Abstract
Sézary syndrome (SzS) is an advanced form of cutaneous T-cell lymphoma associated with involvement of the peripheral blood by malignant T cells. The disease is defined by impaired cell-mediated immunity and the production of interferon-γ (IFN-γ) and interleukin-2 (IL-2), possibly as a result of deficient IL-12 production. To understand the mechanism of this impairment, we examined the composition and function of dendritic cells and monocytes in the blood of SzS patients with different levels of peripheral blood tumor burden. Consistent with our previous observations, numbers of monocytes in SzS patients were comparable to numbers observed in healthy donors. In contrast, decreased IL-12 production correlated with a decrease in the numbers of CD11c+ dendritic cells, which was particularly profound among patients with medium (20%-50% circulating malignant T cells) and high (more than 50% circulating malignant T cells) tumor burden. Furthermore, CD123+ dendritic cells, major producers of IFN-α, were significantly diminished in SzS patients, regardless of the level of tumor burden. Granulocyte macrophage–colony-stimulating factor–treated patients experienced an increase in the number of dendritic cells but not in IFN-α or IL-12 production. However, in vitro stimulation of peripheral blood mononuclear cells from SzS patients with rCD40L and IFN-γ significantly increased the production of IL-12. Thus, our results demonstrate a profound defect in circulating dendritic cells in SzS patients that may contribute to the pathogenesis of the cytokine disorders and to the depressed cellular immunity. Importantly, the ability of rCD40L to potently induce IL-12 production from monocytes and residual dendritic cells of SzS patients could potentially serve as an immune-restorative therapeutic agent.
Introduction
Cutaneous T-cell lymphoma (CTCL) is a lymphoproliferative disorder characterized by the clonal expansion of malignant CD4+ T cells. Early stages of CTCL are characterized by localization of the disease process primarily to the skin, with the presence of patches or plaques. Tumor nodules may also appear. The most common form of this disorder is mycosis fungoides. Progressive disease is associated with the dissemination of malignant T cells to the lymph nodes and peripheral blood. Sézary syndrome (SzS) is a form of the disease manifested by erythroderma and circulating malignant T cells.1,2 Previous studies have demonstrated that progression to Sézary syndrome is associated with a decrease in cell-mediated immunity typified by depressed T-helper type 1 (TH1) responses characterized by defects in the production of interferon-γ (IFN-γ) and interleukin-2 (IL-2). In concert with these findings, the production of IL-4, a TH2 cytokine, is increased and appears to correlate with the burden of circulating malignant T cells.3-6
Recent studies have also demonstrated that peripheral blood mononuclear cells (PBMCs) from SzS patients produce significantly decreased levels of IL-12, an immunoregulatory cytokine that is a key factor in the induction of TH1 responses and a major inducer of IFN-γ production.7 IL-12 is a heterodimeric cytokine composed of covalently linked chains designated p40 and p35, based on molecular weight. The expression of both genes is required for IL-12 (IL-12 p70) biologic activity to occur.8
Dendritic cells and monocytes/macrophages are major producers of IL-12 in response to infectious pathogens.8 However, it has been demonstrated that dendritic cells and monocytes are able to produce IL-12 if they are stimulated by activated T cells through CD40/CD40L interaction.9,10 Activation of this pathway not only stimulates the secretion of IL-12, it enhances the antigen presentation capabilities of dendritic cells and increases the expression of cell surface costimulatory molecules such as CD80 and CD86 on dendritic cells and monocytes, resulting in priming and expansion of CD4+ and CD8+ cytotoxic T cells in response to antigenic stimulation.11-15 It has also been shown that CD40/CD40L interaction is critical for the development of protective tumor immunity.16-18
Dendritic cells are the only antigen-presenting cells (APCs) with the ability to prime naive T cells to an antigen.19In addition to their role in adaptive immunity, they are instrumental in innate immunity by producing cytokines involved in host defense, such as IL-15, IL-18, IFN-α, and IL-12. Moreover, dendritic cells are able to activate natural killer (NK) and NKT cells.20
Recently, 2 distinct lineages of dendritic cells have been defined. Myeloid dendritic cells (DC1) originate from myeloid bone marrow precursors. In human peripheral blood, DC1 are negative for lymphoid cell– and myeloid cell–specific markers (lineage negative; Lin 1−), but they are HLA-DR+/CD11c+.21,22 They are major producers of IL-12 when stimulated with bacterial products or with CD40L. Therefore, it has been accepted that DC1 drive T-cell differentiation into TH1 responses.9,23 The second population consists of plasmacytoid dendritic cells (DC2) of lymphoid origin, which stimulate naive T cells to produce predominantly TH2 cytokines.24 These cells lack myeloid markers and have been described as lin1−/CD11c−/HLA-DR+/CD123+.25Recently, DC2 were recognized as major producers of IFN-α in response to viral infection.26-28
In this report we examine the role of dendritic cells in the pathogenesis of impaired cell-mediated immunity and deficient IL-12 production in SzS patients. We demonstrate that CD123+ and CD11c+ populations of dendritic cells are markedly decreased in SzS patients and that they exhibit an associated decrease in production of IFN-α and IL-12. We also demonstrate in vitro results that suggest that therapy with recombinant CD40L may prove beneficial in the restoration of IL-12 production by monocytes and residual dendritic cells of these patients.
