Abstract
The CD28− subset of CD8+ T cells is associated with cytotoxic T lymphocyte (CTL) effector function. We investigated a potential role for 4-1BB, a costimulatory molecule structurally related to members of the tumor necrosis factor (TNF) receptor family, in the generation and functional activation of CD28− CTLs by using human cord blood (CB) cells composed exclusively of naive CD8+ T cells with few or no CD28− CTLs. The 4-1BB was induced preferentially on the CB CD28−CD8+ T cells when CD28 down-regulation was induced by interleukin 15 (IL-15) and IL-12 stimulation. Anti–4-1BB costimulation induced dramatic phenotypic changes in the CD28− CTLs, including restoration of CD28 expression as well as that of memory markers such as CD45RO and CC chemokine receptor 6 (CCR6). Anti–4-1BB costimulation also promoted long-term survival of CD28− CTLs, which were sensitive to activation-induced cell death upon anti-CD3 stimulation. The memory-type CD28+CTLs induced by anti–4-1BB costimulation acquired a greatly enhanced content of granzyme B, a cytolytic mediator, and enhanced cytotoxic activity as compared with CD28− CTLs. Strong cytotoxicity of memory-type CTLs to a 4-1BB ligand–expressing Epstein-Barr virus (EBV)–transformed B-cell line was almost completely abrogated by 4-1BB–Fc, a soluble form of 4-1BB, suggesting involvement of 4-1BB in cytolytic processes. Taken all together, our results suggest that 4-1BB plays a role in the differentiation of effector memory CTLs.
Introduction
The cytotoxic T lymphocyte (CTL)–mediated immune response plays an essential role in the protection of the host against infectious diseases and cancer. Antigen (Ag) recognition by naive CD8+ T cells triggers a program of proliferation and differentiation that leads to the production of effector lymphocytes able to directly lyse Ag-bearing cells. The lytic mechanism primarily involves release of cytoplasmic granules loaded with perforin (a pore-forming protein) and granzyme B (a serine protease) at the contact site between a CTL and the target cell.1 A large fraction of Ag-specific CTLs are thereafter eliminated to maintain homeostasis. Some cells differentiate into memory cells that are either cytolytic or not cytolytic.2
CD28, a primary costimulatory molecule, is constitutively expressed in naive CD4+ and CD8+ T cells and is involved in initiating T-cell activation. However, the CTL function of human CD8+ T cells has been linked to the loss of CD28 or CD27,3-8 as is often observed during the course of a virus infection9-11 or in tumor development.4 This suggests that CD28 may not be directly involved in CTL functions. This possibility is further suggested by apparent CTL responses in CD28-deficient mice.12 The CD28− subset of CD8+ T cells frequently expresses high amounts of intracellular perforin10 and granzyme B,6,7both essential for killing activity. Thus, CD28−CD8+ T cells may represent a functionally active CTL population.3-5,7 The majority of CD28− effector cells are programmed to die rapidly by apoptosis,13 but small populations of CD28−effector cells survive and form a memory population.14 In contrast to effector cells, memory CD8+ T cells express CD28.5 15 Little is known about this maturation process.
Alternative costimulatory molecules, such as 4-1BB, OX40, CD30, and CD40 ligand (CD40L), all members of the tumor necrosis factor receptor (TNFR) superfamily, that are induced on activated CD4+ or CD8+ T cells have been studied as potential candidates responsible for various effector responses.16-19 Among those costimulatory molecules belonging to the TNFR superfamily, 4-1BB is unique for primarily augmenting CD8+ T cell–mediated antiviral20-22 and antitumor responses,23-31as well as allograft rejection.22,32-34 The effects mediated by 4-1BB are abrogated in mice depleted of CD8+ T cells,33 indicating that CD8+ T cells are important targets for 4-1BB–mediated responses.18,35Although 4-1BB–mediated costimulation has been shown to sustain long-term T-cell survival,16,35 36 its exact role in the developmental pathway of effector or memory CD8+ T cells has not been defined.
In this study, we address the question of how the expression of CD28 and 4-1BB, 2 costimulatory molecules, is coordinated during development of effector or memory CD8+ T cells. Umbilical cord blood (CB) offers a unique source of starting cells for studying the developmental pathway for effector or effector memory CD8+T cells, because CB is composed exclusively of naive CD8+ T cells with few or no cytotoxic CD28− effector cells.37 CD28− CTLs gradually emerge in the peripheral blood of infants after birth.3 In this study, we used the naive CD8+ T cells isolated from CB to develop CD28− CTLs in vitro by stimulating this cell population with 2 proinflammatory cytokines, interleukin 15 (IL-15) and IL-12. Our results demonstrate that 4-1BB is preferentially induced in CD28− CTLs, and the critical phase of differentiation of CD28− CTLs into CD28+ memory-type CTLs is driven by signals delivered by 4-1BB. This differentiation into a population of CD28+ CTLs is accompanied by dramatic increases in the expression of granzyme B and cytotoxic activity, suggesting that CD28− CTLs may represent a transitory population during CTL development and that 4-1BB may be a critical costimulatory molecule for further maturation to more cytotoxic CD28+ CTLs.
Materials and methods
Reagents
Human IL-12 and IL-15 were purchased from Pepro Tech (Rocky Hill, NJ). Anti-CD28–fluorescein isothiocyanate (FITC), anti–4-1BB–phycoerythrin (PE), anti-CD45RO–PE, or annexin V–FITC were purchased from PharMingen (San Diego, CA). Anti-CD8–Tri-Color, anti–granzyme B–PE, isotype control mouse IgG1-FITC, and mouse IgG2a–Tri-Color were purchased from Caltag (Burlingame, CA). Anti–CC chemokine receptor 6 (CCR6)–PE was purchased from R&D Systems (Minneapolis, MN). Anti–human CD3 (OKT3) and 4-1BB–Fc were a kind gift from Ortho Biotech (Raritan, NJ) and Immunex (Seattle, WA), respectively. Monoclonal antibody to 4-1BB (mouse IgG1) was purified from ascites collected from Balb/C mice injected intraperitoneally with the hybridoma 4B4.38 Control mouse IgG1 (MOPC 21) and human IgG1 were purchased from Sigma (St Louis, MO) and Serotec (Oxford, United Kingdom), respectively.
