Abstract
Event-free survival for children with acute lymphoblastic leukemia (ALL) now exceeds 80% in the most effective trials. Failures are due to relapse, toxicity, and second cancers such as therapy-related myeloid leukemia or myelodysplasia (t-ML). Topoisomerase II inhibitors and alkylators can induce t-ML; additional risk factors for t-ML remain poorly defined. The occurrence of t-ML among children who had received granulocyte colony-stimulating factor (G-CSF) following ALL remission induction therapy prompted us to examine this and other putative risk factors for t-ML in 412 children treated on 2 consecutive ALL protocols from 1991 to 1998. All children received etoposide and anthracyclines, 99 of whom received G-CSF; 284 also received cyclophosphamide, 58 of whom also received cranial irradiation. There were 20 children who developed t-ML at a median of 2.3 years (range, 1.0-6.0 years), including 16 cases of acute myeloid leukemia, 3 myelodysplasia, and 1 chronic myeloid leukemia. Stratifying by protocol, the cumulative incidence functions differed (P = .017) according to the use of G-CSF and irradiation: 6-year cumulative incidence (standard error) of t-ML of 12.3% (5.3%) among the 44 children who received irradiation without G-CSF, 11.0% (3.5%) among the 85 children who received G-CSF but no irradiation, 7.1% (7.2%) among the 14 children who received irradiation plus G-CSF, and 2.7% (1.3%) among the 269 children who received neither irradiation nor G-CSF. Even when children receiving irradiation were excluded, the incidence was still higher in those receiving G-CSF (P = .019). In the setting of intensive antileukemic therapy, short-term use of G-CSF may increase the risk of t-ML.
Introduction
Treatment of childhood acute lymphoblastic leukemia (ALL) has made tremendous strides over the past 20 years, but has been complicated by the induction of secondary tumors after some regimens. The cumulative incidence of therapy-related myeloid leukemia and myelodysplastic syndrome (referred to collectively as t-ML) varies widely among treatment protocols, from 1% to 12%.1-5Among children with ALL, t-ML secondary to topoisomerase II inhibitors, characterized by balanced translocations often involving theMLL gene on 11q23, has been the most common t-ML, but t-ML characteristic of that induced by alkylating agents (eg, preceded by myelodysplasia, displaying monosomy 5 or 7) has also been reported.3,6,7 The entity of t-ML occurs not only in survivors of cancer but also in those treated with topoisomerase II inhibitors or alkylators for nonmalignant diseases4,8; thus, risk factors for t-ML among patients with ALL may have relevance for other patients treated with these medications. Because there are many treatment regimens that include high doses of alkylators and topoisomerase II inhibitors that are not associated with a high risk of t-ML,1,4,6 it is clear that there are other factors that cooperate with the primary leukemogen to influence the risk of t-ML. We and others have investigated host risk factors for t-ML,9-12 but the development of t-ML likely depends upon interactions between host and treatment-related risk factors. Heretofore, additional facilitative treatment-related risk factors identified have included irradiation2,5,13 and medications such as asparaginase14,15 and thiopurines,3 9which may enhance the leukemogenic properties of the primary leukemogens: topoisomerase II inhibitors, and alkylators.
Since their discovery, it has been questioned whether granulocyte colony-stimulating factors could facilitate the growth of malignant myeloid cells or could induce a myeloid leukemia.16-20 Granulocyte colony-stimulating factor (G-CSF) induces the growth of primary acute myeloid leukemic blasts in vitro in about 50% of cases,16 and it increases the proliferation and maturation of myeloid progenitors.16However, G-CSF was not leukemogenic in mice,17 induced differentiation of granulocyte precursors, and even had an antileukemic effect in some preclinical models.17 Thus, colony-stimulating factors have now been tested in several randomized phase 3 trials to treat acute myeloid leukemia (AML).21 These trials have failed to show any effect of G-CSF on leukemia-free or overall survival in patients with AML.21-25
There are no controlled trials indicating an excess risk of t-ML among patients treated with G-CSF or granulocyte-macrophage colony-stimulating factor (GM-CSF); however, there are few reports on long-term outcome of any kind in controlled trials of G-CSF or GM-CSF, especially compared with placebo.24,26-28 Although there is a relatively high incidence (5%-27%) of myeloid malignancies following G-CSF use for aplastic anemia or congenital neutropenia,29-35 the underlying predisposition of such patients to develop myeloid malignancy has confounded attempts to determine whether growth factors could contribute to the myelodysplasia or myeloid leukemia in these patients. Thus, it is not currently known whether hematopoietic growth factors are a risk factor for t-ML.
