Abstract
The interleukin 6/glycoprotein 130/signal transducer and activator of transcription 3 (IL-6/gp130/STAT3) pathway has been reported to play an important role in the pathogenesis of multiple myeloma (MM) and for survival of MM cells. However, most data concerning the role of IL-6 and IL-6–triggered signaling pathways were obtained from experiments performed with MM cell lines and without considering the bone marrow microenvironment. Thus, the precise role of IL-6 and its intracellular signaling pathways for survival of human MM cells is still unclear. Here we show that treatment of human MM cells (IL-6–dependent MM cell line INA-6 and primary MM cells) with the IL-6 receptor antagonist Sant7 or with an anti-gp130 monoclonal antibody (mAb) induced apoptosis if the cells were cultured in the absence of bone marrow stromal cells (BMSCs). In contrast, apoptosis could not be observed if the MM cells were cocultured with BMSCs. The analysis of intracellular pathways revealed that Sant7 and anti-gp130 mAb were effectively inhibiting the phosphorylation of gp130 and STAT3 in the absence and presence of BMSCs, whereas ERK1 and ERK2 (ERK1,2) phosphorylation was only slightly affected. In contrast, treatment with the farnesyl transferase inhibitor, FPT III, induced apoptosis in MM cells in the absence or presence of BMSCs and led to a complete inhibition of the Ras/mitogen-activated protein kinase pathway. These observations indicate that the IL-6/gp130/STAT3 pathway is not essential for survival of human myeloma cells if they are grown in the presence of cells from the bone marrow microenvironment. Furthermore, we provide evidence that farnesyl transferase inhibitors might be useful for the development of novel therapeutic strategies for the treatment of MM.
Introduction
Increasing evidence suggests that the bone marrow microenvironment contributes substantially to the malignant growth and survival of multiple myeloma (MM) cells.1,2 Consistent with this hypothesis it has been found that bone marrow stromal cells (BMSCs), in particular, provide a number of factors that might contribute to the growth of myeloma cells.3,4 One of the best characterized BMSC-derived cytokines is interleukin 6 (IL-6), which is thought to play a central role in the pathogenesis of MM. The following observations led to the hypothesis that IL-6 is important and perhaps even essential for growth and survival of MM cells: (1) in a plasmacytoma mouse model IL-6 knock-out mice do not develop plasma cell tumors5; (2) growth of myeloma cell lines is often IL-6 dependent6,7; and (3) primary human myeloma cells can be cultivated for several days in vitro in the presence of IL-6.4 8
IL-6 can trigger at least 2 intracellular signaling pathways: the JAK/signal transducer and activator of transcription 3 (STAT3)9 and the Ras/mitogen-activated protein kinase (MAPK) pathway.10 Binding of IL-6 to the IL-6 receptor (IL-6R; glycoprotein 80 [gp80]) induces binding to and subsequent dimerization of gp130,11 the signal transducing part of the IL-6R complex. On dimerization, gp130 becomes activated and phosphorylated.11 STAT3 can bind to phosphorylated gp130 and is subsequently phosphorylated by JAKs.12 On phosphorylation, STAT3 dimerizes and translocates into the nucleus where it acts as a transcription factor for IL-6–dependent promoters.13 In addition to the activation of the JAK/STAT3 pathway, phosphorylated gp130 might lead to Ras activation, which induces MAPK–ERK1 and ERK2 (ERK1,2) phosphorylation via Raf-1 and MEK14 15 (Ras/MAPK signaling pathway).
Activation of the Ras/MAPK signaling cascade by IL-6 correlates with the proliferative response of MM cells to IL-6. Accordingly, treatment with MAPK antisense oligonucleotides prevents the proliferative effect of IL-6.16
It has been shown recently that STAT3 is constitutively phosphorylated in MM cells, and it has been demonstrated that the inhibition of the IL-6/gp130/STAT3 pathway by IL-6R antagonists, JAK inhibitors, or expression of a dominant-negative STAT3 mutant can induce apoptosis in human MM cell lines in vitro.17-19Therefore, it has been speculated that the IL-6/gp130/STAT3 pathway provides an important antiapoptotic signal in myeloma cells and could be an interesting target for the development of novel therapeutic strategies for this still incurable disease. However, most data concerning the role of IL-6–triggered signaling pathways for the survival and proliferation of human MM cells were obtained through experiments performed with cell lines and without considering the bone marrow microenvironment. Thus, the question of how crucial IL-6, the STAT3 pathway, and the Ras/MAPK pathway are for the survival and malignant growth of MM cells is still unanswered. Consequently, the question of whether targeting of these pathways could be an effective therapeutic strategy is still unanswered as well.
To address these questions we treated human MM cells with different inhibitors of the IL-6/gp130/STAT3 and the Ras pathway either in the presence or in the absence of BMSCs. We could show that inhibition of the IL-6/gp130/STAT3 pathway induced apoptosis in the absence of BMSCs, whereas no induction of apoptosis was observed in the presence of BMSCs. In contrast, inhibition of Ras activation by treatment with the farnesyl transferase inhibitor (FTI) FPT III led to induction of apoptosis in MM cell lines and primary MM cells even in the presence of BMSCs.
Materials and methods
Cell lines
The IL-6–dependent human MM cell line INA-6 was recently described as a model for cytokine-regulated plasmacytoma.7INA-6 cells were kept at 37°C and 5% CO2 in RPMI 1640 (Seromed-Biochrom, Berlin, Germany), supplemented with 20% (vol/vol) heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM sodium pyruvate, 2 mM glutamine (all from Life Technologies, Karlsruhe, Germany) and 2 ng/mL IL-6. The MM cell line U266 and the Burkitt lymphoma cell line Namalwa were kept at 37°C and 5% CO2 in RPMI 1640, supplemented with 10% (vol/vol) heat-inactivated FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM sodium pyruvate, 2 mM glutamine and without addition of IL-6.
Enrichment of primary MM cells
Isolation of MM cells was performed as described previously.20 In brief, mononuclear cells from bone marrow aspirates of 18 different MM patients were separated by Ficoll-Hypaque density gradient centrifugation, labeled with anti-CD138 magnetic beads, and separated using magnetic-activated cell sorting (MACS) columns. Primary MM cells were cultured in RPMI 1640 supplemented with 20% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM sodium pyruvate, 2 mM glutamine and, in the absence of BMSCs, 2 ng/ml IL-6. Cytospins of enriched cells were subjected to Pappenheim stain and controlled by morphology. In every sample at least 90% purity could be achieved. Samples were taken from routine diagnostic specimens after informed consent of patients was obtained and permission was granted by the local ethics committee.