Patients, materials, and methods
Patients
Mycosis fungoides and Sézary syndrome (SzS) were diagnosed on the basis of clinical, histopathologic, and immunohistologic criteria.29 Patients' circulating malignant T cells were analyzed on 1-μm sections of formalin-fixed peripheral blood buffy coats by the detection of mononuclear cells with cerebriform nuclear morphology.29 Additionally, for this report, SzS patients were divided into 3 groups based on tumor load. Those with 5% to 20% circulating Sézary cells were defined as having low tumor burden, those with 20% to 50% circulating Sézary cells were defined as having medium tumor burden, and those with more than 50% circulating Sézary cells were defined as having high tumor burden. Patients with mycosis fungoides had only skin involvement without evidence of circulating malignant T cells. White blood cell counts of SzS patients selected for these studies ranged from 3.5 × 106 to 5 × 106 cells/mL. All patients with SzS underwent identical treatment consisting of the use of extracorporeal photopheresis approximately every 4 weeks.30 None of the patients studied had been treated with chemotherapy, radiation, or other immunosuppressive drugs. As controls, blood samples from age-matched healthy donors were used. Donation of blood by patients and healthy donors conformed to the University of Pennsylvania institutional review board–approved protocols, and informed consent was obtained.
Mononuclear cells
Phenotypic and functional analyses of dendritic cell populations in CTCL patients and healthy donors were performed on viably frozen PBMCs, unless indicated otherwise. PBMCs were collected from blood as previously described, aliquoted, and stored in liquid nitrogen.7 On the day of the test, cells were transferred to culture media (RPMI 1640; Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum (Gibco-BRL, Grand Island, NY) and penicillin/streptomycin. PBMCs were then divided into 2 groups, and cells were stained and analyzed by flow cytometry or cultured under conditions appropriate for stimulating the production of cytokines.
Flow cytometric analysis
To detect CD11c+ and CD123+ subsets of peripheral blood dendritic cells, approximately 106 PBMCs per sample, resuspended in Dulbecco phosphate-buffered saline (PBS; BioWhittaker, Walkersville, MD) with 5% fetal calf serum, were stained with lineage cocktail (Lin 1–fluorescein isothiocyanate [FITC], containing antibodies against CD3, CD14, CD16, CD19, CD20, CD56; BD Biosciences, San Jose, CA), anti–HLA-DR PerCP (BD Biosciences), and anti-CD11c–phycoerythrin (PE) (Caltag, Burlingame, CA) or anti-CD123–PE (BD Biosciences). Murine immunoglobulins of appropriate isotypes were used as a control. Cells were incubated with antibodies for 30 minutes on ice in the dark, then washed twice with PBS containing 0.1% gelatin, resuspended in 1% paraformaldehyde, and analyzed. To analyze monocytes, PBMCs were stained with anti-CD14–PE (PharMingen, San Diego, CA).
Intracellular IL-12 staining was performed on whole blood from patients or healthy donors as described.31 32 Briefly, 1 mL blood, diluted 1:1 with RPMI 1640 medium, was cultured with fixedStaphylococcus aureus Cowan strain 1 (SAC), 1:10 000 wt/vol (Pansorbin; Calbiochem-Behring, La Jolla, CA) in the presence of Brefeldin A (10 μg/mL; Sigma, St Louis, MO) for 5.5 hours followed by 30 minutes of incubation on ice with 30 μL of 0.5 M EDTA (ethylenediaminetetraacetic acid) added to each sample. Aliquots of blood were then stained for 15 minutes in the dark with Lin 1–FITC, HLA-DR–PerCP, CD11c-APC (BD Biosciences), or appropriate isotype controls, treated with fixation medium A (Fix & Perm; Caltag) and permeabilization medium B (Caltag), stained with anti–IL-12 p40/p70–PE antibody (PharMingen), and followed by a wash with PBS. To analyze monocytes, blood samples were stained with anti-CD14–APC (BD Bioscience) and anti–IL-12 p40/p70–PE (PharMingen). To analyze CD80 expression on dendritic cells and monocytes (anti-CD80–PE; PharMingen), whole blood was stimulated with rCD40L (2 μg/mL; Immunex, Seattle, WA) in the absence of Brefeldin A for 8 hours followed by the staining procedure previously described. Cells were analyzed with the FACScalibur (Becton Dickinson) flow cytometer. We collected 150 000 and 50 000 events to analyze dendritic cells and monocytes, respectively, using CellQuest software (Becton Dickinson).
Cytokine assays
To induce cytokine production, PBMCs from patients and healthy donors were cultured in 48-well plates at a density of 1 × 106/mL per well in the presence of SAC, 14 HAU influenza virus (PR8), or allantoic fluid. Previously frozen PBMCs were cultured for 48 hours, and freshly isolated PBMCs were cultured for 24 hours. Supernatants were harvested and tested for the presence of IL-12 by a 2-sided radioimmunoassay, as described previously, with two antibody pairs provided by the Wistar Institute—C11.79 and C8.6 (sensitivity, 50-100 pg/mL) for IL-12 p40 and 20C2 and C8.6 (sensitivity, 10 pg/mL) for IL-12 p70.7 Recombinant IL-12, used as a standard, was kindly provided by Genetics Institute (Cambridge, MA). IFN-α was assayed through enzyme-linked immunosorbent assay (ELISA) using a kit from Endogen (Woburn, MA) according to the manufacturer's recommendations (sensitivity, 10 pg/mL). Recombinant IFN-α from R&D Systems (Minneapolis, MN) was used as a standard.
Statistical analysis
To determine statistical significance, percentages of dendritic cells and monocytes and levels of cytokines were compared between healthy donors and patients using the unpaired Student ttest. Level of significance assumed in these comparisons wasP < .05.