Cell stimulation
Adult peripheral blood (PB) and umbilical CB were obtained, respectively, from the local blood center (Central Indiana Regional Blood Center) and the Wishard Hospital (Indianapolis, IN). Buffy coats collected from whole blood by centrifugation at 900g for 10 minutes were mixed with an equal volume of phosphate-buffered saline (PBS). The mixture was layered over Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden), and cells at the interface were collected. T cells were purified by negative selection with the use of a combination of monoclonal antibodies and complement (Lympho-Kwik; One Lambda, Canoga Park, CA). The purity of the T cells exceeded 90%. CD8+ T cells were isolated either by fluorescence-activated cell sorting (FACS) or by negative selection on magnetic beads (StemCell, Vancouver, BC, Canada) containing antibodies to CD4, CD14, CD16, CD19, CD56, and glycophorin A, resulting in more than 99% and 90% purity, respectively. Purified T cells or CD8+ T cells were incubated in complete RPMI 1640 medium containing 10% fetal bovine serum and 50 μM β-mercaptoethanol with or without a combination of IL-12 (2 ng/mL) and IL-15 (10 ng/mL) for 5 days in 5% CO2 at 37°C. Cells were then primed with anti-CD3 (1 μg/mL) in complete RPMI 1640 medium on ice for 30 minutes, and washed twice with cold medium to remove free antibodies, and immediately placed in 24-well plates (Costar, Corning, NY). The resulting cells were harvested and analyzed for surface antigens or sorted by FACS. For determining the effects of anti–4-1BB costimulation, cells were incubated in plates that were precoated with isotype control IgG (mouse IgG1, MOPC 21), anti-CD28 (clone CD28.2), or anti–4-1BB (clone 4B4) at 10 μg/mL plus anti-CD3 (1 μg/mL). After 3 days, cells were analyzed for the expression of CD28, 4-1BB, granzyme B, and memory markers in CD8+ T cells. These cells were also used for51Cr release assays following cell isolation by FACS.
Flow cytometry analyses
For multiple-color staining, 5 to 10 × 105 cells were incubated with anti-CD8–Tri-Color plus the indicated antibodies conjugated with either FITC or PE in 50 μL staining buffer (PBS containing 1% bovine serum albumin) for 30 minutes at 4°C. The cells were washed 2 times with staining buffer and analyzed in a FACSCalibur cytofluorograph (Becton Dickinson, Mountain View, CA). Three-color staining was performed to detect CD28, 4-1BB, CD45RO, and CCR6 on CD8+ T cells with the use of FITC- or PE-conjugated corresponding antibodies with anti-CD8–Tri-Color. For staining intracellular granzyme B, cell-surface CD28 and CD8 were stained with anti-CD28–FITC and anti-CD8–Tri-Color and subsequently fixed and permeabilized with a kit (Fix and Perm; Caltag) according to the manufacturer's manual, followed by a PE-conjugated anti–granzyme B monoclonal antibody (mAb). Apoptosis assay by annexin V–FITC surface staining was undertaken according to the manufacturer's manual. Expression of 4-1BB ligand on Epstein-Barr virus (EBV)–transformed B cells was measured by staining the cells with 4-1BB–Fc and subsequently detecting the 4-1BB–Fc bound to the 4-1BB ligand with PE-conjugated anti–human Fc. Human IgG1was used as a control for 4-1BB–Fc.
Anti-CD3 redirected cytotoxicity assay
Cytotoxicity was determined in a standard 4-hour51Cr release assay using an EBV-transformed B-cell line, kindly provided by Dr Karen Pollok (Indiana University, Indianapolis), as target cells. Target cells (2 × 106 cells) were cultured 2 hours in 0.4 mL RPMI culture medium containing 500 μCi (18.5 MBq) Na251CrO4 and washed 3 times with PBS to remove free Na251CrO4. The target cells were then plated in 96-well round-bottom plates at 5000 cells per well. CD8+ T cells were mixed with target cells at effector-to-target cell ratios of 10:1, 5:1, 2:1, and 1:1. In some cases, 4-1BB–Fc was added to the target cells at the designated concentrations for 15 minutes at room temperature before the target cells were mixed with effector cells in 96-well plates. Human IgG1, a negative control for 4-1BB–Fc, was added to the target cells in a similar fashion to determine a specific effect of 4-1BB–Fc on CD8+ T cell–mediated cytotoxic activity. The plates were briefly centrifuged at 200g and incubated at 37°C in the presence or absence of anti-CD3 (1 μg/mL). After 4 hours, supernatants were counted in a gamma counter. The percentage of specific lysis was calculated by the following formula:
Results
Cytotoxic defect of CD8+ T cells from CB can be overcome by stimulation with IL-12 and IL-15
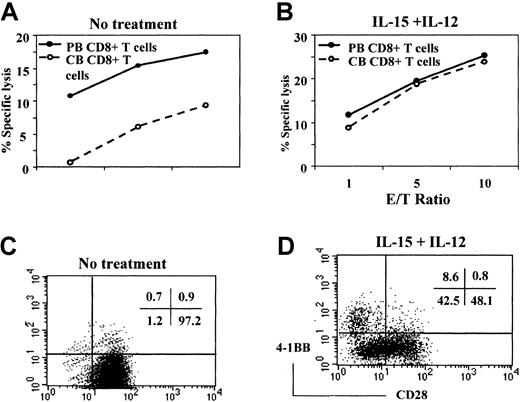
In contrast to CD8+ T cells from adult PB, CD8+ T cells from CB are composed almost exclusively of naive cells, with few effector or memory cells. Exogenous cytokines, such as IL-12 and IL-15, are known to promote the effector functions of CB CD8+ T cells.39,40 We asked whether the lower amount of cytotoxic activity by CB CD8+ T cells might be related to the lack of effector CD8+ T cells in CB, and whether proinflammatory cytokines might induce the development of effector CD8+ T cells. To test this possibility, we compared the cytotoxic activity of CD8+ T cells obtained from adult PB and CB before and after stimulation with a combination of IL-12 and IL-15 for 5 days in a reverse antibody-dependent cell-mediated cytotoxicity (ADCC) assay using an EBV-transformed B-cell line as target cells. Cytotoxicity was initiated by adding anti-CD3 mAb, which will bind to the FcR on the B-cell line and stimulate the CD8+ T cells. Consistent with our previous report,37 fresh CB CD8+ T cells have less cytotoxic activity as compared with those obtained from PB (Figure 1A). In contrast to freshly isolated cells, CB CD8+ T cells stimulated with IL-12 and IL-15 demonstrated markedly enhanced cytotoxic activity that was almost indistinguishable from that mediated by stimulated PB CD8+ T cells (Figure 1B). Therefore, naive CD8+ T cells in CB can be differentiated into cytotoxic effector cells by proinflammatory cytokines such as IL-12 and IL-15.