Preparation of BMSCs
Primary human BMSCs were isolated as described previously.20 21 Briefly, mononuclear cells from bone marrow aspirates from patients with acute myeloid leukemia (BMSC#1), Hodgkin disease (BMSC#2), and MM (BMSC#3 + #4) were separated by Ficoll-Hypaque density gradient sedimentation. Adherent cells were long-term cultured and expanded in Dulbecco modified Eagle medium (DMEM; Seromed-Biochrom), supplemented with 20% (vol/vol) heat-inactivated FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM sodium pyruvate, and 2 mM glutamine at 37°C and 5% CO2. For coculture assays BMSCs were used within 2 to 15 weeks. Samples were taken from routine diagnostic specimens after informed consent of patients was obtained and permission was granted by the local ethics committee.
Cocultures
Cocultures were performed as described before.20Briefly, 1 × 104 BMSCs were seeded and cultured in a 24-well plate overnight prior the addition of 1 × 105 MM cells. Cells from patient 1 were cocultivated with BMSC#4 (Pat#1/BMSC#4). Other combinations were: Pat#2/BMSC#2, Pat#3/BMSC#3, and Pat#4/BMSC#2. Additionally, MM cells from 14 patients were cocultivated with BMSCs: cells from 4 patients were cocultivated with BMSC#1, from 5 patients with BMSC#2, and from 5 patients with BMSC#3 (data not shown). Three independent experiments were performed with INA-6 cocultured with BMSC#1, BMSC#2, and BMSC#3. Once MM cells were attached to the stromal cell layer the inhibitors (Sant7, anti-gp130 monoclonal antibody [mAb], FPT III) were added. After 3 days (INA-6) or 1 week (primary MM cells) of treatment with inhibitors, the MM cells were detached for apoptosis analysis by rinsing the culture dishes with RPMI 1640 medium. To analyze the effects on signal transduction, the same procedure was performed to harvest INA-6 cells after 12 hours of treatment with the inhibitors. To quantify contamination of MM cell samples with BMSCs, and to assess phagocytosis of MM cells by BMSCs, the MM cells were stained with PKH26 red (Sigma, Taufkirchen, Germany) and the BMSCs with CFDA-SE green (Molecular Probes, Leiden, The Netherlands), measured on a FACSCalibur flow cytometer and analyzed with CELLQuest software (Becton Dickinson, Heidelberg, Germany; data not shown). After 3 days of INA-6/BMSC#2 coculture and 1 week of Pat#1/BMSC#4 coculture, the MM cells were detached from the stromal cell layer without a detectable contamination with BMSCs. Also, phagocytosis of MM cells by BMSCs was not observed.
Detection of apoptosis
After 3 days (cell lines) or 1 week (primary MM cells) of treatment with either Sant7, anti-gp130 mAb (INA-6, primary MM cells), or FPT III (INA-6, U266, Namalwa, primary MM cells), apoptosis was determined by flow cytometry analysis. Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining was performed according to the manufacturer's instructions (Bender Medsystems, Vienna, Austria). Then, 1 × 105 MM cells were stained with 2.5 μL annexin V-FITC and 5 μg/mL PI, measured on a FACSCalibur flow cytometer, and analyzed with CELLQuest software (Becton Dickinson). Early apoptosis is characterized by a positive annexin V-FITC staining. Cells in a late apoptotic stage lose their membrane integrity and additionally incorporate PI. Viable cells are negative for both annexin V-FITC and PI. Untreated cells served as controls. In the untreated controls (with or without BMSCs) numbers of viable MM cells were nearly identical.
Western blot analysis
The MM cells were cultured with inhibitors (Sant7, anti-gp130 mAb, or FPT III) for 12 hours and lysed in 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.9), 350 mM NaCl, 1 mM MgCl2, 0.5 mM EDTA (ethylenediaminetetraacetic acid), 0.1 mM EGTA (ethyleneglycoltetraacetic acid), 1% NP40, 0.5 mM dithiothreitol (DTT), 50 mM NaF, 1 mM Na3VO4, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), and 1 μg/mL aprotinin. The lysates were cleared by centrifugation, their protein content was measured by Bradford assay (Bio-Rad, München, Germany), and they were subjected to sodium dodecyl sulfate (SDS) gel electrophoresis. Proteins were transferred onto nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany) and the blots were incubated with antibodies specific for Tyr705-phosphorylated STAT3, for Thr202/Tyr204-phosphorylated ERK1,2, or Ser473-phosphorylated Akt according to standard procedures. For detection a secondary antirabbit horseradish peroxidase (HRP)–conjugated antibody and an enhanced chemoluminescence detection system (ECL; Amersham, Freiburg, Germany) were used. Equal loading was controlled using nonphosphospecific antibodies against STAT3, ERK1,2 or Akt. The same secondary antirabbit-HRP antibody and ECL system were used for detection.
EMSA
Preparation of nuclear protein extracts and electrophoretic mobility shift assays (EMSAs) were performed as previously described.17 Briefly, INA-6 cells were cultivated either with BMSCs or IL-6 (2 ng/mL) with or without Sant7 overnight. Cells were lysed and the nuclei were pelleted. The pelleted nuclei were incubated in 20 mM HEPES (pH 7.9), 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.2 mM PMSF, and 1μg/mL aprotinin. EMSAs were performed with the double-stranded 32P-labeled oligonucleotide sie (sis inducible element) probe (5′-AGCTTCATTTCCCGTAAATCCCTA-3′), which is derived from the c-fos gene promoter and contains a consensus binding site for STAT3,17 or with an Oct-1 probe (5′-GATCCAAGGGCTGGGGATTCCCCATCTCCACAGG-3′) as control.22 Protein-DNA complexes were subjected to a 5% nondenaturating polyacrylamide gel electrophoresis (PAGE) and analyzed by autoradiography. Supershift assays were performed using a rabbit polyclonal antibody against STAT3. For competition, nuclear extracts were incubated with a 50-fold molar excess of unlabeled oligonucleotide probe.
Immunoprecipitation
INA-6 cells were starved for 2 hours and cultivated either with BMSCs or IL-6 (2 ng/mL) with or without Sant7 overnight. The cells were pelleted and lysed in 50 mM Tris (tris(hydroxymethyl)aminomethane)/HCl (pH 7.5), 100 mM NaCl, 50 mM NaF, 3 mM Na3VO4, 1% Brij96, 1 mM PMSF, 1 μg/mL aprotinin, and 1 μg/mL leupeptin. Preclearing of the lysate was carried out by adding 10 μL normal rabbit serum. After 1 hour, nonspecific immune complexes were removed with protein A Sepharose CL-4B (Amersham). The supernatants were then incubated with a rabbit anti-gp130 antibody at 4°C overnight prior to adding protein A Sepharose. After 4 hours the immunoprecipitates were washed, incubated with SDS sample buffer at 98°C, subjected to SDS gel electrophoresis, and transferred to nitrocellulose membrane. Phosphorylation of gp130 was detected by staining with a mouse antiphosphotyrosine primary antibody and an antimouse-HRP secondary antibody.
