Abstract
Allogeneic stem cell transplantation (SCT) is frequently considered as treatment for relapsed childhood acute lymphoblastic leukemia (ALL). For patients without a matched sibling donor, SCT from unrelated donors (UD-SCT) has been increasingly performed during the past years. However, UD-SCT–related mortality and morbidity is still considerable, and the question remains as to which patients are at such high risk of recurrence that UD-SCT is indicated and, conversely, which patients do not require transplantation for long-term disease control. A matched-pair analysis was performed among patients treated according to Acute Lymphoblastic Leukemia Relapse Berlin-Frankfurt-Münster (ALL-REZ BFM) Study Group protocols after first relapse with chemotherapy or UD-SCT. Altogether 81 pairs were identified that could be matched exactly for site of relapse and immunophenotype, and as closely as possible for duration of first remission, age, diagnosis date, and peripheral blast cell count at relapse. No significant difference in the probability of event-free survival (pEFS) between UD-SCT and chemotherapy existed regarding 28 pairs with an intermediate prognosis (0.39 ± 0.10 vs 0.49 ± 0.11,P = .105), whereas the pEFS was significantly different in the 53 pairs with a poor prognosis (0.44 ± 0.07 vs 0.00 ± 0.00, P < .001). The major reasons of treatment failure among patients who underwent UD-SCT were therapy-related death (TRD; 24/81) and relapses (20/81). In contrast, TRD rarely occurred in patients treated with chemotherapy alone (3/81), but relapse was much more common (62/81). In conclusion, UD-SCT provides better event-free survival for children with high-risk relapsed ALL. However, there is no clear advantage of UD-SCT in patients with intermediate prognosis.
Introduction
The most frequent indication for allogeneic stem cell transplantation (SCT) in children is recurrent acute lymphoblastic leukemia (ALL). Regarding patients with an intermediate prognosis at relapse after conventional chemotherapy, it is also the most controversial.
Since 1983, in Germany and Austria nearly all children with ALL relapse are treated according to protocols of the ALL-REZ Berlin-Frankfurt-Münster (BFM) Relapse Study Group. There are 101 centers that take part in these multicenter trials and allogeneic SCT is performed in 18 associated transplantation centers.
The first successful unrelated donor SCT (UD-SCT) was performed more than 20 years ago. However, only in recent years have sufficient numbers of transplants been performed in children to permit comparison with alternative treatment strategies. A comparison of the outcome of SCT with chemotherapy requires a prolonged follow-up, due to late subsequent leukemia recurrences. In patients treated with chemotherapy, such recurrences generally occur within 5 years after relapse; in patients treated with SCT, subsequent relapse most often occurs within 2 years. In this report we present data on a matched-pair analysis comparing UD-SCT with chemotherapy for children with ALL in second complete remission (CR) with a follow-up period of more than 10 years.
Patients and methods
Between June 1983, and April 2001, 1556 patients up to 18 years of age with first relapse of ALL were enrolled in the multicenter trials of ALL-REZ BFM after written consent. Approval was obtained from the institutional review board of the Humboldt-University of Berlin, Charité. Informed consent was provided according to the Declaration of Helsinki. Of these patients, 1188 were given conventional chemotherapy/radiotherapy and 334 underwent either related allogeneic (190), unrelated allogeneic (95), or autologous (49) SCT after remission induction with chemotherapy. Postremission therapy was not yet definitely specified for 34 patients. In the ALL-REZ BFM studies, stratification groups were defined as given in Table1: standard-risk group (SR; 5% of all study patients), intermediate-risk group (IR; 55% of all patients), and high-risk group (HR; 40% of all patients).1,2 Early combined relapses are stratified to the IR group because they are associated with a more favorable prognosis than early isolated bone marrow relapses.3
By definition, late relapses occur more than 6 months after cessation of chemotherapy, early relapses at least 18 months after diagnosis but less than 6 months after cessation of chemotherapy, and very early relapses within 18 months after diagnosis.
Patients of the high-risk group are at a very high risk of suffering a subsequent relapse if treated with chemotherapy alone.4Since trial ALL-REZ BFM 95 they were obligatorily allocated to allogeneic SCT, if a second CR could be achieved. For patients with an intermediate prognosis, matched unrelated donor (MUD)–SCT was not compulsorily indicated, but it was not regarded as a violation of the protocol. The decision to perform SCT in this group was made at the treatment centers by the physicians and the patients and their parents. The final selection of candidates varied according to the policies of the transplantation teams. Additionally, in recent times subgroups of patients of the IR group have been defined with high peripheral blast cell count at relapse and without extramedullary involvement. These patients received transplants from MUDs more frequently. This study compares 2 treatment groups chosen from 1283 children with relapsed ALL treated with chemotherapy/radiotherapy alone or UD-SCT.