Results
CTCL patients demonstrate a stage-dependent decrease in numbers of circulating dendritic cells
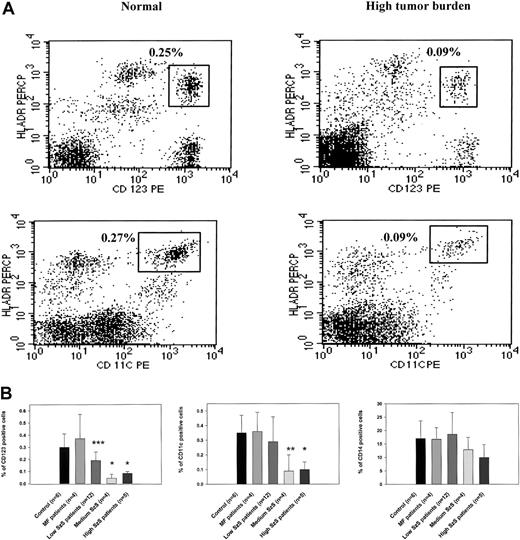
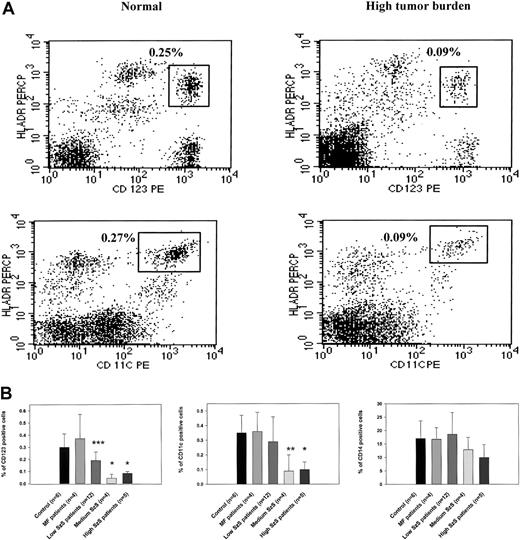
To study the mechanisms underlying decreased cell-mediated immunity and IL-12 production in patients with Sézary syndrome (SzS), we examined the cellular composition of CD11c+ and CD123+ dendritic cells. Flow cytometric analysis of PBMCs from patients with a high burden of malignant T cells in peripheral blood demonstrated significantly decreased numbers of CD123+ and CD11c+ dendritic cells than in healthy donors (Figure 1A). We examined the peripheral blood of healthy donors, patients with CTCL with skin disease but without overt involvement of the peripheral blood (mycosis fungoides), and patients with SzS determined to have low, medium, or high circulating burdens of malignant T cells for the presence of CD123+, CD11c+, and CD14+ cells. Although CTCL patients with skin-restricted disease were shown to have comparatively normal populations of dendritic cells and CD14+ monocytes, SzS patients were observed to have a decrease in circulating dendritic cells that correlated with the relative level of tumor burden (Figure 1B). The decrease in the CD123+ population of dendritic cells ranged from a 1.5-fold decrease among patients with a low tumor burden of circulating malignant T cells (P < .034) to a 3- to 6-fold decrease among patients with a medium or heavy circulating burden of malignant T cells, respectively (P < .002). Although CD11c+ dendritic cell levels were not significantly lower in the circulation of patients with low tumor burden (0.35% in control and 0.29% in patients), a 3-fold decrease was observed among patients with more advanced disease (0.09% among patients with medium tumor burden and 0.1% among patients with high tumor burden;P < .01 and P < .002, respectively). In contrast to significant decreases in peripheral blood dendritic cells among patients with SzS, circulating numbers of CD14+monocytes were observed to be comparable to normal among all patients with SzS, which is consistent with previous observations.7
CD123 and CD11c populations of dendritic cells, but not CD14 monocytes, are significantly depleted in patients with SzS.
(A) PBMCs from a healthy donor and an SzS patient with high tumor burden were excluded of all lineage-positive cells (cells stained with Lin 1–FITC cocktail, containing antibodies against CD3, CD14, CD16, CD19, CD20, CD56), whereas lineage-negative cells were analyzed for the coexpression HLA-DR and CD11c or CD123. The right upper quadrant numbers represent the percentage of dendritic cells expressing HLA-DR and CD11c or CD123. (B) PBMCs from age-matched healthy donors, mycosis fungoides patients, and SzS patients with different levels of tumor burden were analyzed for the presence of dendritic cells, as described in panel A. Monocytes were defined by the expression of CD14, and only CD14high cells were analyzed. Data represent means (±SD) of tested patients and are presented as a percentage of all PBMCs gated on forward and side light scatter. *P < .001; **P < .01; ***P < .05 compared with healthy donors. Control antibodies stained 0.1% of PBMCs.
CD123 and CD11c populations of dendritic cells, but not CD14 monocytes, are significantly depleted in patients with SzS.
(A) PBMCs from a healthy donor and an SzS patient with high tumor burden were excluded of all lineage-positive cells (cells stained with Lin 1–FITC cocktail, containing antibodies against CD3, CD14, CD16, CD19, CD20, CD56), whereas lineage-negative cells were analyzed for the coexpression HLA-DR and CD11c or CD123. The right upper quadrant numbers represent the percentage of dendritic cells expressing HLA-DR and CD11c or CD123. (B) PBMCs from age-matched healthy donors, mycosis fungoides patients, and SzS patients with different levels of tumor burden were analyzed for the presence of dendritic cells, as described in panel A. Monocytes were defined by the expression of CD14, and only CD14high cells were analyzed. Data represent means (±SD) of tested patients and are presented as a percentage of all PBMCs gated on forward and side light scatter. *P < .001; **P < .01; ***P < .05 compared with healthy donors. Control antibodies stained 0.1% of PBMCs.