Ras membrane translocation assay
Membrane protein preparations were performed as previously described.23 INA-6 and U266 cells were incubated with or without FPT III for 12 hours, washed with ice-cold PBS, and lysed for 30 minutes in a hypotonic buffer containing 10 mM Tris-HCl (pH 8.0), 0.1 mM DTT, and 1 mM PMSF. The cell membranes were disrupted in a Dounce tissue grinder. After pelleting the nuclei, the supernatant was centrifuged at 100 000g to pellet the membranes. The pellet was dissolved in buffer containing 1% NP40, 1 mM PMSF, and Laemmli sample buffer. SDS-PAGE was performed and the proteins blotted onto nitrocellulose filters. Membrane-associated Ras protein was detected using a monoclonal anti-Ras antibody.24 To control that FPT III specifically targets only the farnesylation process, the effect of FPT III on nonfarnesylated proteins was analyzed by detection of the major histocompatibility complex (MHC; class I) using a mouse monoclonal anti-MHC primary antibody (HC-10).
Ras activation assay
The Ras activation assay was performed as previously described.25 Briefly, INA-6 and U266 cells were lysed in 125 mM HEPES (pH 7.5), 750 mM NaCl, 5% Igepal CA-630, 50 mM MgCl2, 5 mM EDTA, and 10% glycerol. Ras protein was precipitated with Raf-1 RBD, an agarose-bound GST fusion protein corresponding to the human Ras-binding domain (residues 1-149) of Raf-1 (Upstate Biotechnology, Lake Placid, NY). SDS-PAGE was performed, and the proteins were transferred onto nitrocellulose and incubated with a monoclonal anti-Ras antibody (clone RAS10, Upstate Biotechnology). Incubation with a secondary antimouse HRP-conjugated antibody and the ECL system were used for detection.
Antibodies and reagents
Rabbit phosphospecific and nonphosphospecific anti-STAT3, anti-ERK1,2 and anti-Akt antibodies were purchased from Cell Signaling Technologies (Beverly, MA); antirabbit-HRP and antimouse-HRP antibodies, rabbit anti-gp130 antibody, and antiphosphotyrosine-specific antibody were purchased from Santa Cruz Biotechnology (Heidelberg, Germany). The mouse monoclonal anti-Ras antibody that was used for membrane-bound Ras detection was purchased from Transduction Laboratories (Lexington, KY), and the mouse monoclonal anti-Ras antibody (clone RAS10) was purchased from Upstate Biotechnology. The mouse monoclonal anti-MHC (class I) antibody was a kind gift from Dr Armin Rehm (Max-Delbrück-Center for Molecular Medicine, Berlin). Recombinant human IL-6 and the recombinant IL-6R antagonist Sant719,26,27 were produced inEscherichia coli. The anti-gp130 mAb (clone IKR3) was purchased from Immunokontact (Wiesbaden, Germany). This antibody has been shown to block the activity of IL-6, IL-11, ciliary neurotrophic factor (CNTF), and oncostatin M (OSM) as well as the binding of CNTF and OSM to gp130.28 The anti-CD138 magnetic beads and cell separation columns were purchased from Miltenyi Biotech (Bergisch Gladbach, Germany). FTI FPT III7 was purchased from Calbiochem (Bad Soden, Germany).
Results
BMSCs protect human MM cells from apoptosis induced either by the IL-6R antagonist Sant7 or by an anti-gp130 mAb
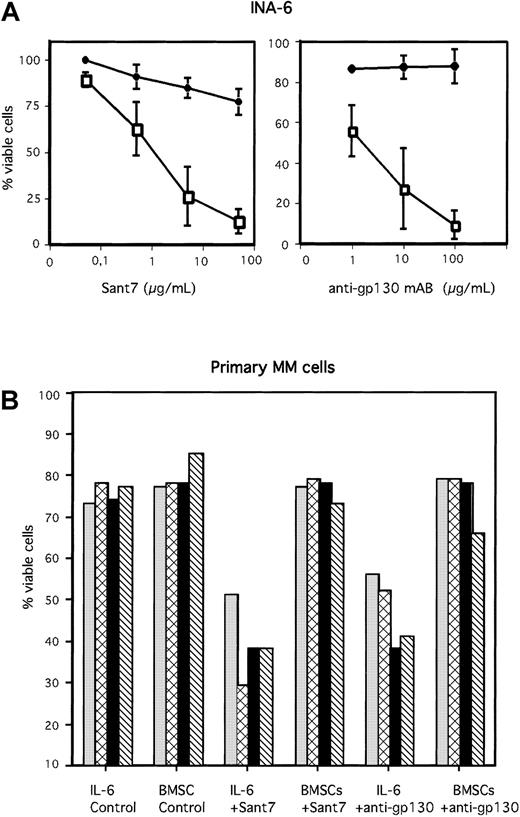
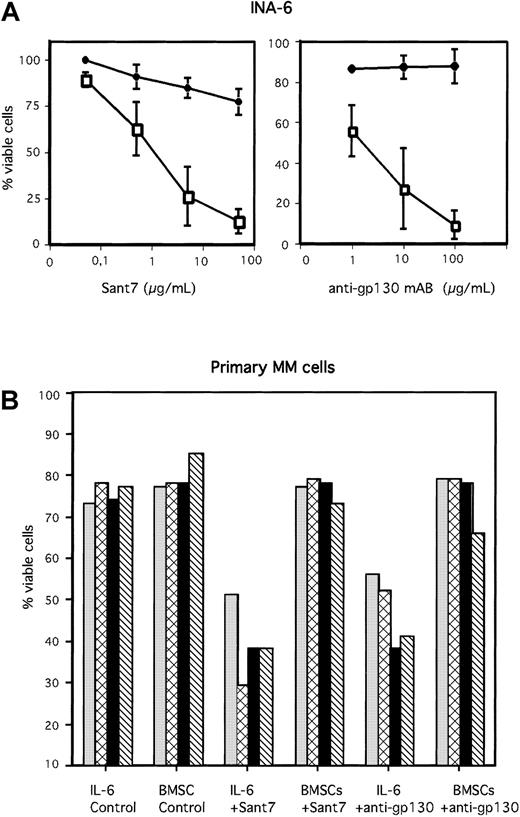
The IL-6R antagonist Sant7 was generated by mutating IL-6 at the predicted sites of receptor interaction. It is a highly specific receptor antagonist that is able to completely abrogate association of the IL-6/IL-6R/gp130 complex. The binding of IL-6 to the IL-6R is inhibited due to a 70-fold higher binding affinity of Sant7 for the receptor. The binding of IL-6R to gp130 and the subsequent dimerization of gp130 is completely inhibited.19,26 27 We analyzed the IL-6–dependent human MM cell line INA-6 as well as primary human MM cells either in the absence or in the presence of viable primary human BMSCs. MM cells were treated with Sant7 or with a blocking anti-gp130 mAb. In the absence of BMSCs nearly 100% of INA-6 cells died after 3 days of IL-6 inhibition due to Sant7 (50 μg/mL) or anti-gp130 mAb (100 μg/mL) treatment (Figure 1A). Of the primary MM cells freshly isolated from bone marrow aspirates of 4 different patients, about 50% underwent apoptosis after treatment with high concentrations of Sant7 (50 μg/mL) or an anti-gp130 mAb (100 μg/mL) if cells were grown in the absence of BMSCs (Figure 1B). In contrast, if cocultured with BMSCs, both INA-6 and primary MM cells were resistant toward Sant7-induced or anti-gp130–induced apoptosis. The BMSC–mediated protection from apoptosis was observed with primary MM cells derived from 15 different patients without exception. Examples from 4 patients are shown in Figure 1B. Primary human BMSCs were isolated from bone marrow aspirates of different donors (see “Materials and methods”). Interestingly, the biologic effects observed in our coculture experiments were independent of the BMSC donors. Thus, coculture experiments performed with different MM cells and different BMSCs led to comparable results.