Patients treated with chemotherapy
The chemotherapy group consisted of 81 patients, selected from a larger group of 1188 patients with first relapse. Of the eligible patients, 1028 (87%) had initially been treated according to BFM (70%) or Cooperative Study Group for Childhood Acute Lymphoblastic Leukemia (CoALL; 17%) frontline protocols.5,6 According to the ALL-REZ BFM protocols, relapse treatment consisted of alternating courses of polychemotherapy. At the end of intensive systemic chemotherapy, local radiotherapy was applied to the central nervous system (CNS) at an age-dependent dose. Children received conventional maintenance therapy with daily oral 6-thioguanine and intravenous methotrexate (MTX) every second week for up to 2 years.4 Of these 1188 patients, 81 met the match criteria of those patients who underwent UD-SCT as described below (Table 2).
Patients treated with UD-SCT
Of the 95 patients with UD-SCT, 89 (94%) had initially been treated according to BFM (76%) or CoALL (18%) frontline protocols. For remission induction, prior to SCT, all children were treated according to ALL-REZ BFM relapse protocols. For 14 patients with SCT, no match partner was found. Hence, for 81 patients a partner could be found meeting the match criteria. For these patients, the interval between achievement of second remission and transplantation varied from 34 to 349 days (median, 139 days).
Conditioning regimens were not uniform: most (n = 50 [of 71 available patient data], 70%) included fractionated total body irradiation (12 Gy) and etoposide and/or cyclophosphamide. There were 21 patients who received other conditioning regimens including different drug combinations with (25%) or without (4%) total body irradiation (TBI). In 10 patients no data were available. Graft-versus-host disease (GVHD) prophylaxis consisted of a short course of MTX, daily doses of cyclosporine A, and interventional doses of steroids in most patients. The method of T-cell depletion as well as data on HLA disparity between unrelated bone marrow (BM) donors and patients in this study are given in Table2. Usually HLA typing for class I was done serologically and for class II by molecular genetic methods.7 However, recently class I antigens have been typed by high-resolution moleculargenetic methods as well in some centers.
Analysis
Preliminary studies identified variables associated with treatment failure in both groups.1-3,8 The main determinants of outcome are duration of first remission (very early, early, late relapse), the immunophenotype of leukemic cells (T-cell, non–T-cell), and the site of relapse (isolated bone marrow, combined bone marrow, isolated extramedullary). Other variables that are independent prognostic determinants in multivariate analyses are age at relapse (< 10 years, ≥ 10 years) and the peripheral blast cell count at relapse (< 1/μL, 1-10 000/μL, ≥ 10 000/μL).9 We selected pairs of patients treated with either chemotherapy or SCT by matching the above-mentioned variables exactly respecting the categories given in brackets. The chemotherapy recipient of each pair was selected from patients in whom the second remission was at least as long as the interval between achievement of second remission and transplantation for the transplant recipient. If more than one patient in the chemotherapy group was eligible for matching with a patient in the transplantation group, we selected the chemotherapy patient with a diagnosis date closest to that of the transplant recipient. Molecular biologic parameters such asBCR/ABL and TEL/AML1 were not included in the match procedure because these data are not determined for a substantial part of the patients. There were 81 matched pairs who met these criteria.
Follow-up is defined as duration of second CR for patients still remaining in continuous CR. The probability of event-free survival (pEFS) was calculated by Kaplan-Meier life-table analysis, and is given at 5 years. Differences were rated by the log-rank test and considered as significant at a P value of .05 or less. Treatment failures, such as therapy-related death in CR, subsequent relapse, or second malignancy were counted as adverse events. Follow-up was calculated for all patients being in continuous CR as usually recommended.10 The overall statistical power to detect a difference of 20% in outcome between the matched-patient groups was 83% for the total group, 40% for the IR group, and 65% for the HR group.
Results
Chemotherapy group
After a median follow-up of 7.9 years (range, 1.4-18.6 years) from achieving a second complete remission, pEFS at 5 years is .17 ± .05 for the entire group of patients receiving chemotherapy. The death rate related to chemotherapy was 4% (3/81). A subsequent relapse was experienced by 62 patients (77%); 4 of these are still alive. Continuous CR was achieved by 16 patients (20%). For IR patients, the 5-year estimates for EFS were .49 ± .11 versus .00 ± .00 for HR patients (Figures 1, 2). The numbers of patients in continuous CR after chemotherapy in the different subgroups and the respecting pEFS are shown in Table 3.