IL-12 and IL-15 induction of cytotoxic activity and CD28− CB CD8+ T cells.
CD8+ T cells freshly isolated from PB and CB were compared for cytotoxic activity against an EBV-transformed B-cell line by a standard 51Cr release assay (A). The reaction mixtures included anti-CD3 at 1 μg/mL for redirected antibody-dependent T cell–mediated cytotoxicity. Data are expressed as the percentage of specific lysis at the indicated effector-to-target cell ratios. Cytotoxic activity was driven largely by anti-CD3 because the percentage of specific lysis without anti-CD3 remains lower than 15% of that obtained with anti-CD3. Also, CD8+ T cells isolated from PB and CB were incubated with IL-12 (2 ng/mL) and IL-15 (10 ng/mL) for 5 days and used as effectors against the same targets (B). The CD8+ T cells cultured without (C) or with (D) IL-15 and IL-12 were then analyzed for surface expression of CD28 and 4-1BB by 2-color staining with anti-CD28–FITC and anti–4-1BB–PE. The numbers in the upper right corners of the plots represent the percentage of the cells in each quadrant. These results are representative of 3 experiments.
IL-12 and IL-15 induction of cytotoxic activity and CD28− CB CD8+ T cells.
CD8+ T cells freshly isolated from PB and CB were compared for cytotoxic activity against an EBV-transformed B-cell line by a standard 51Cr release assay (A). The reaction mixtures included anti-CD3 at 1 μg/mL for redirected antibody-dependent T cell–mediated cytotoxicity. Data are expressed as the percentage of specific lysis at the indicated effector-to-target cell ratios. Cytotoxic activity was driven largely by anti-CD3 because the percentage of specific lysis without anti-CD3 remains lower than 15% of that obtained with anti-CD3. Also, CD8+ T cells isolated from PB and CB were incubated with IL-12 (2 ng/mL) and IL-15 (10 ng/mL) for 5 days and used as effectors against the same targets (B). The CD8+ T cells cultured without (C) or with (D) IL-15 and IL-12 were then analyzed for surface expression of CD28 and 4-1BB by 2-color staining with anti-CD28–FITC and anti–4-1BB–PE. The numbers in the upper right corners of the plots represent the percentage of the cells in each quadrant. These results are representative of 3 experiments.
The subset of CD8+ T cells generated in cord blood by IL-12 plus IL-15 lacks CD28 but expresses 4-1BB
Most CD8+ and CD4+ T cells freshly isolated from CB express CD28, a primary costimulatory molecule for T-cell activation.3,5 Because the loss of CD28 is correlated with the effector function of CD8+ T cells,4,5 we asked whether treatment of CB CD8+ T cells with a combination of IL-15 and IL-12 down-regulated expression of CD28. We also examined the possible reciprocal induction of 4-1BB, an inducible costimulatory molecule, known to promote various CD8+ T cell–mediated effector functions, such as antiviral20-22 or antitumor responses.23-31 Expression of CD28 and 4-1BB on the CB CD8+ T cells was assessed by 2-color flow cytometry after incubation of CB CD8+ T cells for 5 days in the presence or absence of IL-12 plus IL-15. CD8+ T cells cultured in the absence of cytokines were essentially all CD28+ (Figure1C). As shown in Figure 1D, more than half the CB CD8+ T cells cultured in IL-12 plus IL-15 were CD28− or CD28low cells (51.1%). However, under the same culture conditions, CD28−CD4+ T cells were not induced (data not shown). The 4-1BB induction is known to be dependent on CD3 stimulation.16 Interestingly, these proinflammatory cytokines alone could also induce 4-1BB without CD3 engagement (8.6% of the CD8+ T cells). The 4-1BB was detected exclusively in the CD28− population, suggesting that the CD28− CTLs are a primary cell type where 4-1BB may be involved in activating effector function. In contrast, very little 4-1BB was detected in the CB CD8+ T cells cultured in the absence of IL-12 plus IL-15 (fewer than 2% of CD8+ T cells). Our results suggested the possibility that the lower cytotoxic activity of CB CD8+ T cells compared with that of PB CD8+ T cells might be related to lack of CD28−effector cells and that the gain of cytotoxicity by IL-15 and IL-12 might be attributed to an induction of the CD28−effector T-cell population. During culture with IL-15 and IL-12, there was little cell proliferation or death of cord blood CD8+ T cells unless anti-CD3 was added. Therefore, induction of the CD28−CD8+ T cells after IL-15 and IL-12 stimulation was probably due to the down-regulation of CD28 expression and not to selective expansion or survival of a CD28−population existing but not detected in the starting population of CB CD8+ T cells.
