Abstract
Autoimmune hemolytic anemia (AIHA) in children is sometimes characterized by a severe course, requiring prolonged administration of immunosuppressive therapy. Rituximab is able to cause selective in vivo destruction of B lymphocytes, with abrogation of antibody production. In a prospective study, we have evaluated the use of rituximab for the treatment of AIHA resistant to conventional treatment. Fifteen children with AIHA were given rituximab, 375 mg/m2/dose for a median of 3 weekly doses. All patients had previously received 2 or more courses of immunosuppressive therapy; 2 patients had undergone splenectomy. After completing treatment, all children received intravenous immunoglobulin for 6 months. Treatment was well tolerated. With a median follow-up of 13 months, 13 patients (87%) responded, whereas 2 patients did not show any improvement. Median hemoglobin levels increased from 7.7 g/dL to a 2-month posttreatment level of 11.8 g/dL (P < .001). Median absolute reticulocyte counts decreased from 236 to 109 × 109/L (P < .01). An increase in platelet count was observed in patients with concomitant thrombocytopenia (Evans syndrome). Three responder patients had relapse, 7, 8, and 10 months after rituximab infusion, respectively. All 3 children received a second course of rituximab, again achieving disease remission. Our data indicate that rituximab is both safe and effective in reducing or even abolishing hemolysis in children with AIHA and that a sustained response can be achieved in the majority of cases. Disease may recur, but a second treatment course may be successful in controlling the disease.
Introduction
Autoimmune hemolytic anemia (AIHA) in children is usually characterized by a good prognosis; the disease often presents as an acute, self-limited illness, with good response to short-term steroid therapy in nearly 80% of patients.1 However, in some cases, AIHA can be characterized by a chronic course and an unsatisfactory control of hemolysis, thus requiring prolonged immunosuppressive therapy. Especially in children younger than 2 years of age or in teenagers, the clinical course of the disease may show either resistance to steroids or dependence on high-dose steroids,2 with subsequent development of severe side effects on growth, bone mineralization, and the endocrine system. The mortality rate in these children with primary AIHA has been reported to be on the order of 10%.1
Splenectomy, administration of immunosuppressive drugs such as azathioprine, cyclosporine A, or cyclophosphamide, or immunomodulating agents, such as intravenous immunoglobulin, have been used frequently, alone or in combinations, with the aim of reducing steroid dependence and controlling hemolysis. However, these therapies are not consistently effective and present a nonnegligible risk of infectious complications due to their profound immunosuppression.1
Rituximab is a chimeric, human, IgG1/κ monoclonal antibody (MoAb) specific for the CD20 antigen, expressed on the surface of B lymphocytes. This antibody has induced rapid in vivo depletion of both normal B lymphocytes and lymphoma B cells.3 4
Its in vivo mechanisms of action include complement-mediated cytotoxicity, antibody-dependent cytotoxicity, inhibition of B-cell proliferation, and induction of apoptosis.4
Rituximab has demonstrated good clinical activity in the treatment of relapsed, low-grade B-cell CD20+ non-Hodgkin lymphoma (NHL), particularly in patients with follicular NHL.5-8
The good therapeutic efficacy, coupled with its limited toxicity, consisting primarily of infusion-related events, has led to the recent use of this agent for the treatment of autoimmune disorders, with the aim of interfering with or, at best, abolishing autoantibody production.9-17
Preliminary results of this application appeared encouraging9-17 and prompted us to evaluate, in a prospective multicenter study, the efficacy of rituximab for treatment of children affected by AIHA resistant to conventional immunosuppressive therapy or requiring continuous high-dose steroid treatment.
Patients and methods
Patient characteristics
Five boys and 10 girls, with a median age at diagnosis of 2 years (range, 0.3-14 years), were prospectively enrolled in this study, approved by the institutional review boards of the participating centers. Written informed consent was obtained from the parents. Data on one of these patients have been previously reported.18
Nine children were affected by AIHA, and the other 6 patients had Evans syndrome. We found no evidence of primary T- or B-lymphocyte deficiency in these patients.
In 13 cases, warm-reactive autoantibodies of IgG type were demonstrated by the direct antiglobulin test (DAT), whereas in another case IgM cold agglutinins were present, and in another DAT remained repeatedly negative.
In 4 children a concomitant autoimmune disease was present at time of AIHA onset: systemic lupus erythematosus in 1, rheumatoid arthritis in 2, and vitiligo in 1. In one child, AIHA developed after allogeneic bone marrow transplantation, performed for treatment of mucopolysaccharidosis.
Further details on the clinical features of the patients enrolled and on pretreatment hematologic values are reported in Table1.