We performed one of the few placebo-controlled randomized studies of G-CSF in a relatively large group (n = 164) of children with ALL,36 and because of effective chemotherapy a large percentage (> 75%) of patients are long-term survivors. The occurrence of cases of t-ML among patients with ALL who received G-CSF prompted us to perform the current analysis of whether G-CSF could be a risk factor for t-ML. Because other factors (eg, irradiation) might have also impacted the risk of t-ML, we extended the analysis to include a relatively large cohort of consecutively treated children with ALL (n = 412) to account for potentially confounding risk factors.
Patients and methods
Patients with newly diagnosed ALL were enrolled on St Jude Children's Research Hospital protocol Total XIIIA (1991-1994)37 or on Total XIIIB (1994-1998) (Tables1-2). Patients or their parents (as appropriate) gave informed consent to participate. Both protocols, and the current analysis of risk factors for t-ML, were approved by the institutional review board. The 2 treatment arms (low and intermediate/high) of each protocol had identical cumulative doses and frequency of administration of topoisomerase II inhibitors and alkylating agents. A total of 164 of the 167 patients enrolled on Total XIIIA were stratified by their up-front methotrexate treatment dose and risk group (based on age, initial leukocyte count, presence of the Philadelphia chromosome, and DNA content of the leukemic blasts) and randomized to receive 10 μg/kg/d G-CSF subcutaneously (80 patients) or placebo (84 patients) for 15 days after remission induction that included etoposide, or until the neutrophil count was higher than 1 × 109/L for 2 days.36 The dose of 10 μg/kg was based on the fact that pharmacokinetic data in children indicated that G-CSF exposure was dose-related over the 5 to 10 μg/kg dosage range,39 and that early data had suggested that children might benefit from the higher 10 μg/kg dose. The optimal dosage in children has not been established.22This was the only use of G-CSF among patients on Total XIIIA for the duration of their ALL treatment. G-CSF use on Total XIIIB was not randomly assigned, but was at the discretion of the treating physician, and was generally given to patients with prolonged or severe neutropenia to attempt to hasten neutrophil recovery following chemotherapy. To confirm use on Total XIIIA, and to identify use on Total XIIIB, we performed comprehensive computer searches for all prescriptions and orders for hematologic growth factors for patients treated on Total XIIIA and B from December of 1991 to January 2000, and then performed detailed medical record reviews to verify the administration, dose, and duration of growth factor use (all growth factors were G-CSF) given during the time period of front-line protocol therapy. The 14% of patients at higher risk of central nervous system (CNS) relapse37 received irradiation at one year of therapy on both protocols (Tables 1-2).
We defined the event of t-ML to include AML, relapse with a lineage switch, myelodysplastic syndrome, and chronic myeloid leukemia. Competing events included induction failure, isolated CNS relapse, testicular relapse, all other relapses, and death. Patients were classified as having received G-CSF or irradiation if G-CSF or irradiation was given prior to an event. Patients remaining in complete remission were classified as having received G-CSF or irradiation if G-CSF or irradiation was ever given.
The t-ML–free survival was calculated from the initial complete remission date to the date of the diagnosis of the t-ML for those who experienced a t-ML, and from the initial remission date to the date of a competing event for patients experiencing a competing event. Patients who were still alive and free of any kind of event were censored at the time of last follow-up. Patients who had stem cell transplantation before any kind of event at the time of analysis were censored at the time of transplantation, as their therapy then changed substantially; no patients who developed t-ML underwent stem cell transplantation prior to their t-ML. The cumulative incidence of t-ML was estimated using the method described by Kalbfleisch and Prentice,40 and the cumulative incidence functions were compared using the method described by Gray.41 Because asparaginase use has been linked to the risk of t-ML,14 15 and protocol Total XIIIA had more asparaginase than Total XIIIB (Tables 1-2), analyses were stratified by treatment protocol.