Ability to induce 4-1BB by CB CD8+ T-cell is coupled to IL-15 and IL-12 stimulation
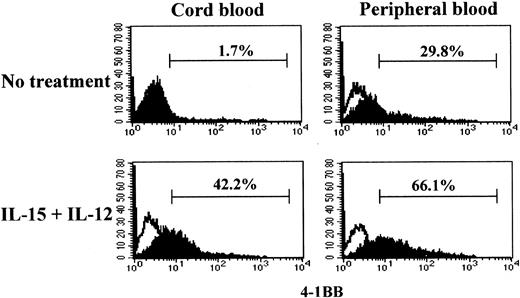
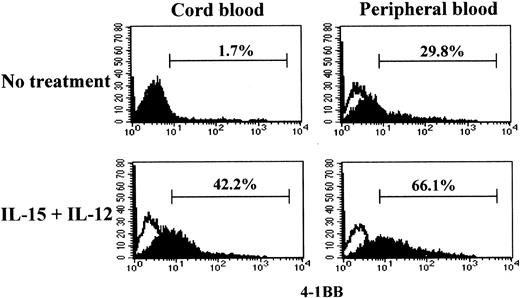
The cytotoxicity induced by IL-15 and IL-12 in CB CD8+T cells was driven by anti-CD3 stimulation. Naive and effector/memory CD8+ T cells may have different responsiveness to anti-CD3; naive CD8+ T cells may require more prolonged or stronger anti-CD3 stimulation compared with effector/memory cells in order to activate a program for killing target cells.41 To determine whether the differential cytotoxicities in CB and PB CD8+ T cells were correlated with the ability to induce 4-1BB upon anti-CD3 stimulation, we compared 4-1BB expression on CB and PB CD8+ T cells after stimulation with or without IL-15 and IL-12 for 5 days under conditions similar to those used for the cytotoxicity assay. We employed a suboptimal anti-CD3 stimulation condition as described in “Materials and methods” to better distinguish the distinctive responsiveness to anti-CD3 in 4-1BB induction in naive and effector/memory CD8+ T cells. Under this anti-CD3 stimulation condition, CB CD8+ T cells, consisting exclusively of naive cells, failed to induce 4-1BB (1.7%) (Figure 2). However, CB CD8+ T cells stimulated with IL-15 and IL-12 responded to anti-CD3 and expressed 4-1BB (42.2%). In contrast, PB CD8+T cells expressed 4-1BB without such cytokine treatment in response to anti-CD3 stimulation (29.8%). However, the induction of expression of 4-1BB on PB CD8+ T cells was further enhanced after IL-15 and IL-12 stimulation (66.1%). The induction of the differential cytotoxic activities of PB and CB CD8+ T cells shown in Figure 1A-B occurred essentially parallel with the induction of 4-1BB. These results indicated that IL-15 and IL-12 induced the maturation process toward acquisition of cytotoxic activity as well as the expression of 4-1BB.
Effect of IL-15 and IL-12 stimulation on induction of 4-1BB in CD8+ T cells.
IL-15 and IL-12 stimulation is required for induction of 4-1BB on CB but not on PB CD8+ T cells. Purified CD8+ T cells from CB and PB were cultured for 5 days in the presence or absence of IL-15 and IL-12 followed by a 3-day stimulation with anti-CD3 as described in “Materials and methods.” The resulting cells were analyzed for 4-1BB expression by staining with anti–4-1BB–PE as shown by filled histograms. The open histograms indicate staining with the isotype control mouse IgG1-PE. The percentages shown indicate the 4-1BB+ CD8+T cells.
Effect of IL-15 and IL-12 stimulation on induction of 4-1BB in CD8+ T cells.
IL-15 and IL-12 stimulation is required for induction of 4-1BB on CB but not on PB CD8+ T cells. Purified CD8+ T cells from CB and PB were cultured for 5 days in the presence or absence of IL-15 and IL-12 followed by a 3-day stimulation with anti-CD3 as described in “Materials and methods.” The resulting cells were analyzed for 4-1BB expression by staining with anti–4-1BB–PE as shown by filled histograms. The open histograms indicate staining with the isotype control mouse IgG1-PE. The percentages shown indicate the 4-1BB+ CD8+T cells.
4-1BB costimulation induces a subset of CD28+CD8+ T cells with greater cytotoxic activity
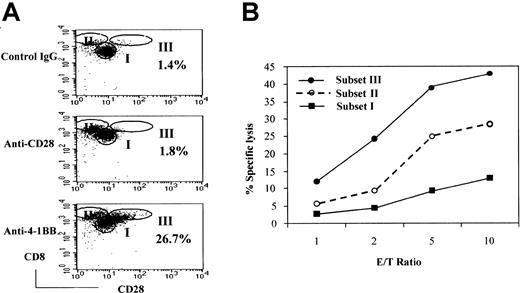
Because cytotoxic activities were correlated with the expression of 4-1BB, we asked whether 4-1BB costimulation could cause expansion of the CD28− subset of CD8+ T cells, a prototype CTL, or elicit any phenotypic changes in this population. To this end, we analyzed the CD8+ T-cell population for CD28− cells after stimulation of naive CB CD8+T cells with IL-15 and IL-12 followed by anti–4-1BB costimulation with anti-CD3 for 3 days. We included anti-CD28 and isotype control IgG as controls to evaluate the specificity of anti–4-1BB costimulation. The resulting cells consisted largely of 3 populations based on the relative intensities of expression of CD28 and CD8 (Figure3A). We designated the 3 populations as subsets I, II, and III. Subset I corresponded to naive CD8+T cells that expressed CD28 but no 4-1BB. Subset II identified cells that expressed little or no CD28. This population was similar to that generated after stimulation with IL-12 and IL-15, as seen in Figure 1D. Anti-CD28 costimulation increased numbers of subset II cells. Interestingly, anti–4-1BB costimulation induced an additional population of CD28+ cells, designated subset III cells (26.7%). Subset III cells were barely detected after incubation with control IgG (1.8%) or anti-CD28 (1.4%). Subset II and III cells showed brighter CD8 staining compared with cells in subset I. Thus, costimulation with CD28 and 4-1BB contributed to an expansion of phenotypically different CD8+ T-cell subpopulations and increased the proportion of cells in subsets II and III, respectively. At this time, it was not clear whether subset III cells originated from the subset II population.