BMSCs protect MM cells from apoptosis induced by Sant7 or an anti-gp130 mAb.
The IL-6–dependent MM cell line INA-6 (A) and primary MM cells derived from 4 different patients (B) were treated with Sant7 (50 μg/mL) or with a blocking anti-gp130 mAb (100 μg/mL) either (with 2 ng/mL IL-6) in the absence or presence of primary BMSCs derived from 3 different donors (see “Materials and methods”). After incubation periods of 3 days (INA-6) or up to 7 days (primary MM cells), apoptosis was analyzed by annexin V-FITC/PI staining. Fraction of viable cells, negative for both annexin V and PI, is indicated as percentage of untreated control (A) or as percentage of total cells (B). SDs of 3 independently performed experiments (INA-6/BMSC#1, INA-6/BMSC#2, INA-6/BMSC#3) are shown in panel A). Panel A: ● indicates +BMSCs; ■, −BMSCs. Panel B: ░ indicates patient 1; ▩, patient 2; ▪, patient 3; and ▧, patient 4.
BMSCs protect MM cells from apoptosis induced by Sant7 or an anti-gp130 mAb.
The IL-6–dependent MM cell line INA-6 (A) and primary MM cells derived from 4 different patients (B) were treated with Sant7 (50 μg/mL) or with a blocking anti-gp130 mAb (100 μg/mL) either (with 2 ng/mL IL-6) in the absence or presence of primary BMSCs derived from 3 different donors (see “Materials and methods”). After incubation periods of 3 days (INA-6) or up to 7 days (primary MM cells), apoptosis was analyzed by annexin V-FITC/PI staining. Fraction of viable cells, negative for both annexin V and PI, is indicated as percentage of untreated control (A) or as percentage of total cells (B). SDs of 3 independently performed experiments (INA-6/BMSC#1, INA-6/BMSC#2, INA-6/BMSC#3) are shown in panel A). Panel A: ● indicates +BMSCs; ■, −BMSCs. Panel B: ░ indicates patient 1; ▩, patient 2; ▪, patient 3; and ▧, patient 4.
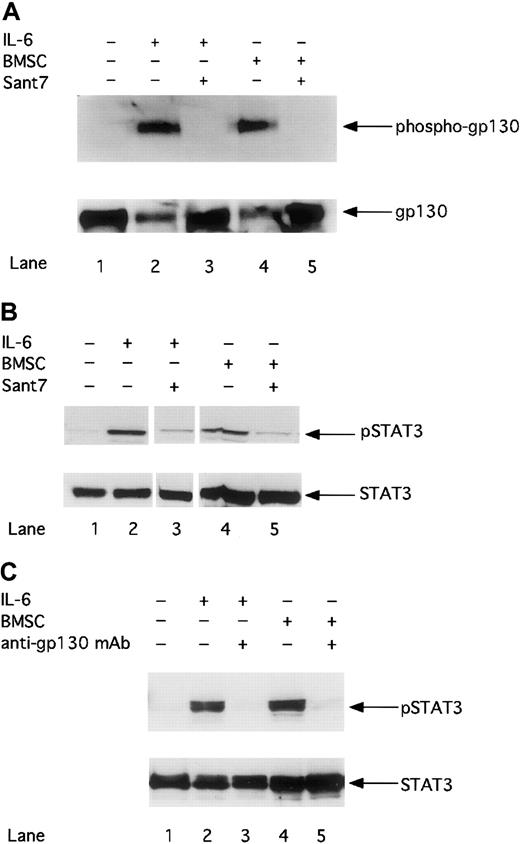
Treatment with Sant7 or an anti-gp130 mAb inhibits the gp130/STAT3 pathway in MM cells cultured in the absence or in the presence of BMSCs
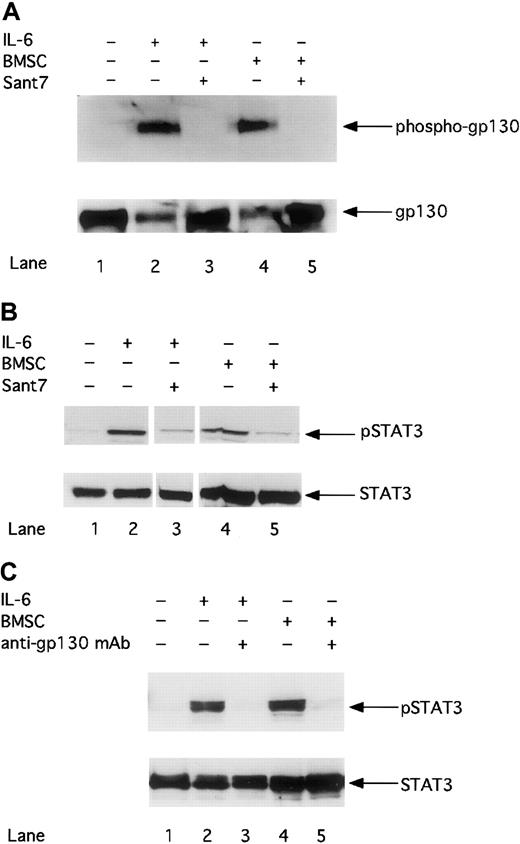
To clarify whether BMSC–mediated resistance of MM cells to apoptosis might be due to insufficient inhibition of gp130–mediated signaling, we analyzed the gp130/STAT3 pathway after treatment with Sant7 or an anti-gp130 mAb. First we investigated whether Sant7 treatment could inhibit the activation of the gp130 receptor. Figure 2A shows that gp130 phosphorylation in INA-6 MM cells can be induced with 2 ng/mL exogenously added IL-6 or through the cocultivation with BMSCs. Treatment with Sant7 (50 μg/mL) leads to complete inhibition of gp130 phosphorylation in cells cultured in the absence or in the presence of BMSCs. Thus, Sant7 treatment efficiently inhibits the activation of gp130 regardless of the presence of BMSCs.