Acquired point mutations in exon 17 of the G-CSF receptor have been linked by some but not others33,34 42 to the development of t-ML in patients who have received G-CSF. Intron 16 and exon 17 were amplified with 2 separate polymerase chain reactions (PCRs) using DNA from bone marrow collected at the time of diagnosis of t-ML, using primer pairs AACAGCTCAGAGACCTGTGGCCT and GGCCATTGGGTGGGGGCTGGAT or TGCCCAGAATCATGGAGGAG and TGGAGTCACAGCGGAGATAG. Amplification conditions were 95°C for 5 minutes, 40 cycles at 95°C for 45 seconds, 61°C for 1 minute, and 70°C for 1 minute. Sequence was obtained from at least 4 separate amplifications for each sample in both forward and reverse orientations.
Results
Presenting features of the 412 patients are indicated in Table3. With a median follow-up of 5.7 years, 20 patients have developed a t-ML, with a median onset of 2.3 years (range, 1.0-6.0 years) from the start of ALL therapy. Of these, 16 presented with acute myeloid leukemia, 3 with myelodysplasia, and 1 with chronic myeloid leukemia. Among the 99 patients who received G-CSF, the median daily dose of G-CSF was 10 mcg/kg/d (coefficient of variation of only 1.9% in daily dose), median duration of use was 9 days, and the median time that G-CSF started relative to ALL therapy was 34 days (corresponding to the day following the last etoposide/cytarabine of remission induction therapy). The distribution of G-CSF use and irradiation therapy among patients on each protocol is listed in Table 4.
Our goal was to evaluate whether G-CSF was associated with the risk of t-ML, accounting for other risk factors that were variant in the antileukemic therapy for these 412 patients. All patients received epipodophyllotoxins. Among patients who received neither G-CSF nor irradiation, there was no evidence of a difference in the incidence of t-ML for patients on the intermediate/high risk versus those on the low-risk arms of Total XIIIA (4.9% ± 2.8% vs 0%, respectively;P = .55) or of Total XIIIB (1.5% ± 1.5% vs 1.0% ± 1%, respectively; P = .72). When patients treated in both protocols were combined for analysis, there was also no evidence for a difference (P = .52) in the risk of t-ML between those on the intermediate/high-risk and low-risk arms (3.7% ± 2.0% vs 0.9% ± 0.9%, respectively), despite the fact that patients on the intermediate/high-risk arms of both studies received more doses of epipodophyllotoxins and cyclophosphamide (Table2). Thus, when evaluating the possible role of irradiation and G-CSF on the development of t-ML, we did not stratify for risk group. Because use of asparaginase has been linked to the risk of t-ML,14 15 and asparaginase was more extensively used in Total XIIIA than in Total XIIIB (Table 2), analyses were stratified for asparaginase use by stratifying for treatment protocol.
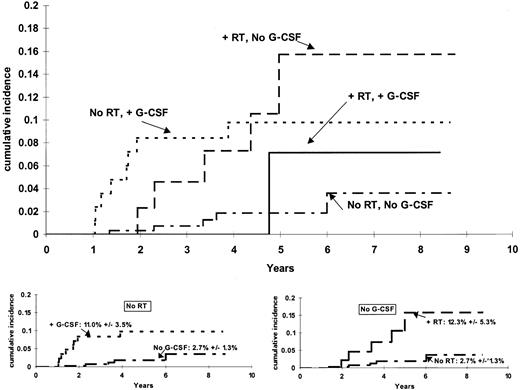
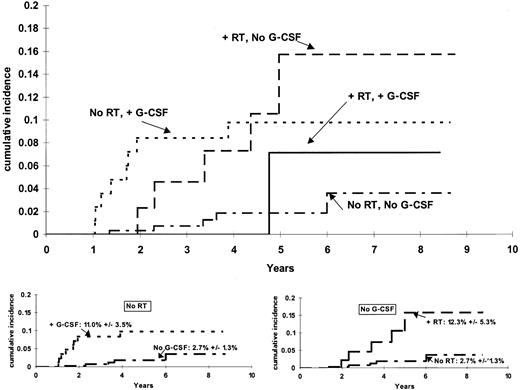
The cumulative incidence functions for risk of t-ML differed significantly among patients grouped by their G-CSF and irradiation treatment (P = .017, stratified by protocol) (Figure1). Among those who did not receive G-CSF, there was a higher incidence (P = .0038) of t-ML among those who received irradiation (6-year estimate, 12.3% ± 5.3%) than among those who did not receive irradiation (6-year estimate, 2.7% ± 1.3%). Among those who did not receive irradiation, there was a higher incidence (P = .019) of t-ML among those who did versus did not receive G-CSF (11.0% ± 3.5% vs 2.7% ± 1.3%). The cumulative incidence of t-ML was similar (P = .87) among patients who had only one risk factor: 6-year estimates of 12.3% ± 5.3% for those who received irradiation but no G-CSF and 11.0% ± 3.5% for those who received G-CSF but no irradiation.