Effect of 4-1BB costimulation on the generation of a cytotoxic effector cell population derived from CB CD8+ T cells.
Costimulation by 4-1BB results in the generation of a highly cytotoxic effector-cell population derived from CB CD8+ T cells. CB CD8+ T cells were cultured with IL-15 and IL-12 for 5 days. The cells were then stimulated for 3 days in plates precoated with anti-CD28 (or control IgG) and anti–4-1BB plus anti-CD3 and analyzed for cell surface levels of CD28 and CD8 by FACS. Cells were divided into 3 subsets—I, II, and III—on the basis of the intensity of staining for CD28 and CD8 (A). The percentages shown in the dot plots represent the proportion of the cells in subset III. These results are representative of 6 experiments. Cells from subsets I, II, and III were separated by cell sorting and used as effectors in a standard51Cr release assay with EBV-transformed B cells as target cells at the indicated effector-to-target cell ratios (B). The reaction mixtures included anti-CD3 at 1 μg/mL for redirected antibody-dependent T-cell–mediated cytotoxicity. The data are displayed as the percentage of specific lysis. These results are representative of 3 experiments.
Effect of 4-1BB costimulation on the generation of a cytotoxic effector cell population derived from CB CD8+ T cells.
Costimulation by 4-1BB results in the generation of a highly cytotoxic effector-cell population derived from CB CD8+ T cells. CB CD8+ T cells were cultured with IL-15 and IL-12 for 5 days. The cells were then stimulated for 3 days in plates precoated with anti-CD28 (or control IgG) and anti–4-1BB plus anti-CD3 and analyzed for cell surface levels of CD28 and CD8 by FACS. Cells were divided into 3 subsets—I, II, and III—on the basis of the intensity of staining for CD28 and CD8 (A). The percentages shown in the dot plots represent the proportion of the cells in subset III. These results are representative of 6 experiments. Cells from subsets I, II, and III were separated by cell sorting and used as effectors in a standard51Cr release assay with EBV-transformed B cells as target cells at the indicated effector-to-target cell ratios (B). The reaction mixtures included anti-CD3 at 1 μg/mL for redirected antibody-dependent T-cell–mediated cytotoxicity. The data are displayed as the percentage of specific lysis. These results are representative of 3 experiments.
Because of a positive correlation between the level of 4-1BB expression and cytotoxic activity, we postulated that subset III specifically induced by 4-1BB costimulation would be the most cytotoxic. To test this hypothesis, we separated each subpopulation by cell sorting and compared the cytotoxic activity against an EBV-transformed B-cell line as target cells (Figure 3B). As shown, subset III cells were the most cytotoxic, subset II cells were intermediate, and subset I cells were the least cytotoxic. We did not detect distinguishable differences in the cytotoxic activity of populations I and II when comparing treatment with anti–4-1BB and treatment with anti-CD28 (data not shown). Because 4-1BB has been shown by others to promote various cytotoxic effector functions, such as antitumor and allogeneic responses, subset III cells may be likely candidate cells for those 4-1BB–mediated CTL responses.21,22,27 33
Subset III cells may represent effector memory CTLs
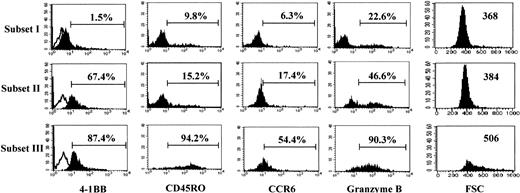
As subset III cells were the most potent cytotoxic subpopulation of CD8+ T cells, we analyzed the phenotypes of subset III cells in comparison with subset I and II cells (Figure4). As expected, subset III cells demonstrated the highest expression of 4-1BB (87.4%), compared with subset II cells, which exhibited somewhat less 4-1BB (67.4%). Little 4-1BB was detected in the subset I population, which had elicited the least cytotoxic activity. We noticed several other unique phenotypes for subset III, as compared with subset I and II cells. CD45RO and CCR6 are known as prototype memory cell markers for T cells.42These memory markers were expressed predominantly on subset III, compared with subset I and II cells, suggesting that the subset III population may represent memory-type cytotoxic CD8+ T cells. Granzyme B is an important cytolytic mediator for natural killer (NK) and CTL cells. The highest levels of granzyme B were detected in the subset III cells. This abundance of granzyme B in subset III may account for the strong cytotoxic activity of these cells, as shown in Figure 3B. Notably, subset III cells were extraordinarily large as estimated by a high mean fluorescence intensity of FSC (506 for subset III versus 368 and 384 for subsets I and II, respectively). These results indicate that anti–4-1BB costimulation gave rise to interesting phenotypic changes in the cells present in subset III. These changes correlated with the induced functional activities of these cells.
Subset III cells.
Subset III cells differ from subset I and II cells in the expression of memory markers and have a high intracellular granzyme B content. Each subset of CD8+ T cells was analyzed for cell surface levels of 4-1BB, CD45RO, CCR6, and intracellular granzyme B by 3-color staining along with staining for CD28 and CD8 expression. Expression of markers in each gated subset of cells (I, II, and III) is displayed by the filled histograms. Open histograms represent the cells stained with the isotype control Ab. The percentages represent the CD8+ T cells positive for each marker. The cell size of each subset was assessed by the forward scatter (FSC) intensity; these particular numbers represent the mean intensity of FSC as a parameter for cell size. The results in this figure are representative of 4 experiments.
Subset III cells.