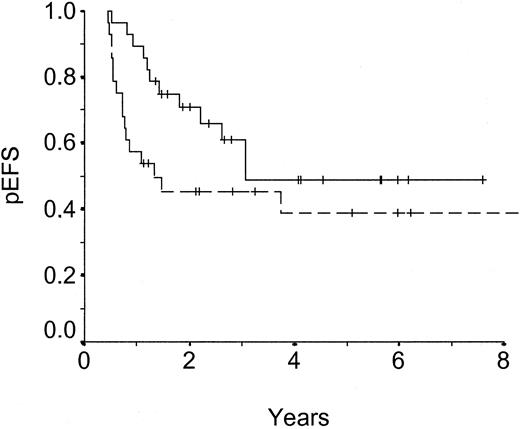
Probability of event-free survival (pEFS) of matched pairs, intermediate-risk group.
Dashed line: unrelated donor SCT, n = 28, censored = 12, pEFS = .39 ± .10. Solid line: chemotherapy, n = 28, censored = 16, pEFS = .49 ± .11. P = .105.
Probability of event-free survival (pEFS) of matched pairs, intermediate-risk group.
Dashed line: unrelated donor SCT, n = 28, censored = 12, pEFS = .39 ± .10. Solid line: chemotherapy, n = 28, censored = 16, pEFS = .49 ± .11. P = .105.
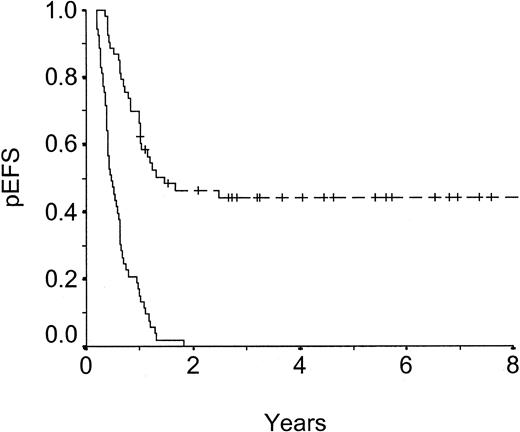
Probability of event-free survival (pEFS) of matched pairs, high-risk group.
Dashed line: unrelated donor SCT, n = 53, censored = 24, pEFS = .44 ± .07. Solid line: chemotherapy, n = 53, censored = 0, pEFS = .00 ± .00. P < .001.
Probability of event-free survival (pEFS) of matched pairs, high-risk group.
Dashed line: unrelated donor SCT, n = 53, censored = 24, pEFS = .44 ± .07. Solid line: chemotherapy, n = 53, censored = 0, pEFS = .00 ± .00. P < .001.
Transplant recipients
After a median follow-up of 4.1 years (range, 1.1-13.1 years) from achieving a second remission, pEFS at 5 years is .42 ± .06 for patients who received a transplant. Of 58 evaluable patients, 18 (31%) suffered from grades III-IV GVHD. Death rate related to transplantation was 30% (24/81). Therapy-related death (TRD) was due to acute GVHD, (n = 8), graft failure or rejection (n = 2), sepsis (n = 2), aspergillosis (n = 2), or veno-occlusive disease (VOD)/cardiotoxicity/hemorrhage/multiorgan failure (n = 5). In 6 cases there were no data. In 20 patients (25%) subsequent relapses occurred, 6 of those patients are still alive. One patient (1%) developed a second malignancy. Of the 81 patients who received a transplant, 36 (44%) are in continuous CR. The 28 patients with an IR prognosis had a pEFS of .39 ± .10 compared with .44 ± .07 in 53 children of the HR group (Figures 1, 2). The numbers of patients who received a transplant who are in continuous CR in the different subgroups are given in Table 3.
Comparison of groups
All 81 pairs are exactly matched for the above given categorical parameters. Furthermore, a high up to very high correlation of continuous risk parameters could be achieved with a Pearson product moment correlation coefficient (PC) of 0.71 for duration of first CR, 0.72 and 0.79 for age at initial and at relapse diagnosis, and 0.80 for the peripheral blast cell count at relapse (2 missing values). Observation time was significantly different in HR patients (P < .001), whereas in IR patients a high correlation of the parameter could be achieved (PC = 0.93).
pEFS was significantly superior for patients receiving UD-SCT as compared with those receiving chemotherapy (P < .001). This difference is based on a highly significant better outcome in HR patients (P < .0001, Figure 1). In contrast, there is no difference of pEFS in IR patients (P = .105, Figure2).