Cumulative incidence of t-ML.
The incidence of t-ML differed among the 14 patients who received radiation therapy (RT) and G-CSF, the 44 patients who received RT and no G-CSF, the 85 patients who received G-CSF but no RT, and the 269 patients who received neither G-CSF nor RT (P = .017). For those who did not receive RT (bottom left), there was a higher incidence (P = .0192) of t-ML top among those who did versus did not receive G-CSF (6-year estimates, 11.0% ± 3.5% versus 2.7% ± 1.3%). For those who did not receive G-CSF (bottom right), there was a higher incidence (P = .0038) of t-ML among those who received RT (6-year estimate, 12.3% ± 5.3%) than among those who did not receive RT (6-year estimate, 2.7% ± 1.3%).
Cumulative incidence of t-ML.
The incidence of t-ML differed among the 14 patients who received radiation therapy (RT) and G-CSF, the 44 patients who received RT and no G-CSF, the 85 patients who received G-CSF but no RT, and the 269 patients who received neither G-CSF nor RT (P = .017). For those who did not receive RT (bottom left), there was a higher incidence (P = .0192) of t-ML top among those who did versus did not receive G-CSF (6-year estimates, 11.0% ± 3.5% versus 2.7% ± 1.3%). For those who did not receive G-CSF (bottom right), there was a higher incidence (P = .0038) of t-ML among those who received RT (6-year estimate, 12.3% ± 5.3%) than among those who did not receive RT (6-year estimate, 2.7% ± 1.3%).
In our prior studies,2 43 all patients treated with epipodophyllotoxins and alkylators for higher risk ALL also received irradiation, thereby precluding our ability to assess the impact of irradiation on t-ML risk. In the current study, only a fraction of the higher risk patients received radiation. When we restricted the analysis to the 193 patients on the higher-risk arms of Total XIIIA and XIIIB who did not receive G-CSF (all of whom received cyclophosphamide and a large number of etoposide doses [Tables 1-2] and 23% of whom received irradiation), irradiation therapy was associated with a higher risk of t-ML, with 6-year estimates of 12.3% ± 5.3% vs 3.7% ± 2.0% (P = .0320). When we restricted the analysis to the 164 patients on Total XIIIA, for whom G-CSF was administered as part of the randomized trial, there was no statistically significant difference in the risk of t-ML in those who did versus did not receive G-CSF (10.0% ± 3.4% vs 6.0% ± 2.6%; P = .33, stratifying for radiation therapy).
Among the 5 t-ML cases who received irradiation but no G-CSF, 2 had myelodysplastic syndrome (one with an 11q23 translocation), 1 had chronic myelogenous leukemia (CML) with a t(9;22), and 2 had acute myeloid leukemia with 11q23 translocations. Among the 9 t-ML patients who received G-CSF but no irradiation, 1 developed myelodysplastic syndrome and 8 developed acute myeloid leukemia (7 with 11q23 translocations). Among the 5 t-ML patients who received neither G-CSF nor irradiation, all 5 had acute myeloid leukemia (4 with 11q23 translocations). The single case of t-ML among the 14 patients who received irradiation and G-CSF had acute myeloid leukemia with an 11q23 translocation.
No heterozygous or homozygous mutations were detected in exon 17 of the G-CSF receptor in DNA from bone marrow obtained at the time of diagnosis of t-ML in any of the 20 patients who developed t-ML.