Subset III cells differ from subset I and II cells in the expression of memory markers and have a high intracellular granzyme B content. Each subset of CD8+ T cells was analyzed for cell surface levels of 4-1BB, CD45RO, CCR6, and intracellular granzyme B by 3-color staining along with staining for CD28 and CD8 expression. Expression of markers in each gated subset of cells (I, II, and III) is displayed by the filled histograms. Open histograms represent the cells stained with the isotype control Ab. The percentages represent the CD8+ T cells positive for each marker. The cell size of each subset was assessed by the forward scatter (FSC) intensity; these particular numbers represent the mean intensity of FSC as a parameter for cell size. The results in this figure are representative of 4 experiments.
CD28+ CTLs induced by 4-1BB costimulation are a direct precursor of CD28− CTLs
Although circumstantial evidence supported the possibility that CD28+ subset III cells may have originated from CD28− subset II cells, no direct evidence for this was shown. To address this possibility, we started with FACS-purified subset II cells from which we attempted to induce subset III cells after anti–4-1BB costimulation. We also included anti-CD28 or control IgG in this study to evaluate the specificity of anti–4-1BB costimulation. Purified subset II cells were cultured with anti-CD3 under different costimulatory conditions. The resulting cells were subjected to FACS analysis for induction of subset III cells. We chose up-regulation of CD28 as a marker for subset III cells. As shown in Figure 5A, a significant increase of CD28 expression was detected in the purified population of subset II cells after anti–4-1BB costimulation (66.2%) as compared with those cultured with isotype control IgG (15.2%) or anti-CD28 (21.3%). To rule out the possibility that the failure of CD28 induction after stimulation with anti-CD28 might be caused by residual anti-CD28 released from the plates, which could block our anti-CD28 staining process, we repeated the experiments with biotin-conjugated anti-CD28 to test for the presence of biotin-conjugated anti-CD28 on the cell surface by staining with streptavidin-PE. Our flow cytometry results indicated that there was no detectable amount of biotin-conjugated anti-CD28 on the cell surface when compared with control cells simultaneously incubated in the plates coated with biotin-conjugated isotype control IgG (data not shown). From these experiments, we confirmed that anti–4-1BB, but not anti-CD28 costimulation, induced CD28 expression in subset II cells generating a subset III–like phenotype. Subset III cells were also characterized by a remarkably high content of granzyme B. The level of granzyme B induced by anti–4-1BB costimulation was comparable to that seen in the subset III cells shown in Figure 4. Granzyme B content was not increased by either control IgG or anti-CD28. Up-regulation of CD28 and granzyme B expression suggests that the CD28− subset II cells are a direct precursor of CD28+ subset III cells. These results also suggest a unique role for 4-1BB in the process of differentiation from CD28− CTLs to CD28+CTLs.
Effect of anti–4-1BB costimulation on the differentiation of subset II cells.
Anti–4-1BB costimulation promotes the differentiation of subset II into III cells. Subset II cells were isolated by cell sorting from anti-CD28–stimulated CB CD8+ T cells after a 5-day culture in the presence of IL-15 and IL-12 as described in Figure 3A, and stimulated on plates precoated with isotype control IgG, anti-CD28, or anti–4-1BB plus anti-CD3 for 3 days. The resulting cells were analyzed for expression of CD28 and intracellular granzyme B as described in Figure 4. The cells were also analyzed for apoptosis by staining with annexin V (A). Percentages shown in the histograms represent the positive cells. Cells stimulated with the different antibodies were used as effectors in a standard 51Cr release assay at an effector cell–to–target cell ratio of 2:1 (B), as described above. The 51Cr release assays were performed in the presence or absence of anti-CD3 (1 μg/mL). The data are presented as the percentage of specific lysis. Subset II and III cells were purified and subjected to 51Cr release assays with the use of K562 cell line as target cells at an effector-to-target cell ratio of 2:1 in the presence or absence of anti-CD3. These data are representative of 3 experiments.
Effect of anti–4-1BB costimulation on the differentiation of subset II cells.
Anti–4-1BB costimulation promotes the differentiation of subset II into III cells. Subset II cells were isolated by cell sorting from anti-CD28–stimulated CB CD8+ T cells after a 5-day culture in the presence of IL-15 and IL-12 as described in Figure 3A, and stimulated on plates precoated with isotype control IgG, anti-CD28, or anti–4-1BB plus anti-CD3 for 3 days. The resulting cells were analyzed for expression of CD28 and intracellular granzyme B as described in Figure 4. The cells were also analyzed for apoptosis by staining with annexin V (A). Percentages shown in the histograms represent the positive cells. Cells stimulated with the different antibodies were used as effectors in a standard 51Cr release assay at an effector cell–to–target cell ratio of 2:1 (B), as described above. The 51Cr release assays were performed in the presence or absence of anti-CD3 (1 μg/mL). The data are presented as the percentage of specific lysis. Subset II and III cells were purified and subjected to 51Cr release assays with the use of K562 cell line as target cells at an effector-to-target cell ratio of 2:1 in the presence or absence of anti-CD3. These data are representative of 3 experiments.
Consistent with reports by others,13 the majority of CD28− subset II cells underwent apoptosis as cells were cultured for 7 more days with control IgG (65.3% apoptosis) or anti-CD28 (63.1% apoptosis) after analysis of CD28 and granzyme B (Figure 5A). In contrast, anti–4-1BB costimulation significantly protected the CD28− subset II cells from cell death (29.3% of the cells positive for annexin V staining). Therefore, the apoptosis-prone subset II cells probably require survival signals delivered by 4-1BB–costimulation in order to differentiate into subset III cells. Finally, we performed cytotoxicity assays with each resulting cell population derived from the purified subset II cells after stimulation with anti–4-1BB, control IgG, or anti-CD28 in the presence of anti-CD3 for 3 days. Cells stimulated with anti–4-1BB showed far greater cytotoxic activity than those stimulated with control IgG or anti-CD28 (Figure 5B). Because we measured cytotoxic activity on the basis of defined effector-to-target ratios, the enhanced cytotoxic activity in response to 4-1BB ligation was due to increased cytotoxicity at the individual cell level. The cytotoxic activity (greater than 30% specific lysis) of the subset II cells stimulated with anti–4-1BB was comparable to that detected with subset III cells shown in Figure 3B. Interestingly, unlike subset II cells, subset III cells did not require anti-CD3 to initiate cytotoxic activity to the target cells during the cytotoxic assay. This lymphokine-activated killer (LAK)–like cytotoxic activity of subset III cells was further manifested by their ability to kill K562 cells, the prototypic NK-sensitive target cell line expressing very low levels of major histocompatibility complex (MHC) class I. However, we did not detect this LAK-like activity from subset II cells (Figure 5C). Considering all the results together, we suggest that CD28− CTLs may represent an immediate precursor for a further maturated and more active form of CTL and that 4-1BB may be a key factor influencing this differentiation pathway. Subset III cells produced as a consequence of 4-1BB stimulation showed unique cytotoxic activity in a relatively MHC-unrestricted fashion.