Decreased number of peripheral blood dendritic cells in SzS correlates with impaired production of IL-12 and IFN
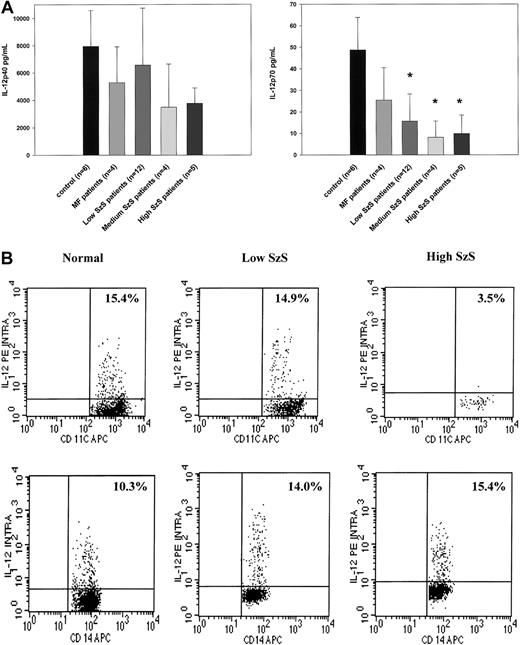
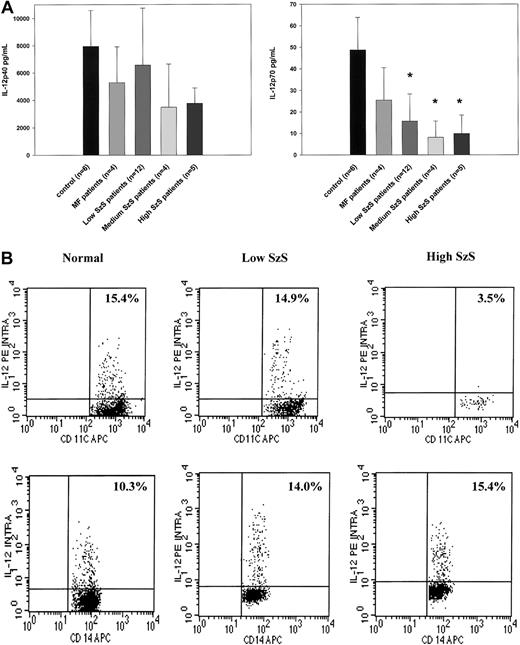
Because dendritic cells are critical producers of IL-12 and IFN-α, we examined whether decreased numbers of peripheral blood dendritic cells could be correlated with decreased production of these cytokines by PBMCs stimulated by SAC or influenza virus, respectively, in patients with SzS. PBMCs from patients with mycosis fungoides or SzS with different levels of peripheral blood tumor burden produced decreased levels of IL-12 p40 not statistically significantly different than those from healthy donors (Figure2A). In contrast, though the PBMCs of patients with mycosis fungoides produced levels of IL-12 p70 comparable to those of healthy donors, levels of PBMC production in SzS patients were profoundly decreased in relation to peripheral blood tumor burden (Figure 2A). Although the PBMCs of healthy controls on average produced a mean of 49 ± 15 pg/mL IL-12 p70 in response to SAC, the PBMCs of patients with low tumor burden produced a mean of 15.9 ± 12 pg/mL IL-12 p70, and the PBMCs of patients with medium and high tumor burden produced a mean of 8.1 ± 7 pg/mL and 9.9 ± 8 pg/mL, respectively (Figure 2A). Flow cytometric analysis of intracellular IL-12 production on whole blood from selected SzS patients further confirmed a marked reduction in the ability of CD11c+ dendritic cells from patients with high tumor burden to produce IL-12 in response to SAC (Figure 2B). The anti–IL-12 antibody used for intracellular staining does not distinguish between IL-12 p40 and IL-12 p70. In a representative experiment, 15.4% of CD11c+ cells from a healthy donor and 14.9% of CD11c+ cells from a patient with low tumor burden demonstrated intracellular IL-12, but only 3.5% of CD11c+ cells from a patient with high tumor burden were positive for intracellular IL-12 (Figure 2B). In contrast to a decrease in CD11c+ dendritic cell production of IL-12 among patients with SzS, a similar percentage of CD14+ cells in the blood of healthy donors and patients with SzS produced IL-12.
PBMCs from SzS patients exhibit the significantly decreased ability to produce IL-12 p70, whereas production of IL-12 p40 is not significantly impaired.
(A) PBMCs from healthy donors, mycosis fungoides patients, and SzS patients with different levels of tumor burden were stimulated with SAC for 48 hours. Cytokines were measured in cell-free supernatants by radioimmunoassay. Data represent means (±SD) of tested patients. *P < .001 compared with healthy donors. (B) Blood from healthy donors and from patients with low and high tumor burden was stimulated with SAC for 5.5 hours and then stained, and lineage-negative cells were analyzed for coexpression of HLA-DR/CD11c and IL-12 p40/p70 or CD14 and IL-12 p40/p70. Right upper quadrant numbers represent the percentages of double-positive cells.
PBMCs from SzS patients exhibit the significantly decreased ability to produce IL-12 p70, whereas production of IL-12 p40 is not significantly impaired.
(A) PBMCs from healthy donors, mycosis fungoides patients, and SzS patients with different levels of tumor burden were stimulated with SAC for 48 hours. Cytokines were measured in cell-free supernatants by radioimmunoassay. Data represent means (±SD) of tested patients. *P < .001 compared with healthy donors. (B) Blood from healthy donors and from patients with low and high tumor burden was stimulated with SAC for 5.5 hours and then stained, and lineage-negative cells were analyzed for coexpression of HLA-DR/CD11c and IL-12 p40/p70 or CD14 and IL-12 p40/p70. Right upper quadrant numbers represent the percentages of double-positive cells.