Discussion
Multiple therapy-related and host-specific risk factors are likely to contribute to the development of secondary leukemias,2-6,9-11,13,15,44-47 in addition to the well-known contribution of topoisomerase II inhibitors and alkylating agents to t-ML.44,47,48 In the present study, topoisomerase II inhibitors were given to all children. Our goal was to identify additional treatment-related factors, besides topoisomerase II inhibitors, that contributed to the risk of t-ML. Several studies have attempted to identify other “coleukemogens” that affect the risk of t-ML, suggesting that irradiation5,13,43 and concurrent asparaginase use14 15 may increase the risk of t-ML following potentially leukemogenic multiagent chemotherapy. In the current analysis of 412 patients treated on 2 consecutive front-line ALL trials, even after stratifying for asparaginase use and accounting for irradiation, we found that G-CSF use was associated with an increased risk of t-ML.
A proleukemogenic effect of hematologic growth factors has long been a theoretical concern.16-19 Despite shortening the need for acute hospitalization in patients with AML,49,50 G-CSF has demonstrated no effect on event-free survival or overall survival.21,22,24,25,50 Thus, it is possible that any potentially improved treatment response due to G-CSF or its effects on delivery of chemotherapy (which was observed as a higher complete remission rate in one study)25 could be nullified by its proliferative effects on leukemic clones. A few case reports in patients with t-ML have included withdrawal and rechallenge with G-CSF, indicating that G-CSF use was temporally associated with proliferation of myeloid leukemia.51-53 However, promoting the proliferation of an existing myeloid leukemia may involve different mechanisms than promoting the genesis of t-ML.
If G-CSF can facilitate leukemogenesis of chemotherapy, given its widespread use,54,55 it might be argued that more cases of t-ML should have been reported. The incidence of t-ML overall is not known, and such documentation is confounded because many myeloid malignancy trials exclude t-ML cases, and most front-line randomized studies of hematopoietic growth factors lack long-term follow-up. A study of acute myeloid leukemia, which did not exclude patients previously treated with growth factors or with chemo- or radiotherapy, reported that 24% (50/211) of its patients presented as t-ML, although only a few had been documented to have received prior myeloid growth factors.24 Although G-CSF is widely used in patients with cancer, few studies have evaluated long-term outcome,24,26and only rarely have they specifically reported a lack of t-ML.26 Most randomized studies have been of patients who are at high risk of relapse of their primary malignancy; in a randomized study of G-CSF with relatively long follow-up (up to 4 years), the median survival in the G-CSF and placebo groups was only 6 and 9 months (P = .71), respectively,24 far shorter than the median onset time for t-ML. Overall 3-year survival was only 21% and 19% in G-CSF versus placebo arms in another trial.23
In nonrandomized trials of G-CSF during potentially leukemogenic chemotherapy, a higher-than-expected frequency of t-ML has occasionally been reported,45,46,56 and it is possible that G-CSF could have contributed to t-ML following intensification of therapy for women with breast cancer.57,58 By inhibiting apoptosis of hematopoietic precursors, affecting differentiation, or enhancing proliferation of clones carrying leukemogenic genomic fusions,58 growth factors could contribute to leukemogenesis initiated by other potentially leukemogenic stimuli (eg, radiation, alkylators, and topoisomerase II inhibitors). Because the dose intensity of leukemogenic anticancer therapy has been increased in parallel with the introduction of G-CSF in many nonrandomized studies,56,57 it has not been possible to distinguish the contribution of intensified therapy versus G-CSF. However, there are conflicting data as to whether t-ML is related to either intensity or cumulative doses of chemotherapy.2,6,43,45,59 60 Thus, higher frequencies of t-ML observed on “modern” trials, which include both growth factors and “more intense” chemotherapy, cannot necessarily be ascribed to the more intense chemotherapy. We acknowledge that when the current analysis was restricted to only the 164 children on Total XIIIA, in which use of G-CSF was solely based upon randomization, there was no statistically significant association of G-CSF with risk of t-ML. Because of the relatively small sample size in that subset, the fact that irradiation was confounding and its use was not randomized, and that administration of etoposide-containing chemotherapy was not altered by the administration of G-CSF, we think it is appropriate to present results for the entire cohort of 412 patients (those who did and did not receive either G-CSF or irradiation, respectively, whether randomized or not). Because of the now widespread (and therefore undocumented) use of G-CSF, it will become increasingly difficult to track any potential long-term effects of the agent in cancer trials.