The phosphorylation of gp130 and STAT3 is down-regulated by Sant7 or an anti-gp130 mAb in the absence or presence of BMSCs.
INA-6 cells were cultivated without IL-6 (lane 1), with 2 ng/mL IL-6 (lanes 2 and 3), or with BMSCs (lanes 4 and 5), and treated either with Sant7 (50 μg/mL; A,B: lanes 3 and 5) or with a blocking anti-gp130 mAb (100 μg/mL; C: lanes 3 and 5) for 12 hours. The phosphorylation of gp130 was analyzed by Western blotting and staining of immunoprecipitated gp130 with an antiphosphotyrosine antibody (A). The phosphorylation of STAT3 after treatment with Sant7 (B) or an anti-gp130 mAb (C) was detected on Western blots with a mAb against phosphorylated STAT3 at residue Tyr705. Western blot analysis of unphosphorylated gp130 or STAT3 served as a loading control.
The phosphorylation of gp130 and STAT3 is down-regulated by Sant7 or an anti-gp130 mAb in the absence or presence of BMSCs.
INA-6 cells were cultivated without IL-6 (lane 1), with 2 ng/mL IL-6 (lanes 2 and 3), or with BMSCs (lanes 4 and 5), and treated either with Sant7 (50 μg/mL; A,B: lanes 3 and 5) or with a blocking anti-gp130 mAb (100 μg/mL; C: lanes 3 and 5) for 12 hours. The phosphorylation of gp130 was analyzed by Western blotting and staining of immunoprecipitated gp130 with an antiphosphotyrosine antibody (A). The phosphorylation of STAT3 after treatment with Sant7 (B) or an anti-gp130 mAb (C) was detected on Western blots with a mAb against phosphorylated STAT3 at residue Tyr705. Western blot analysis of unphosphorylated gp130 or STAT3 served as a loading control.
Next we asked whether blocking gp130 activation inhibits STAT3 phosphorylation. The phosphorylation of tyrosine residue 705 is reported to be essential for the dimerization and nuclear translocation of STAT3.13 Panels B and C in Figure 2 demonstrate that STAT3 in INA-6 cells is activated by IL-6 or by cocultivation with BMSCs. The inhibition of STAT3 phosphorylation by Sant7 (50 μg/mL; panel B) or anti-gp130 mAb (100 μg/mL; panel C) treatment could be observed to a similar extent in INA-6 cells cultured in the absence or in the presence of BMSCs.
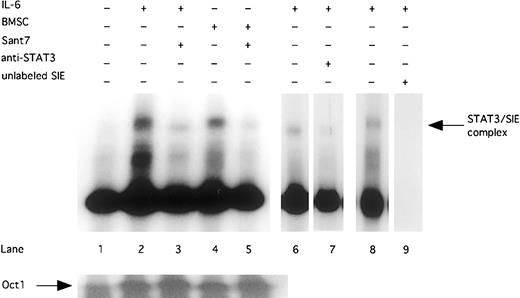
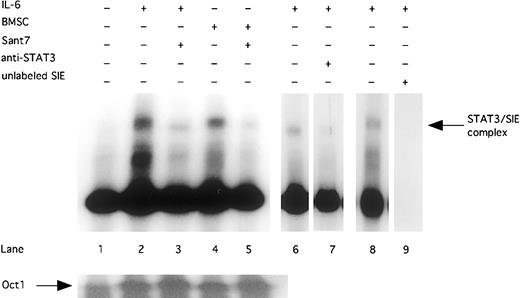
Finally, we could show that the inhibition of STAT3 phosphorylation resulted in a reduced DNA-binding activity. STAT3 DNA-binding activity was induced by exogenously added IL-6 (2 ng/mL) or cocultivation with BMSCs. Treatment with Sant7 (50 μg/mL) inhibited DNA-binding activity of STAT3 in INA-6 cells cultured in the absence and in the presence of BMSCs (Figure 3). By using an anti-STAT3 antibody, which blocks DNA binding of STAT3, we could show that the DNA-binding complex in the EMSA contained STAT3.
Sant7 treatment of INA-6 cells leads to a reduced STAT3 DNA-binding activity.
INA-6 cells were cultivated without IL-6 (lane 1), with 2 ng/mL IL-6 (lanes 2, 3, and 6-8), or with BMSCs (lanes 4 and 5), and treated with Sant7 (50 μg/mL; lanes 3 and 5) for 12 hours. EMSA with double-stranded 32P-labeled oligonucleotide probe (which contains a consensus-binding site for STAT3) were performed to show DNA-binding activity of STAT3. Preincubation with an anti-STAT3 antibody inhibits the binding of STAT3 protein to the SIE probe (lane 7). To control binding specificity of the 32P-labeled SIE probe, a competition with a 50-fold excess of unlabeled probe was performed (lane 9). A probe for the transcription factor Oct-1 served as a control.
Sant7 treatment of INA-6 cells leads to a reduced STAT3 DNA-binding activity.
INA-6 cells were cultivated without IL-6 (lane 1), with 2 ng/mL IL-6 (lanes 2, 3, and 6-8), or with BMSCs (lanes 4 and 5), and treated with Sant7 (50 μg/mL; lanes 3 and 5) for 12 hours. EMSA with double-stranded 32P-labeled oligonucleotide probe (which contains a consensus-binding site for STAT3) were performed to show DNA-binding activity of STAT3. Preincubation with an anti-STAT3 antibody inhibits the binding of STAT3 protein to the SIE probe (lane 7). To control binding specificity of the 32P-labeled SIE probe, a competition with a 50-fold excess of unlabeled probe was performed (lane 9). A probe for the transcription factor Oct-1 served as a control.
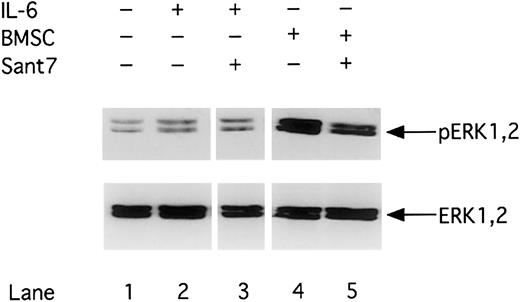
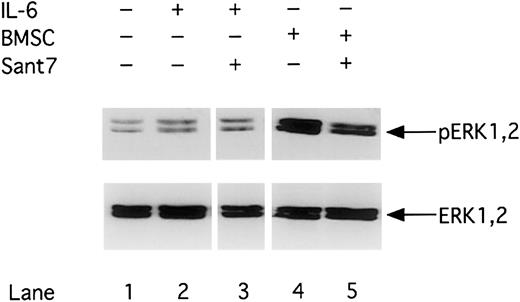
Phosphorylation of ERK1,2 is up-regulated in the presence of BMSCs
The Ras/MAPK-ERK1,2 pathway has been reported to be another gp130–triggered pathway in MM cells. We therefore analyzed whether inhibition of gp130 by Sant7 or an anti-gp130 mAb treatment not only inhibits STAT3 but also ERK1,2 activity. A constitutive ERK1,2 phosphorylation signal could be detected in INA-6 cells in the absence of IL-6 and BMSCs. ERK1,2 phosphorylation is enhanced by cocultivating INA-6 cells with primary BMSCs, whereas the presence of IL-6 (2 ng/mL) alone had only minor effects. Consequently, Sant7 (50 μg/mL) treatment did not lead to down-regulation of ERK1,2 phosphorylation in MM cells cultivated in the presence of BMSCs (Figure4). Similar results could be observed after anti-gp130 mAb (100 μg/mL) treatment (data not shown).