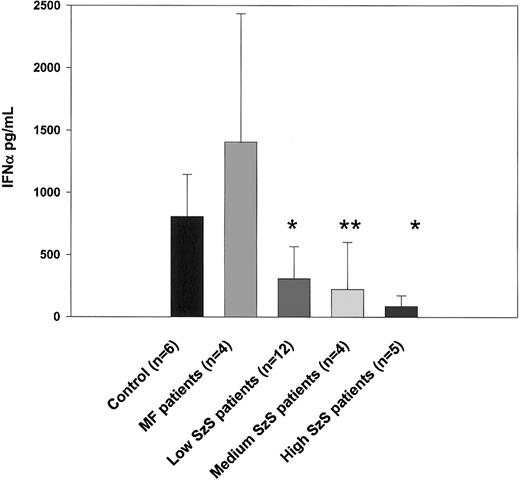
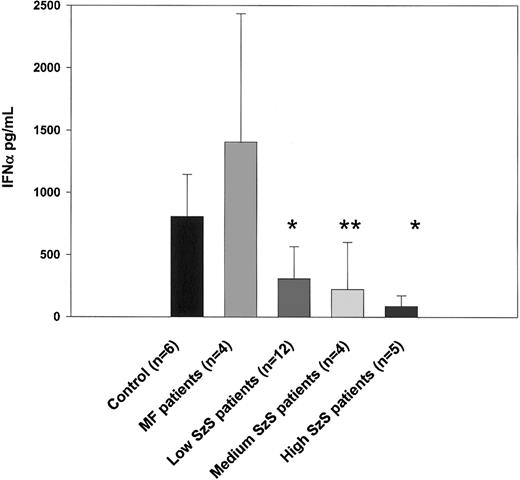
As shown in Figure 1, the number of IFN-α–producing CD123+ dendritic cells was significantly decreased in all 3 groups of patients with SzS (P < .05) but not in patients with mycosis fungoides. Consistent with this observation, IFN-α production induced by influenza virus was also profoundly impaired in patients with SzS but not in those with mycosis fungoides (Figure3). Compared with levels in healthy controls, 2.5- to 3.6-fold decreases in levels of IFN-α were observed using PBMCs from patients with low and medium tumor burden, and up to a 9.4-fold decrease was observed using the PBMCs of patients with high tumor burden. Allantoic fluid, used as a negative control, did not induce detectable levels of IFN-α (data not shown).
Decreased IFN-α production correlates with low numbers of CD123+ dendritic cells in the circulation.
PBMCs from healthy donors, mycosis fungoides patients, and SzS patients with different levels of tumor burden were stimulated with 14 HAU of influenza virus A/Puerto Rico/8/34 (PR8) or allantoic fluid (data not shown) for 48 hours. Cytokine levels were measured in cell-free supernatants by ELISA. Data represent means (±SD) of tested patients. *P < .001; **P < .01, compared with healthy donors.
Decreased IFN-α production correlates with low numbers of CD123+ dendritic cells in the circulation.
PBMCs from healthy donors, mycosis fungoides patients, and SzS patients with different levels of tumor burden were stimulated with 14 HAU of influenza virus A/Puerto Rico/8/34 (PR8) or allantoic fluid (data not shown) for 48 hours. Cytokine levels were measured in cell-free supernatants by ELISA. Data represent means (±SD) of tested patients. *P < .001; **P < .01, compared with healthy donors.
Intermittent GM-CSF treatment increases the number of peripheral blood dendritic cells in CTCL patients
Studies of animal tumor models have demonstrated that granulocyte macrophage–colony-stimulating factor (GM-CSF)–based therapy can augment cellular immunity that is dependent on CD4+ and CD8+ T cells.33 Furthermore, an important component of its effects is related to its ability to promote the differentiation of hematopoietic precursors to dendritic cells.34 Based on these results, intermittent GM-CSF therapy was introduced to treat some of our patients with SzS. All SzS patients received identical therapy in the form of extracorporeal photopheresis, with 2 consecutive daily treatments administered every 3 to 4 weeks.30 Seven patients with SzS received GM-CSF (Leukine; Immunex) in a dose of 125 μg administered subcutaneously after each photopheresis treatment for a period of at least 6 months. To determine the effect of GM-CSF on the number and function of peripheral blood dendritic cells, cryopreserved PBMCs from patients before and after the initiation of GM-CSF treatment were analyzed for the presence of dendritic cell populations and the ability to produce IL-12 and IFN-α. All 7 SzS patients demonstrated significant increases in peripheral blood dendritic cell numbers during GM-CSF treatment, though increases in dendritic cell numbers occurred predominantly within the CD123+ population. Increases in CD11c+ cells were also observed among patients treated with GM-CSF, but these changes were not statistically significant (Table 1). With the exception of SzS patient 7, who manifested a low circulating burden of malignant T cells and whose number of peripheral blood dendritic cells were within the normal range at the start of GM-CSF treatment, the remaining SzS patients treated with GM-CSF were determined to have either a medium or a high tumor burden along with a decreased number of dendritic cells. GM-CSF treatment of patients with medium and high tumor burden was associated with significant increases in CD123+ cells, with an average increase of 2.3-fold. However, intermittent GM-CSF treatment failed to elevate the percentages of CD123+ cells in patients to levels observed in healthy donors. In patient 7, GM-CSF treatment also increased the number of CD123+ cells by 2.4-fold, from 0.34% of total PBMCs before treatment to 0.84% on the completion of treatment.
Although 4 patients experienced a 2-fold increase in CD11c+ cell numbers, overall this increase was not significant. Despite observing increases in the number of dendritic cells during GM-CSF treatment, the PBMCs of such patients did not manifest an increased ability to produce IL-12 or IFN-α in response to SAC or influenza virus during the period of GM-CSF therapy (data not shown).