Whether the timing or dosing of G-CSF relative to chemotherapy might have an impact on its putative leukemogenic effects is not known. The dose of G-CSF that was used in most patients (10 mcg/kg) in this study is higher than that recommended for most indications in adults,22 and thus a lower dose of G-CSF might not have been similarly associated with t-ML risk. The timing of G-CSF use might also be important: most patients in the current study received G-CSF immediately following topoisomerase II inhibitors at the end of remission induction therapy. We previously “back-tracked” the molecular emergence of t-ML in one of our ALL patients (reported herein). This patient received only 6 doses of topoisomerase II inhibitors, G-CSF before and after the first 3 topoisomerase II inhibitors doses during induction therapy, no alkylators, and no irradiation.61 We found that t-ML emerged early in therapy, after exposure to only the 3 induction doses of topoisomerase II inhibitors with G-CSF. Given that most patients in the current study received G-CSF at this same time point—in the days immediately following etoposide—it is possible that G-CSF affected the differentiation, survival, or proliferation of a t-ML precursor that had already acquired the primary leukemogenic genetic lesion within the first few weeks of therapy. Because G-CSF has immediate pharmacologic effects on its target cells,16,17 chronic administration of G-CSF therapy would not necessarily be a requirement for any cooperative effect of growth factors on leukemogenesis of other therapy. As studies of back-tracking for t-ML have proved,61 the initiating events causing leukemogenic cytogenetic abnormalities can happen over a very short time period of days-to-weeks.
Secondary acute myeloid leukemia is divided into 2 major types: that due to topoisomerase II inhibitors and that due to alkylating agents.48 The former type is characterized by a short onset, the lack of a prodromal phase, and balanced translocations, most often involving MLL on 11q23.2,4,6,47,48Alkylator- and radiation-associated t-ML typically has a longer onset, is often preceded by a myelodysplastic phase, and displays chromosomal deletions, especially of chromosomes 5 and 7.47,62 Herein, 15 of 20 t-ML cases displayed 11q23 translocations, suggesting that most cases were at least partly due to topoisomerase II inhibitors, and 3 of 20 cases presented with myelodysplasia. Interestingly, the t-ML we observed in the patients who received irradiation alone (no G-CSF) plus ALL systemic therapy was more likely to exhibit alkylator-like t-ML characteristics (2 with myelodysplasia) than the other G-CSF/irradiation subgroups. This group also included an unusual case of secondary CML carrying a t(9;22) (in a patient who was negative for the t(9;22) at diagnosis of ALL), a translocation for which fusion products have been induced in vitro by irradiation.63 It is possible that both irradiation and G-CSF can act as cooperative factors for leukemogenesis initiated by other primary leukemogens (eg, topoisomerase II inhibitors, alkylators), and thus the t-ML we observed reflected the molecular signatures of several possible primary leukemogens. The cumulative incidence of t-ML among children who received irradiation but no G-CSF was similar to that observed among children who received G-CSF but no irradiation (P = .87), suggesting that either one of these modalities provided an additional leukemogenic hit that enhanced leukemogenesis. Because only 14 children received both G-CSF and irradiation (in addition to topoisomerase II inhibitors and alkylators), it is not possible to speculate as to whether their putative cooperative leukemogenic effects were “additive” in this setting.
Given the lack of effect of G-CSF on long-term outcomes among cancer patients,23-26 and some contradictory data on its efficacy for short-term outcomes,23-25 64 our observation of a possible contribution of G-CSF to the risk of t-ML dictates that caution be exercised in adding hematopoietic growth factors to intensive antineoplastic regimens.
We thank Michael Hancock, Terreia Jones, Jean Cai, and Yinmei Zhou for analytical assistance; the clinical and laboratory staff at St Jude Children's Research Hospital; and the patients and their families for participating.
None of the funding sources had any role in the design or analysis of these data, nor in the decision to submit this manuscript. The authors have no financial or personal conflicts of interest related to this work.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/blood-2002-08-2405.
Supported by NCI CA 51001, CA 78224, CA 21765, and the National Institutes of Health (NIH)/National Institute of General Medical Sciences (NIGMS) Pharmacogenetics Research Network and Database (U01 GM61393, U01 GM61374 [http://pharmgkb.org/do/serve?id=home.welcome]) from the NIH; by a Center of Excellence grant from the State of Tennessee; and by American Lebanese Syrian Associated Charities (ALSAC). C.-H.P. is American Cancer Society FM Kirby Clinical Research Professor.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mary V. Relling, Department of Pharmaceutical Sciences, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38105; e-mail:mary.relling@stjude.org.