Phosphorylation of MAPK/ERK1,2 is up-regulated in the presence of BMSCs.
INA-6 cells were cultivated without IL-6 (lane 1), with 2 ng/mL IL-6 (lanes 2 and 3), or with BMSCs (lanes 4 and 5) and treated with Sant7 (50 μg/mL; lanes 3 and 5) for 12 hours. Western blot analysis with a Thr202/Tyr204-phosphospecific anti-ERK1,2 antibody was performed to determine the phosphorylation status of ERK1,2 (pERK1,2) in INA-6 cells. Western blot analysis of the unphosphorylated ERK1,2 served as a loading control.
Phosphorylation of MAPK/ERK1,2 is up-regulated in the presence of BMSCs.
INA-6 cells were cultivated without IL-6 (lane 1), with 2 ng/mL IL-6 (lanes 2 and 3), or with BMSCs (lanes 4 and 5) and treated with Sant7 (50 μg/mL; lanes 3 and 5) for 12 hours. Western blot analysis with a Thr202/Tyr204-phosphospecific anti-ERK1,2 antibody was performed to determine the phosphorylation status of ERK1,2 (pERK1,2) in INA-6 cells. Western blot analysis of the unphosphorylated ERK1,2 served as a loading control.
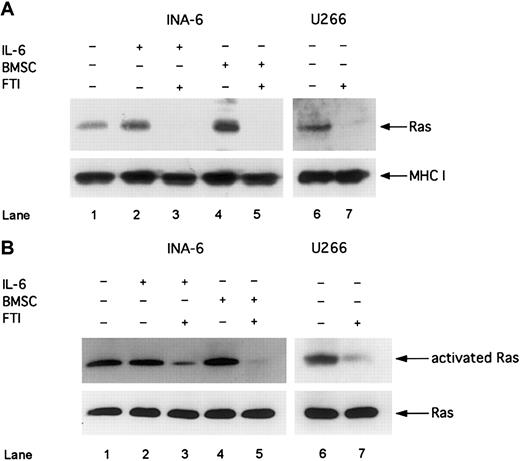
Treatment of MM cells with FPT III blocks the Ras/MAPK pathway and induces apoptosis in the absence and in the presence of BMSCs
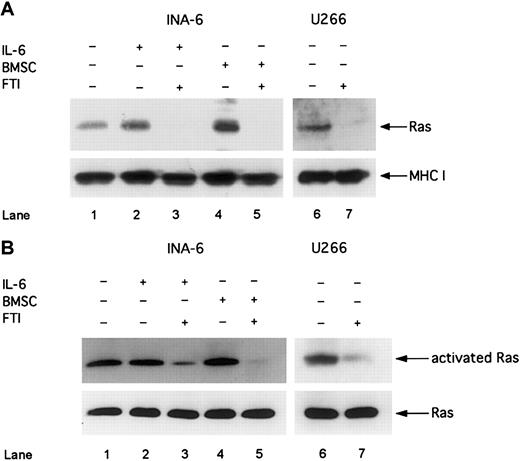
Next we asked whether inhibitors of the Ras/MAPK pathway are more effective than inhibitors of the IL-6/gp130/STAT3 pathway to induce apoptosis in MM cells in the presence of BMSCs. Thus, we treated MM cells with the FTI, FPT III, which has been reported as a specific inhibitor of Ras farnesylation and consequently of Ras activation.29 First, we analyzed whether Ras is activated in MM cells and whether FPT III is effectively inhibiting the Ras/MAPK pathway. Furthermore, we investigated whether pathway activation and sensitivity to FPT III depends on the Ras mutation status. We analyzed 2 different human MM cell lines: INA-6, which has an activating Ras mutation,7 and U266, which expresses wild-type Ras.30 Despite the different mutation status we found constitutively activated Ras as well as phosphorylated ERK1,2 in both INA-6 and U266 cells (Figures 5 and6 and also Figure 4). Treatment with FPT III (50 μM) for 12 hours led to (1) inhibition of membrane translocation of Ras, which is an essential step in Ras activation and mediated by farnesylation; (2) decrease of Ras activation (as shown by decreased Ras bound to the Ras-binding domain of Raf-1); and (3) inhibition of ERK1,2 phosphorylation in both cell lines (Figures 5and 6).
FTI FPT III blocks Ras membrane translocation and Ras activation.
INA-6 cells were cultivated without IL-6 (lane 1), with 2 ng/mL IL-6 (lanes 2 and 3), or with BMSCs (lanes 4 and 5). U266 and INA-6 cells were treated with FTI FPT III (50 μM; lanes 3, 5, and 7) for 12 hours. (A) Western blot analysis of membrane fractions with an anti-Ras mAb was performed to analyze the effect of 50 μM FTI FPT III on membrane translocation of Ras. MHC class I served as a control for nonfarnesylated membrane proteins. (B) Activated Ras was precipitated with a Raf1-(RBD)-GST fusion protein and analyzed by using an anti-Ras antibody (Ras activation assay). Staining for Ras in whole cell lysates without precipitation served as a control. The IL-6–independent cell line U266 was cultivated without IL-6 or BMSCs (lanes 6 and 7).
FTI FPT III blocks Ras membrane translocation and Ras activation.
INA-6 cells were cultivated without IL-6 (lane 1), with 2 ng/mL IL-6 (lanes 2 and 3), or with BMSCs (lanes 4 and 5). U266 and INA-6 cells were treated with FTI FPT III (50 μM; lanes 3, 5, and 7) for 12 hours. (A) Western blot analysis of membrane fractions with an anti-Ras mAb was performed to analyze the effect of 50 μM FTI FPT III on membrane translocation of Ras. MHC class I served as a control for nonfarnesylated membrane proteins. (B) Activated Ras was precipitated with a Raf1-(RBD)-GST fusion protein and analyzed by using an anti-Ras antibody (Ras activation assay). Staining for Ras in whole cell lysates without precipitation served as a control. The IL-6–independent cell line U266 was cultivated without IL-6 or BMSCs (lanes 6 and 7).