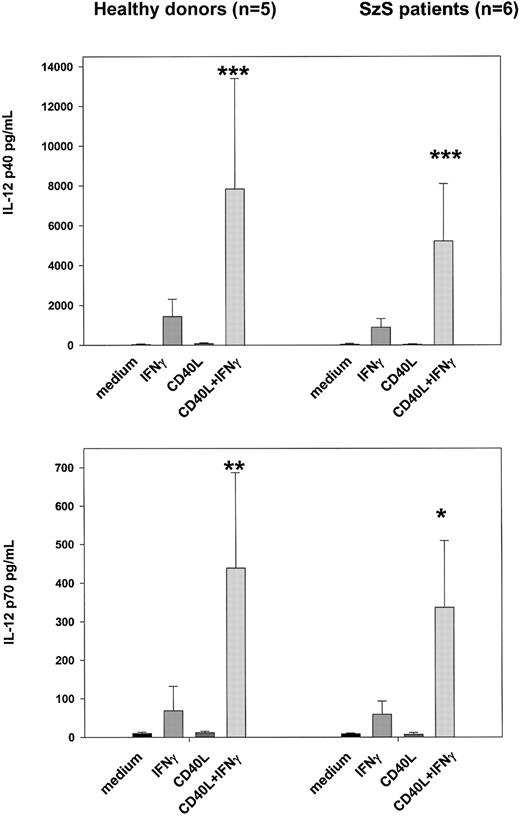
Recombinant CD40L in vitro partially restores IL-12 production by dendritic cells and monocytes of SzS patients
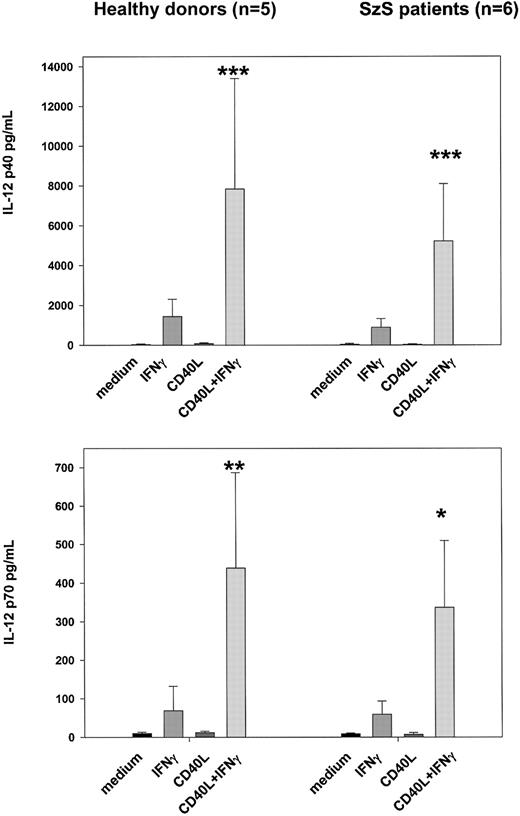
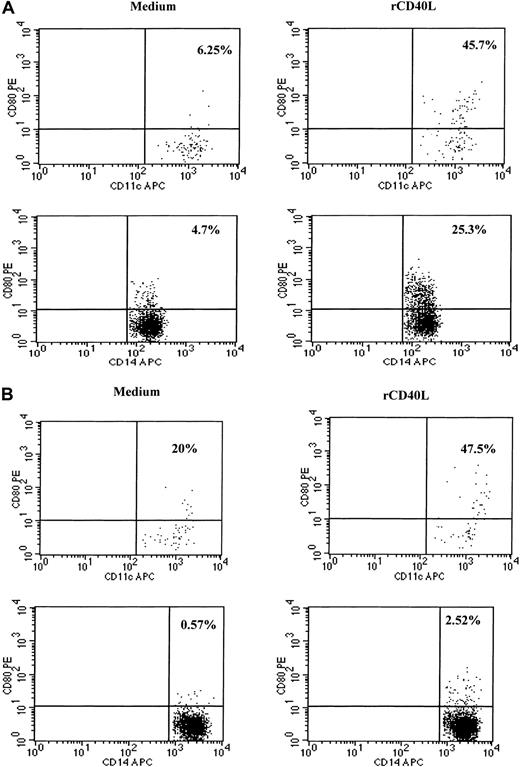
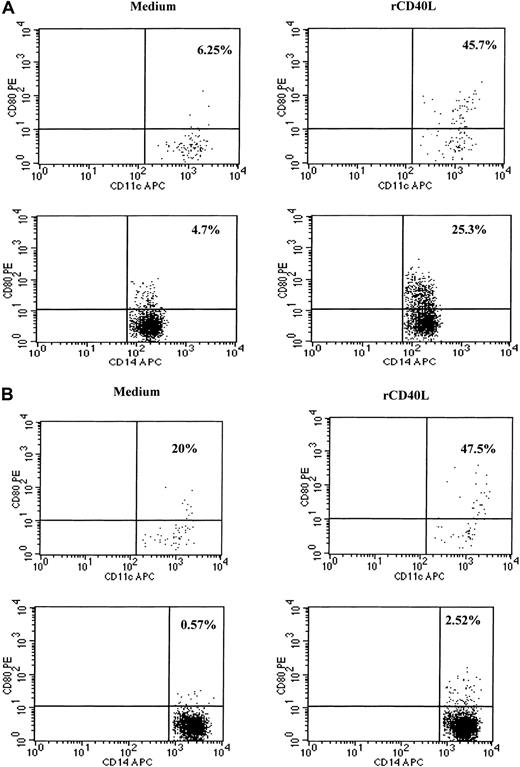
Because CD40–CD40L interactions are known to play an important role in the activation of dendritic cells and in the induction of IL-12 production, we examined whether recombinant CD40L in vitro could stimulate the PBMCs of SzS patients to produce IL-12.9,11-15 23 Following the culture of patient PBMCs with CD40L plus recombinant IFN-γ for 24 hours, high levels of IL-12 p40 and p70 were detected in culture supernatants of patients with low, medium, and high tumor burden (Figure 4). CD40L alone was not capable of inducing the production of IL-12 in PBMCs of healthy donors or SzS patients; however, IFN-γ induced significant levels of IL-12 p40 and IL-12 p70 by the PBMCs of healthy donors and SzS patients. Importantly, the combination of CD40L plus IFN-γ worked synergistically, resulting in a 2- to 10-fold increase in IL-12 production compared with IFN-γ alone. Furthermore, evidence that CD40L can activate CD11c+ dendritic cells in SzS patients was provided following the stimulation of patient PBMCs with rCD40L for 8 hours, which resulted in the significant up-regulation of CD80 cell surface expression (Figure5A,B). A lesser degree of CD80 up-regulation was observed on patients CD14+ monocytes following 8-hour culture with CD40L.