Discussion
SCT from an unrelated donor is a curative approach in children with relapsed ALL. There is a price to pay, however, in terms of significant early morbidity and mortality.11-18 When SCT offers the only chance of cure, then these risks could be easily justified; if on the other hand, another treatment offers an equal opportunity for cure at less risk of therapy-related toxicity, then SCT should be avoided.
In this study, we performed a matched-pair analysis among patients with ALL in second CR treated either with chemotherapy according to the ALL-REZ BFM protocols or with UD-SCT. Because patients with a high risk for subsequent relapse have been preferably allocated to allogeneic SCT, comparison of MUD-SCT and chemotherapy is biased by negative selection of the group undergoing transplantation with respect to risk factors, but by positive selection with respect to time to transplantation. To overcome these biases, for every patient who received a transplant, a partner treated with chemotherapy alone has been selected matching on one side all established risk factors as determinants of EFS and on the other side with a duration of event-free survival at least as long as time to transplantation of the respective partner. A matched-pair analysis is one of several possibilities to compare different treatment modalities. The optimum method would be a prospective randomized allocation with the possibility to assess results according to the intention to treat as well as the treatment received. However, several attempts to randomize SCT versus chemotherapy have failed in the past, mostly due to the personal preferences of the treating physicians as well as the patients and their families to one of the approaches.19 An alternative approach would be a retrospective multivariate Cox regression analysis including all relevant prognostic factors as well as SCT as a time-dependent covariant. However, EFS after chemotherapy or SCT often does not follow proportional hazard functions and requires artificial statistical models for adequate analysis. Important factors, such as tolerance to treatment and individual infectious risk factors, are not considered in all those methods and remain biases of the analysis with impredictable influence on outcome.
Unrelated SCT is a treatment modality of the last 10 years with an increased availability of unrelated donors. Since the beginning of the trial ALL-BFM 96 we tried to find unrelated BM donors for all high-risk patients without a family donor. Therefore, for high-risk patients who recently underwent transplantation, mainly historical controls treated with chemotherapy alone are available. This leads to different lengths of follow up. Nevertheless, if more than one patient in the chemotherapy group was eligible for matching with a patient in the transplantation group, we selected the chemotherapy patient with a diagnosis date closest to that of the transplant recipient.
In this study, the TRD rate of 30% among children treated with UD-SCT was high in comparison with the rates among children treated with chemotherapy alone (4%) but is within the range of rates reported by other groups.10 20-22
The pEFS (.42 at 5 years) of children who received transplants from unrelated donors in second CR in our study is encouraging, taking into account their unfavorable risk profile (65% of patients belonged to the HR group). Event-free survival was 0.44 for patients who underwent transplantation and 0.00 for patients treated with chemotherapy in the HR group (P < .001). Therefore, children with poor prognostic features can clearly benefit from UD-SCT in second CR. The TRD rate of about 30% is still high, but it had to be accepted in face of the otherwise incurable disease.
In contrast, there was no clear advantage of UD-SCT for patients in the IR group with respect to EFS: event-free survival rates at 5 years were 0.39 for patients who received an unrelated transplant and 0.49 for patients treated with conventional chemotherapy, not being significantly different. It must be acknowledged that these conclusions are based on a small number of patients and true differences may not have been detected, especially because the subgroup analyses showed low statistical power values of 40% and 65% for the IR and HR groups, respectively.
In summary, although UD-SCT offers a clear advantage over chemotherapy alone for patients with ALL relapse in the HR group, our results show no significant advantage of UD-SCT for IR patients. Considering the complications potentially associated with UD-SCT, it will be essential to develop strategies to identify subgroups of the IR group who still might benefit from UD-SCT. Risk factors such as initial high blast cell count, the presence of BCR/ABL fusion transcripts, or evidence of minimal residual disease detected by clone-specific moleculargenetic probes after achievement of cytologic remission may allow further stratification of the IR group but have yet to be investigated in a prospective study.9 23-25
We thank the participating medical centers and bone marrow transplantation units, as well as Sabine Brühmüller and Andrea Kretschmann, Berlin, for preparing the data of the BFM relapse studies.