4-1BB may be directly involved in target cell lysis
Thus far, our data suggested a role for 4-1BB in the CTL differentiation process. Next, we tested the possibility that 4-1BB might actually be involved in the killing mechanism. To this end, we established a cytotoxic assay system in which we could block 4-1BB signals delivered by 4-1BB ligands expressed on the target cells to test a potential role for 4-1BB in killing activity. We used 4-1BB–Fc, a soluble form of 4-1BB consisting of the extracellular part of 4-1BB fused with the Fc portion of human IgG1. We first tested whether 4-1BB–Fc was capable of binding to 4-1BB ligands on the EBV-transformed B-cell line used in our cytotoxic assay. Bound 4-1BB–Fc on the target cells was detected with a PE-conjugated goat F(ab′)2 anti–human Fc. We found that more than 90% of target B cells were positive for 4-1BB ligand (Figure6A). In contrast, human IgG1 gave rise to only a background level of fluorescent intensity, indicating that detection of 4-1BB ligand on the target cells resulted from 4-1BB–Fc's binding the 4-1BB ligand, but not the Fc receptor. This information allowed us to use 4-1BB–Fc to block the majority of 4-1BB ligands on target cells from triggering 4-1BB on effector cells. We then determined whether treatment of target cells with 4-1BB–Fc affected cytotoxic activity by subset III cells, which expressed a high level of 4-1BB. As seen in Figure 6B, target cells pretreated with 4-1BB–Fc at a concentration of 10 μg/mL were almost completely resistant to cytolysis by effector cells. This inhibitory 4-1BB–Fc effect was dose-dependent. In contrast, human IgG1 used as a negative control did not affect cytolysis of the target cells. These results strongly suggest that 4-1BB signals delivered by 4-1BB ligands on the target cells are involved in cytolytic activity. At this time, the exact mechanisms through which 4-1BB signals might trigger the cytolytic process remain unclear. Because EBV-transformed B cells are known to be resistant to Fas-mediated cell death,43 we favor the possibility that 4-1BB–costimulation may be involved in promoting a granule enzyme–mediated cytotolytic pathway.
Effect of 4-1BB–Fc on cell-mediated cytotoxic activity to 4-1BB ligand–expressing target cells by subset III cells.
The 4-1BB–Fc inhibits the cell-mediated cytotoxic activity to 4-1BB ligand–expressing target cells by subset III cells. Levels of 4-1BB ligand on EBV-transformed B cells were measured by staining cells with 4-1BB–Fc followed by a PE-conjugated anti–human IgG Ab (Fc-specific), as shown (A) by the filled histogram. The open histogram shows staining with an isotype human IgG1. Subset III cells were isolated by cell sorting from the CB CD8+ T cells stimulated with anti–4-1BB as described in Figure 3A and used as effectors in a standard 51Cr release assay at an effector cell–to–target cell ratio of 2:1. Increasing amounts of 4-1BB–Fc (at final concentrations of 0, 1, 5, and 10 μg/mL) were added to the target cells for 15 minutes at room temperature before the addition of effector cells (B). Human IgG1 was used as a negative control. The data are presented as the percentage of specific lysis. These results are representative of 4 experiments.
Effect of 4-1BB–Fc on cell-mediated cytotoxic activity to 4-1BB ligand–expressing target cells by subset III cells.
The 4-1BB–Fc inhibits the cell-mediated cytotoxic activity to 4-1BB ligand–expressing target cells by subset III cells. Levels of 4-1BB ligand on EBV-transformed B cells were measured by staining cells with 4-1BB–Fc followed by a PE-conjugated anti–human IgG Ab (Fc-specific), as shown (A) by the filled histogram. The open histogram shows staining with an isotype human IgG1. Subset III cells were isolated by cell sorting from the CB CD8+ T cells stimulated with anti–4-1BB as described in Figure 3A and used as effectors in a standard 51Cr release assay at an effector cell–to–target cell ratio of 2:1. Increasing amounts of 4-1BB–Fc (at final concentrations of 0, 1, 5, and 10 μg/mL) were added to the target cells for 15 minutes at room temperature before the addition of effector cells (B). Human IgG1 was used as a negative control. The data are presented as the percentage of specific lysis. These results are representative of 4 experiments.
