, secondary, and tertiary ELTCs, as evaluated by limiting dilution (Figure 2H); at the right, serial single-cell ELTC protocol. (B) Microscopy analysis of a very large Hem-End colony generated by blasts grown in tertiary unicellular ELTC (original magnifications: left, × 40; right, × 200). (C) RT-PCR analysis of a Hem-End colony split in 2 halves, which were seeded in secondary endothelial or hematopoietic (not shown) culture.
Extensive self-renewal of hemangioblasts in serial single-cell ELTC.
(A) At the left, LTC-IC frequency in primary, secondary, and tertiary ELTCs, as evaluated by limiting dilution (Figure 2H); at the right, serial single-cell ELTC protocol. (B) Microscopy analysis of a very large Hem-End colony generated by blasts grown in tertiary unicellular ELTC (original magnifications: left, × 40; right, × 200). (C) RT-PCR analysis of a Hem-End colony split in 2 halves, which were seeded in secondary endothelial or hematopoietic (not shown) culture.
Discussion
Previous studies indicate that HSC-enriched populations in humans8,9 (Figure 1) and mice10 11 comprise precursors for hematopoietic and endothelial lineages. The present findings newly identify the human postnatal hemangioblast in a small subset of CD34+KDR+ cells, which are uniquely endowed with long-term proliferative potential and bilineage differentiation capacity.
The frequency of hemangioblasts in CB and adult BM is very low—approximately 5 hemangioblasts per 106 MNCs, assuming 1% CD34+ cells in the MNC population and 1% KDR+ cells in the CD34+ subset. This frequency level has consistently been observed in different assay systems reported here. Although further improved culture conditions might increase the number of Hem-End colonies, it is apparent that, within CD34+KDR+ cells, the frequency of hemangioblasts is lower than that of primitive hematopoietic8 or endothelial9 cells.
The relationship between hemangioblasts, primitive HPCs, and primitive endothelial progenitors is a key issue. In early embryonic life, hemangioblasts generate the progenitor cells of hematopoietic and endothelial lineages.1-4 Hypothetically, this hierarchical relationship may persist in postnatal life, and, though exceedingly rare, hemangioblasts may function throughout the lifetime as a reservoir and/or a steady state source of primitive hematopoietic and endothelial cells.
Sibling cell studies indicate that postnatal hemangioblasts generate either bilineage or unilineage colonies, depending on the specific culture conditions. The gene program of hemangioblasts may be flexible and modulated by microenvironment stimuli to undergo either bilineage or unilineage differentiation. Accordingly, hemangioblasts may function as unipotent hematopoietic or endothelial progenitor/stem cells if tested in assays permissive for either hematopoietic8 or endothelial9 differentiation, respectively.
Preliminary experiments suggest that a subset of CD34+KDR+ cells is endowed with plasticity for the generation of mesodermic, ectodermic, and endodermic tissues (eg, skeletal muscle, neural, and hepatic cells) in diverse assay systems (eg, murine blastocyst, regenerating skeletal muscle) (results not shown). Within the CD34+KDR+ population, the cell subsets endowed with hemangioblast activity and plastic capacity may partially or totally overlap—postnatal hemangioblasts hypothetically represent residual embryonic stem cells with wide-spectrum differentiation potential. Future studies based on hemangioblast purification and in vivo assay will be required to verify this attractive model.
Finally, hemangioblasts may play a role in cellular mechanisms underlying abnormal hematopoiesis. A hematopoietic cell line with endothelial features was recently isolated from a patient with a transformed myeloproliferative syndrome.20 In chronic myeloid leukemia, the bcr-abl fusion protein is apparently present not only in hematopoietic cells but also in endothelial cells.21 These findings suggest that hemangioblasts may be targeted by the oncogenic hit in selected myeloproliferative disorders.
We thank Dr S. Rafii for providing the endothelial cell subculture medium and A. M. Cerio for skillful technical help. We also thank M. Blasi, M. Fontana, and A. Zito for editorial help.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-05-1511.
Supported in part by National Institutes of Health grant 1R01HL63168.
E.P. and M.V. contributed equally to this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Cesare Peschle, Kimmel Cancer Center, Room 609, Thomas Jefferson University, 233 S 10th St, Philadelphia, PA 19107-5541; e-mail: cesare.peschle@mail.tju.edu.