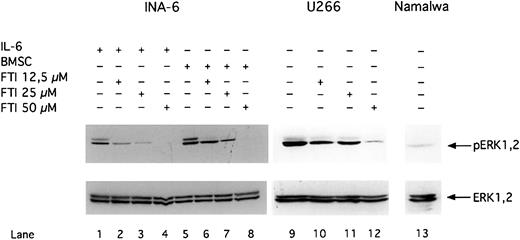
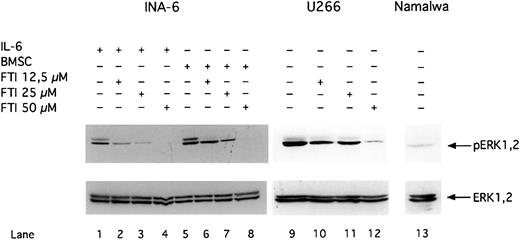
FTI FPT III blocks phosphorylation of ERK1,2.
INA-6 cells were cultivated with 2 ng/mL IL-6 (lanes 1-4) or with BMSCs (lanes 5-8). The IL-6–independent cell lines U266 (lanes 9-12) and Namalwa (lane 13) were cultivated without IL-6 or BMSCs. U266 (lanes 10-12) and INA-6 (lane 2-4 and 6-8) cells were treated with FPT III for 12 hours. Western blot analysis with a Thr202/Tyr204-phosphospecific anti-ERK1,2 antibody was performed to analyze phosphorylation of ERK1,2 (pERK1,2) in INA-6, U266, and Namalwa cells. Western blot analysis of the unphosphorylated ERK1,2 indicates equal loading.
FTI FPT III blocks phosphorylation of ERK1,2.
INA-6 cells were cultivated with 2 ng/mL IL-6 (lanes 1-4) or with BMSCs (lanes 5-8). The IL-6–independent cell lines U266 (lanes 9-12) and Namalwa (lane 13) were cultivated without IL-6 or BMSCs. U266 (lanes 10-12) and INA-6 (lane 2-4 and 6-8) cells were treated with FPT III for 12 hours. Western blot analysis with a Thr202/Tyr204-phosphospecific anti-ERK1,2 antibody was performed to analyze phosphorylation of ERK1,2 (pERK1,2) in INA-6, U266, and Namalwa cells. Western blot analysis of the unphosphorylated ERK1,2 indicates equal loading.
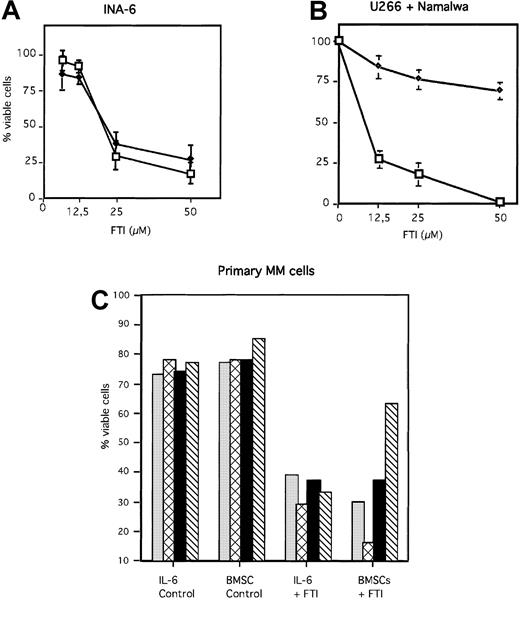
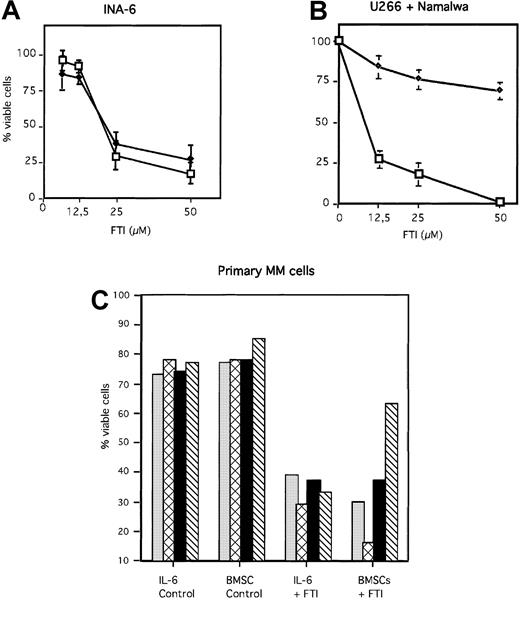
Inhibition of the Ras/MAPK pathway by FPT III was accompanied by induction of apoptosis in INA-6 and U266 cells (Figure7A and B). In contrast, the Burkitt lymphoma cell line Namalwa, which has no constitutive ERK1,231 activity (Figure 6), was resistant toward apoptosis induced by FPT III (Figure 7B). In addition, we could show that FPT III is effective in blocking the activity of the Ras/MAPK pathway in the absence and in the presence of BMSCs (Figures 5 and 6). This inhibition leads to strong induction of apoptosis in both MM cells cultured in the absence or in the presence of BMSCs (Figure 7). This effect could be observed with INA-6 cells as well as with primary MM cells from 7 different patients. Cells from one patient were partially protected from FPT III–induced apoptosis if cultured in the presence of BMSCs. We compared the efficacy of Sant7, the anti-gp130 mAb, and FPT III on primary MM cells derived from 4 different patients. Each patient sample was split in thirds and treated with each single inhibitor (Sant7 or anti-gp130 mAb or FPT III). Results of these 4 samples are shown in Figure 1B (Sant7 or anti-gp130 mAB) and Figure 7C (FPT III). Due to limited cell numbers, samples from 3 additional patients could be treated with FPT III only (data not shown). FPT III induced apoptosis in all samples in the presence and in the absence of BMSCs (Figure 7C).
FTI FPT III induces apoptosis in MM cells in the absence and presence of BMSCs.
INA-6 (A), and U266 and Namalwa (B) cells were treated for 3 days with different FPT III concentrations as indicated, and primary MM cells (C) derived from 4 patients (same patients as shown in Figure 1) for 7 days. Primary MM cells were treated with 50 μM FTI FPT III. BMSCs used for coculture assays with INA-6 and primary MM cells were taken from 3 different donors (Figure 1 and see “Materials and methods”). U266 and Namalwa cells were cultured without BMSCs. Apoptosis was analyzed by annexin V-FITC/PI staining. The fraction of viable cells, negative for both annexin and PI, is shown as percentage of untreated control (A + B) or as percentage of total cells (C). Panel A: ♦ indicates +BMSCs; ■, −BMSCs. Panel B: ♦ indicates Namalwa; ■, U266. Panel C: ░ indicates patient 1; ▩, patient 2; ▪, patient 3; and ▧, patient 4. SDs of 3 experiments are shown in panels A and B.
FTI FPT III induces apoptosis in MM cells in the absence and presence of BMSCs.