Discussion
The down-regulation of CD28 on CD8+ T cells often occurs during an acute virus infection or during tumor development.5 Recently, low expression of CD28 on the cell surface has been used for the identification of cytotoxic effector T cells.3,44 CD28− effector cells exhibit various features related to cytolytic function, such as expression of perforin, granzyme A and B, and Fas ligand.5 Human memory CD8+ T cells are known to express CD28.5 A highlight of our present study is the finding that 4-1BB is preferentially expressed on CD28−CD8+ T cells and that 4-1BB plays a key costimulatory role in the differentiation of CD28− CTLs into more cytotoxic, memory-type CD28+ CTLs. Thus, potential vaccines based on the induction of memory CTLs may require differentiation of CD28− effector cells into posteffector memory CD28+ T cells.45
The differentiation pathway from CD28− effector to CD28+ posteffector memory CTLs by 4-1BB costimulation offers an explanation as to why CB CD8+ T cells exhibit such a low level of cytotoxic effector activity.3,46 CB CD8+ T cells contain little if any of the CD28− population. Therefore, it is difficult to produce highly cytotoxic memory-type CD28+ CTLs since de novo induction requires a 2-step stimulation, initially through proinflammatory cytokines, and subsequently through a specific costimulatory molecule, such as 4-1BB. CB cells are known to be defective in the production of IL-15 and IL-12,39,40,47and as we have shown in Figure 2, they also lack expression of 4-1BB. Therefore, the defect in cytotoxic activity of CB CD8+ T cells may be attributed to an impaired ability to induce these essential molecules. Consistent with this, the risk of graft-versus-host disease (GVHD) associated with CB stem cell transplantation is lower than that associated with transplantation of adult bone marrow.48,49 In mice, GVHD is regulated by ligation of 4-1BB on alloreactive effector CD8+ T cells present in the donor transplantation inoculum.34 50 It would be of great interest to examine whether delayed or impaired induction of the memory-type CD28+ CD8+ T cells that we identified in this study are associated with a low incidence of GVHD after CB transplantation.
Our results strongly suggest that 4-1BB plays an important role in maintaining survival during the CTL differentiation pathway, particularly from CD28− effector to memory-type CD28+CD8+ T cells. The massive increase in CD28− effector cells during an acute antiviral immune response51 is followed by their apoptotic deletion.14 The loss of expression of CD28 in cytotoxic effector cells may provide the basis for the cells to be prone to apoptosis upon repetitive antigen exposure. Some selected CD28−CD8+ T cells that escape apoptosis may mature into CD28+ memory-type cells, critical for an effective long-term immune reaction. T-cell memory depends on survival factors, such as 4-1BB, that regulate the death of CD8+ T cells. Therefore, as previously suggested by others in another context,35 rescue of CD28−CD8+ T cells (subset II) from apoptosis by 4-1BB costimulation may be an important element required for differentiation into memory-type CD28+ effector cells (subset III). Two types of human memory CD8+ T cells have been defined: CCR7+“central” and CCR7− “effector memory” cells.52 Subset III cells were mostly CD45RO+CCR6+CCR7− (data not shown). Therefore, the subset III cells that we described in this study may represent so-called “effector memory” or another form of terminally differentiated effector CD8+ T cells. The subset III cells were so potent in their cytotoxic activity that these cells could kill the 4-1BB–expressing target cells even without anti-CD3 stimulation.
One basic pathological problem for HIV-infected patients is defective HIV-specific CTL responses, although most HIV-infected patients have sustained HIV-specific cytotoxic T-cell expansion. We speculate that this immune incompetence may be related to impaired CTL differentiation. Many of these HIV-specific CD8+ T cells are not functional, even with a high viral burden.53-56Importantly, the balance between CD28− effector and CD28+ memory CTLs is closely associated with viral pathogenesis.57 For example, the majority of functionally defective anti-HIV CTLs exhibit the CD28− phenotype but lack CD28+ HIV-specific CTLs.57 This suggests that most HIV-specific CD8+ T cells have not fully differentiated into CD28+ memory-type CTLs, and as a result, HIV continuously replicates. In contrast to HIV, virus-specific CD8+ T cells against EBV and influenza virus, which usually enter a latent phase after the acute mononucleosis syndrome or are cleared after recovery from disease, are essentially all CD28+, although the CD28− phenotype is dominant at an initial infection stage. These findings strongly suggest that regulation of the differentiation pathway to CD28+ memory CD8+ T cells from the CD28− phenotype CTL population is critical for antiviral immunity. In many cases, tumor-specific CD8+ T cells in patients with metastatic tumors are often profoundly anergic.58 This antitumor immune incompetence may also be associated with inefficient differentiation from CD28−effector cells to memory-type CD28+ effectors. In addition, antitumor CD8+ T cells may be defective in the exocytosis of granzymes into target cells as well.58 In this context, it is of interest to recognize the potential effects of 4-1BB not only on the differentiation process but also for cytolytic activity. Preclinical studies seem justified for possible clinical usage of 4-1BB signals in antiviral and antitumor immunotherapy. These studies will probably need to include ex vivo 4-1BB costimulation for Ag-specific CD8+ T cells before adoptive transfer back to the patients or by vaccination with cells genetically manipulated to constitutively express 4-1BB ligand.59 IL-12 is known to elicit antitumor activity.60 The 4-1BB–mediated antitumor responses are known to be amplified by IL-12.30 31 This cooperative IL-12 effect on 4-1BB–mediated antitumor responses can be explained by a crucial effect of IL-12 on 4-1BB expression.
In conclusion, 4-1BB specifically regulates the differentiation of a CD28− subset of CD8+ T cells into memory-type CD28+ CTLs. Moreover, 4-1BB costimulation appears to be involved in the cytolytic process. Thus, 4-1BB costimulation may be useful as a therapeutic tool to promote antitumor or antiviral CTL responses. Conversely, blocking reagents, such as 4-1BB–Fc, may be a useful tool to prevent undesirable CTL-mediated responses, such as autoimmunity or allograft rejection after transplantation.
We thank Dr Karen E. Pollok for providing the EBV-transformed B-cell line. We are also grateful to Ortho Biotech and Immunex for providing anti-CD3 and 4-1BB–Fc, respectively.
Prepublished online as Blood First Edition Paper, July 5, 2002; DOI 10.1182/blood-2001-11-0136.
These studies were supported by US Public Health Service grants RO1 HL56416, RO1 HL67384, RO1 DK53674 (H.E.B.), and RO1 AI46455 (R.R.B.) from the National Institutes of Health, and by a grant from the Phi Beta Psi Sorority (H.E.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hal E. Broxmeyer, Walther Oncology Center, Indiana University School of Medicine, Bldg R4, Rm 302, 1044 W Walnut St, Indianapolis, IN 46202-5254; e-mail: hbroxmey@iupui.edu.