rCD40L synergizes with IFN-γ to induce production of IL-12 by PBMCs from healthy donors and SzS patients.
PBMCs from the 5 healthy donors and 6 patients with different tumor loads were stimulated with rCD40L (2 μg/mL) with or without IFN-γ (1000 U/mL) for 18 hours. IL-12 p40 and IL-12 p70 were measured in cell-free supernatants by radioimmunoassay. Data represent means (±SD) of tested persons. *P < .001; **P < .01; ***P < .05 compared with responses induced by IFN-γ alone.
rCD40L synergizes with IFN-γ to induce production of IL-12 by PBMCs from healthy donors and SzS patients.
PBMCs from the 5 healthy donors and 6 patients with different tumor loads were stimulated with rCD40L (2 μg/mL) with or without IFN-γ (1000 U/mL) for 18 hours. IL-12 p40 and IL-12 p70 were measured in cell-free supernatants by radioimmunoassay. Data represent means (±SD) of tested persons. *P < .001; **P < .01; ***P < .05 compared with responses induced by IFN-γ alone.
rCD40L induces activation of dendritic cells and monocytes as evidenced by up-regulation of CD80 molecules.
Blood from a healthy donor (A) and patient with low tumor burden (B) was stimulated with medium or rCD40L for 8 hours and then stained, and lineage-negative cells were analyzed for the coexpression of HLA-DR/CD11c and CD80 or CD14 and CD80. Right upper quadrant numbers represent the percentages of double-positive cells.
rCD40L induces activation of dendritic cells and monocytes as evidenced by up-regulation of CD80 molecules.
Blood from a healthy donor (A) and patient with low tumor burden (B) was stimulated with medium or rCD40L for 8 hours and then stained, and lineage-negative cells were analyzed for the coexpression of HLA-DR/CD11c and CD80 or CD14 and CD80. Right upper quadrant numbers represent the percentages of double-positive cells.
Discussion
Patients with Sézary syndrome demonstrate a stage-related impairment of cell-mediated immunity, including a decrease in IFN-γ production and NK activity. We have previously reported that the decreased ability of SzS patients to produce IL-12, a cytokine critically linked with TH1 responses and cell-mediated immunity, contributes to this impairment.7 Here, we demonstrate that deficient IL-12 production and stage-related depression in cell-mediated immunity is associated with a decreased number of dendritic cells in the peripheral blood of SzS patients. A particularly marked decrease in dendritic cell numbers was observed among patients with medium and high tumor burden. Both populations of dendritic cells (CD123+ and CD11c+) were affected, but the CD123+population of IFN-α–producing dendritic cells was also significantly decreased in the peripheral blood of SzS patients with low tumor burden. The mechanisms underlying the decrease in dendritic cells in SzS patients remain unclear. A progressive loss of IFN-α–producing cells has also been observed in patients with other hematologic disorders, including hairy cell leukemia and idiopathic CD4 lymphopenia, and in patients with HIV infection, though in HIV this decrease could be related to a loss of dendritic cells from viral infection.35 36
It remains to be determined whether a diminution in dendritic cell numbers in the circulation reflects elimination of these cells or a different pattern of dendritic cell trafficking in SzS patients. Contrary to dendritic cell populations, the number of CD14+monocytes was not significantly decreased in the blood of patients with advanced CTCL in comparison with those in healthy age-matched donors.
An examination of IL-12 production by the PBMCs of our SzS patients revealed that the levels of IL-12 p40 detected in culture supernatants, though decreased 3-fold in some of the patients with medium and high tumor burden, were on average similar in healthy donors and patients. This observation suggests that most IL-12 p40 may be produced by monocytes because dendritic cell numbers were significantly reduced. Furthermore, intracellular staining for IL-12 induced by SAC demonstrated almost no difference between the monocytes of healthy donors and patients. Nevertheless, the percentage of CD11c+dendritic cells producing IL-12 p40 was lower in patients, proportional to an overall lower number of dendritic cells.
In contrast to the near normal findings among SzS patients with regard to IL-12 p40 production, the production of biologically active IL-12 p70 was profoundly decreased by the PBMCs of all patients with SzS, even among those with a low tumor burden, suggesting that SzS patients exhibit a marked defect in forming IL-12 p70. Unless IFN-γ was used in culture, PBMCs from SzS patients were unable to produce IL-12 p70, whereas healthy donors under the same conditions of stimulation produced IL-12 p70. These results suggest that decreased endogenous IFN-γ may also be a factor responsible for defects in the production of biologically active IL-12 and that the addition of exogenous IFN-γ may at least partially contribute to the restoration of IL-12 p70 production. The enhancing effect of IFN-γ on IL-12 p40 and p35 and the resultant IL-12 p70 production has been previously demonstrated.37-40 The positive-feedback up-regulation of IL-12 production mediated by IFN-γ may be blunted in the PBMCs of SzS patients for a number of reasons. Decreased expression of the β2 signaling receptor for IL-12 on the normal T cells of SzS patients and its complete absence on malignant T cells results in a lack of IL-12–dependent IFN-γ production, particularly among patients with medium and heavy tumor burden.41,42 Furthermore, our previous observation that the T cells of patients with SzS are unable to up-regulate CD40L (L.E.F., manuscript in preparation) suggests a disruption of IL-12–dependent and IL-12–independent IFN-γ production stimulated by the CD40–CD40L interaction.43 In addition, a recent report suggests that human dendritic cells are able to secrete IFN-γ that might provide an early stimulus to increase IL-12 production.44 However, it is entirely possible that this pathway may also be impaired in SzS patients because of decreased numbers of peripheral blood dendritic cells.