INA-6 (A), and U266 and Namalwa (B) cells were treated for 3 days with different FPT III concentrations as indicated, and primary MM cells (C) derived from 4 patients (same patients as shown in Figure 1) for 7 days. Primary MM cells were treated with 50 μM FTI FPT III. BMSCs used for coculture assays with INA-6 and primary MM cells were taken from 3 different donors (Figure 1 and see “Materials and methods”). U266 and Namalwa cells were cultured without BMSCs. Apoptosis was analyzed by annexin V-FITC/PI staining. The fraction of viable cells, negative for both annexin and PI, is shown as percentage of untreated control (A + B) or as percentage of total cells (C). Panel A: ♦ indicates +BMSCs; ■, −BMSCs. Panel B: ♦ indicates Namalwa; ■, U266. Panel C: ░ indicates patient 1; ▩, patient 2; ▪, patient 3; and ▧, patient 4. SDs of 3 experiments are shown in panels A and B.
Discussion
Here we show that inhibition of the IL-6/gp130/STAT3 pathway either with the IL-6R–specific antagonist Sant7 or with a blocking anti-gp130 mAb induces apoptosis in the human MM cell line INA-6 and in primary MM cells freshly isolated from the bone marrow aspirates of patients. These results are in good accordance with data showing that inhibition of the IL-6/STAT3 pathway induces apoptosis in the human MM cell lines U266, XG-1, and XG-2.17-19
However, in our experiments no apoptosis could be induced if MM cells were cocultivated with primary human BMSCs, a major cellular constituent of the bone marrow microenvironment. Again, this observation could be made with cells from the human MM cell line INA-6 and with primary MM cells derived from the bone marrow aspirates of 15 different patients. Because Sant7 and anti-gp130 mAb treatment led to strong down-regulation of the phosphorylation of gp130 and STAT3 in the absence and also in the presence of BMSCs, it appears that the IL-6/gp130/STAT3 pathway is not essential for the survival of MM cells in the presence of cells from the bone marrow microenvironment. Thus, it seems that MM cells become independent of the IL-6/gp130/STAT3 pathway in the presence of BMSCs. These findings support the idea that the bone marrow microenvironment provides additional factors activating different signaling pathways, which might substitute for the antiapoptotic function of the IL-6/gp130/STAT3 pathway.
Clinical phase I studies have been performed with murine or chimeric anti-IL-6 mAbs to evaluate the effects of IL-6 inhibition in vivo. Despite a strong reduction of C-reactive protein, an in vivo surrogate marker for IL-6, no clinical response could be documented.32 However, due to low patient numbers and high tumor burden, these studies did not provide enough data to prove this approach ineffective. On the other hand, the lack of clinical responses in these trials is in good accordance with our data showing that inhibition of the IL-6/gp130/STAT3 pathway in coculture assays does not induce apoptosis. Nevertheless, Sant7 or other IL-6/IL-6R–blocking compounds might be of potential therapeutic use because we previously demonstrated that blocking of the IL-6R can overcome drug resistance of MM cells.20
Our observation that BMSCs enhance the constitutive ERK1,2 signal in INA-6 cells, which is not down-regulated by Sant7, is a hint that Ras–triggered pathways might be more important for the survival of MM cells than the IL-6/gp130/STAT3 pathway. In accordance with this hypothesis we could demonstrate that FPT III was able to induce apoptosis in MM cells either in the presence or in the absence of BMSCs to a similar extent. In contrast, the inhibitors of the gp130 pathway effectively induce apoptosis in the absence but not in the presence of BMSCs. This could be observed with MM cell lines as well as with primary MM cells.
In addition, we could demonstrate that FTI treatment of 2 different human MM cell lines, INA-6 and U266, led to the inhibition of (1) Ras membrane translocation, (2) Ras activity, and (3) ERK1,2 phosphorylation. Furthermore, we could demonstrate that FTI–induced inhibition of the Ras/MAPK pathway was paralleled by the induction of apoptosis in INA-6 and U266 cells. INA-6 contains an activating N-Ras mutation,7 whereas U266 contains wild-type Ras.30 However, in both cell lines Ras and MAPK are constitutively activated. Thus, the Ras/MAPK pathway in MM cells might be activated by different mechanisms (eg, mutations, serum, environment). In contrast to MM cells, the Burkitt lymphoma cell line Namalwa, which lacks constitutive ERK1,2 activity was resistant toward apoptosis induced by FTI treatment. Thus, these experiments support the idea that FTI sensitivity depends on the activation status of the Ras/MAPK pathway rather than on the Ras mutation status.
FTIs are a novel class of pharmacologic molecules that have been developed to inhibit the growth of Ras-dependent tumors.33,34 These molecules can block the farnesylation of Ras, which is an essential posttranslational modification step for the activation of Ras, and consequently inhibit Ras-triggered pathways (such as the ERK1,2 pathway).29 FTIs have been shown to inhibit the growth of Ras-dependent tumor cells in vitro and in animal models without causing significant side effects.35Currently, FTIs are being tested in first clinical trials in particular for the treatment of tumors with a high frequency of Ras mutations, such as pancreatic cancer (for a review, see Reuter et al29). Although FTIs have been reported to be effective and specific inhibitors of Ras activation, these inhibitors probably also interfere with other proteins that are farnesylated. Thus, we cannot exclude some unspecific and Ras/MAPK-independent effects of FTIs on MM cells.
Another signaling cascade that deserves attention is the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. This cascade can be stimulated in myeloma cells by IL-6 and insulinlike growth factor 1 (IGF-1) or by functional loss of the tumor suppressor phosphatase and tensin homolog deleted on chromosome 10 (PTEN).36,37 Blocking the PI3K/Akt pathway has been reported to inhibit growth of MM cell lines in the absence of BMSCs.38 We found that BMSCs do not stimulate Akt phosphorylation in INA-6 cells and that FTI treatment does not substantially block Akt phosphorylation (data not shown). Thus, we assume that the effects we have observed (ie, the protective effect of BMSCs and FTI–induced apoptosis) do not depend on this pathway.
Taken together, we show direct experimental evidence that the IL-6/gp130/STAT3 pathway is not essential for the survival of human MM cells in the presence of cells from their bone marrow microenvironment. Furthermore, we provide evidence that FTIs rather than inhibitors of the IL-6/gp130/STAT3 pathway are promising molecules for the development of novel therapeutic strategies for the treatment of MM.
Prepublished online as Blood First Edition Paper, June 28, 2002; DOI 10.1182/blood-2002-01-0102.
Supported in part by grants from the Deutsche Krebshilfe (10-1292-Ba.I) and the Bundesministerium für Bildung und Forschung (0312578). M.C. and D.H. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ralf C. Bargou, Department of Hematology, Oncology, and Tumorimmunology, Robert-Rössle Cancer Center at the Max-Delbrück-Center for Molecular Medicine, Charité, Humboldt University of Berlin Lindenberger Weg 80, D-13122 Berlin, Germany; e-mail: bargou@mdc-berlin.de.