Members of the study committee
M. Albrecht (radiotherapie@km-berlin.de); J. D. Beck (joern.beck@kinder.imed.uni-erlangen.de); U. Bode (bode@ukb.uni-bonn.de); W. Dörffel (hodgkinped@cecon.de); W. Ebell (wolfram.ebell@charite.de); R. Fengler (ruediger.fengler@charite.de); H. Gadner (gadner@ccri.univie.ac.at); U. Göbel (kko4ambz@uni-duesseldorf.de); G. Henze (guenter.henze@charite.de); G. Janka (janka@uke.uni-hamburg.de); T. Klingebiel (tklingebiel@zki.uni-frankfurt.de); E. Koscielnak (cws.study@olgahospital.s.shuttle.de); G. Mann (mann@ccri.univie.ac.at); S. Müller-Weihrich (smw@lrz.tu-muenchen.de); C. Peters (peters@ccri.univie.ac.at); J. Ritter (ritterj@uni-muenster.de); and M. Schrappe (schrappe.martin@mh-hannover.de).
City locations and principal investigators of the participating medical centers
Aachen, Germany (R. Mertens); Aarau, Switzerland (P. Imbach); Augsburg, Germany (P. Heidemann, E. Pongratz); Basel, Switzerland (P. Imbach); Bayreuth, Germany (G. F. Wündisch); Berlin, Germany (G. Henze); Berlin-Buch, Germany (W. Dörffel); Bielefeld (J. Otte); Bonn, Germany (U. Bode); Braunschweig, Germany (G. Mau); Bremen, Germany (H.-J. Spaar); Celle, Germany (M. Kirschstein); Chemnitz, Germany (K. Hofmann); Coburg, Germany (R. Frank); Coesfeld, Germany (E. B. Lang); Cottbus, Germany (D. Möbius); Datteln, Germany (W. Andler); Delmenhorst, Germany (C. Niekrens); Dortmund, Germany (H. Breu); Dresden, Germany (M. Suttorp, V. Scharfe); Düsseldorf, Germany (U. Göbel, P. Zickler); Erfurt, Germany (G. Weinmann); Erlangen, Germany (J. D. Beck); Essen, Germany (W. Havers); Feldkirch, Austria (E. Ludescher) Frankfurt, Germany (B. Kornhuber, V. Gerein); Freiburg, Germany (M. Brandis); Giessen, Germany (F. Lampert); Greifswald, Germany (H. Reddemann); Hamburg, Germany (G. Janka-Schaub, K. Winkler); Hannover, Germany (H. Riehm, A. Reiter); Heidelberg, Germany (K.M. Debatin); Herdecke, Germany (C. Tautz); Homburg, Germany (N. Graf); Innsbruck, Austria (F.M. Fink); Jena, Germany (F. Zintl, J. Hermann); Kaiserslautern, Germany (J. Krüger); Karlsruhe, Germany (G. Nessler); Kassel, Germany (H. Wehinger); Kiel, Germany (R. Schneppenheim); Köln, Germany (F. Berthold, W. Sternschulte); Leipzig, Germany (M. Domula); Linz, Austria (K. Schmitt); Ludwigshafen, Germany (H. C. Dominick); Mainz, Germany (P. Gutjahr); Mannheim, Germany (O. Sauer); Marburg, Germany (C. Eschenbach); Minden, Germany (M. Scharnetzky); München, Germany (C. Bender-Götze, S. Müller-Weihrich, R.J. Haas); Münster, Germany (J. Ritter, H. Jürgens); Nijmegen, the Netherlands (J. Bökkerink); Nürnberg, Germany (A. Jobke); Rostock, Germany (G. Eggers); Saarbrücken, Germany (R. Geib); Sankt Augustin, Germany (K. von Schnakenburg); Stuttgart, Germany (J. Treuner); Trier, Germany (W. Rauh); Tübingen, Germany (D. Niethammer); Ulm, Germany (W. Behnisch); Wien, Austria (H. Gadner); Wuppertal, Germany (B. Dohrn); Würzburg, Germany (J. Kühl); Zürich, Switzerland (E. Frey).
Supported by grants from Deutsche Krebshilfe, Bonn, Germany.
A complete list of the members of the Berlin-Frankfurt-Münster Relapse Study Group appears in the “.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anja Borgmann, Humboldt University of Berlin, Charité, Department of Pediatric Oncology/Hematology, Augustenburger Platz 1, D-13353 Berlin, Germany; e-mail:anja.borgmann@charite.de.