The decrease in peripheral blood CD123+ dendritic cells correlates with the production of significantly lower levels of IFN-α by the PBMCs of SzS patients. IFN-α not only plays an essential role in innate immunity against viruses by directly inhibiting viral replication in infected cells, it also has pleiotropic effects on the immune system, including up-regulation of major histocompatibility complex class 1 molecules, activation of macrophages and NK cells, and increasing NK antitumor immunity, and it contributes to the survival of activated T cells.35,45-47 It has been shown that, as with IL-12, IFN-α induces tyrosine and serine phosphorylation of Stat-4 in activated human T cells and in the human NK cell line.48This was the first indication that IFN-α, by mimicking some of the signal transduction pathways of IL-12, may be able to trigger TH1 cell differentiation. Furthermore, it has recently been shown that plasmacytoid dendritic cells, major producers of IFN-α, are able to drive IFN-γ–dependent differentiation of T cells toward TH1 responses.49
Clearly, IFN-α plays a profound role in the support of effective cell-mediated immune responses. Its known immune-enhancing activity has been the rationale for its use as therapy for SzS. Although it is generally unknown whether SzS patients are deficient in IFN-α production, most IFN-α–treated patients experience significant clinical responses.50 Thus, the ability to restore deficient IFN-α production by the exogenous administration of this cytokine represents an important therapeutic advance for these patients.
Like IFN-α, GM-CSF is recognized for its immunomodulatory effects. Importantly, GM-CSF is also known for its ability to expand and maturate dendritic cells in vitro and in vivo.51,52Numerous studies have confirmed that dendritic cells may develop from human adherent CD14+ blood monocytes cultured with GM-CSF and IL-4.53,54 Thus, our analysis of dendritic cell populations from GM-CSF–treated patients with medium and high tumor burden showed significantly increased numbers of CD123+cells during treatment. Because CD123+ cells lack responsiveness to GM-CSF, it is unlikely that GM-CSF therapy has directly expanded this cell population. Rather, as recent data suggest, GM-CSF stimulates differentiation of CD34+ progenitors into immature CD123+ cells unable to produce IFN-α.55 56 Thus, among our patients, despite the GM-CSF–associated expansion of this population of dendritic cells, IFN-α production by patient PBMCs in response to viral stimulation did not increase, suggesting that this treatment alone may not be sufficient to induce functional maturation of dendritic cells in vivo. It is noteworthy, however, that several of the GM-CSF–treated patients did experience significant improvement in skin lesions and numbers of circulating malignant T cells during this treatment (A.H.R., unpublished observations, October 2000–September 2002).
We have also shown that exogenously added IL-12 improves the cell-mediated immune responses of SzS patients.7 Phase 1 trials of the use of recombinant IL-12 in CTCL have confirmed this observation in vivo and have indicated that IL-12 may have substantial clinical benefit for this patient population.57 Thus, enhancing the ability of SzS patients to secrete endogenous IL-12 is an important goal of anti-CTCL therapy. Although CD40L expression is impaired on the T cells of SzS patients, CD40 expression seems to be comparable to that on normal dendritic cells and monocytes (L.E.F., manuscript in preparation). Therefore, when rCD40L in combination with IFN-γ is added to the PBMCs of SzS patients, significant levels of IL-12 are released into the supernatant. Our results suggest that treatment with rCD40L may enhance immune function and the antitumor responses of SzS patients by inducing endogenous IL-12. Additional benefit may result from the ability of CD40 ligation to promote differentiation of adherent blood monocytes into functional dendritic cells.51
All participating patients in this study were treated with extracorporeal photopheresis (ECP). In this regard, it is important to stress that Berger et al58 have demonstrated the potent capacity of ECP treatment to rapidly induce the differentiation of monocytes into large numbers of functional dendritic cells. This effect likely represents one of the most important mechanistic aspects of ECP therapy; the end result is the enhanced processing of apoptotic malignant T cells by the expanded dendritic cell population, leading to a more efficient “immunization” process against the tumor cells. Although our patients did not experience significant changes in dendritic cell numbers while they were observed (data not shown), it is entirely possible that long-term treatment with ECP prevented a further disease-associated decline in total dendritic cell numbers.
In summary, in this report we show that impaired cell-mediated immunity of SzS patients may result from decreased numbers of dendritic cells and a marked defect in the production of IL-12 p70 and IFN-α, cytokines critically linked with NK activation and TH1 differentiation. The remaining dendritic cells and monocytes of SzS patients are able to produce IL-12 if properly stimulated, though IL-12 p70 production depended on the presence of IFN-γ To improve the immunological status of patients with SzS, future therapies should consider treatments that increase the number of dendritic cells, their rate of maturation, and their production of cytokines that support cell-mediated immunity.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-01-0231.
Supported in part by a grant from the Leukemia and Lymphoma Society and by grants CA 10815 (A.H.R.) and CA 20833 (L.M.) from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alain H. Rook, Department of Dermatology, University of Pennsylvania, 3600 Spruce St, Philadelphia, PA 19104; e-mail: arook@mail.med.upenn.edu.