Patient characteristics
| Patient no. . | Sex . | Age at diagnosis, y . | Diagnosis . | Concomitant disease . | Type of antibody . | Hematologic values before rituximab treatment . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hb level, g/dL . | Reticulocyte count, × 109/L . | Platelet count, × 109/L . | DAT . | IAT . | Transfusion dependence . | ||||||
| Responders | |||||||||||
| 1 | M | 0.3 | AIHA | — | Warm (IgG) | 6.6 | 390 | — | Pos | Pos | Yes |
| 2 | M | 0.3 | Evans | — | Warm (IgG) | 7.1 | 175 | 43 | Pos | Neg | No |
| 3 | F | 1.6 | AIHA | — | Negative | 7.8 | 230 | — | Neg | Neg | Yes |
| 4 | F | 1.8 | AIHA | — | Warm (IgG) | 10.0 | 210 | — | Pos | Pos | No |
| 5 | F | 1.9 | AIHA | Post-BMT | Warm (IgG) | 9.2 | 233 | — | Pos | Neg | No |
| 6 | F | 7.6 | Evans | Rheum arth | Warm (IgG) | 8.0 | 236 | 4 | Pos | Neg | No |
| 7 | F | 11.4 | AIHA | — | Warm (IgG) | 9.8 | 463 | — | Pos | Neg | No |
| 8 | F | 12.5 | Evans | Rheum arth | Warm (IgG) | 7.1 | 433 | 27 | Pos | Neg | No |
| 9 | M | 13.5 | AIHA | — | Warm (IgG) | 5.6 | 374 | — | Pos | Pos | No |
| 10 | F | 13.8 | AIHA | — | Warm (IgG) | 7.4 | 750 | — | Pos | Neg | No |
| Responders who relapsed | |||||||||||
| 11 | M | 1.3 | Evans | Vitiligo | Cold (IgM) | 9.3 | 185 | 61 | Pos | Neg | No |
| 12 | F | 1.3 | Evans | — | Warm (IgG) | 9.7 | 159 | 12 | Pos | Neg | No |
| 13 | F | 10.1 | AIHA | SLE | Warm (IgG) | 3.5 | 118 | — | Pos | Pos | Yes |
| Nonresponders | |||||||||||
| 14 | M | 1.1 | AIHA | — | Warm (IgG) | 6.9 | 240 | — | Pos | Pos | Yes |
| 15 | F | 13.6 | AIHA | — | Warm (IgG) | 7.7 | 428 | — | Pos | Neg | No |
| Median | 1.9 | 7.7 | 236 | 27 | |||||||
| Range | 0.3-13.8 | 3.5-10 | 118-750 | 4-61 | |||||||
| Patient no. . | Sex . | Age at diagnosis, y . | Diagnosis . | Concomitant disease . | Type of antibody . | Hematologic values before rituximab treatment . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hb level, g/dL . | Reticulocyte count, × 109/L . | Platelet count, × 109/L . | DAT . | IAT . | Transfusion dependence . | ||||||
| Responders | |||||||||||
| 1 | M | 0.3 | AIHA | — | Warm (IgG) | 6.6 | 390 | — | Pos | Pos | Yes |
| 2 | M | 0.3 | Evans | — | Warm (IgG) | 7.1 | 175 | 43 | Pos | Neg | No |
| 3 | F | 1.6 | AIHA | — | Negative | 7.8 | 230 | — | Neg | Neg | Yes |
| 4 | F | 1.8 | AIHA | — | Warm (IgG) | 10.0 | 210 | — | Pos | Pos | No |
| 5 | F | 1.9 | AIHA | Post-BMT | Warm (IgG) | 9.2 | 233 | — | Pos | Neg | No |
| 6 | F | 7.6 | Evans | Rheum arth | Warm (IgG) | 8.0 | 236 | 4 | Pos | Neg | No |
| 7 | F | 11.4 | AIHA | — | Warm (IgG) | 9.8 | 463 | — | Pos | Neg | No |
| 8 | F | 12.5 | Evans | Rheum arth | Warm (IgG) | 7.1 | 433 | 27 | Pos | Neg | No |
| 9 | M | 13.5 | AIHA | — | Warm (IgG) | 5.6 | 374 | — | Pos | Pos | No |
| 10 | F | 13.8 | AIHA | — | Warm (IgG) | 7.4 | 750 | — | Pos | Neg | No |
| Responders who relapsed | |||||||||||
| 11 | M | 1.3 | Evans | Vitiligo | Cold (IgM) | 9.3 | 185 | 61 | Pos | Neg | No |
| 12 | F | 1.3 | Evans | — | Warm (IgG) | 9.7 | 159 | 12 | Pos | Neg | No |
| 13 | F | 10.1 | AIHA | SLE | Warm (IgG) | 3.5 | 118 | — | Pos | Pos | Yes |
| Nonresponders | |||||||||||
| 14 | M | 1.1 | AIHA | — | Warm (IgG) | 6.9 | 240 | — | Pos | Pos | Yes |
| 15 | F | 13.6 | AIHA | — | Warm (IgG) | 7.7 | 428 | — | Pos | Neg | No |
| Median | 1.9 | 7.7 | 236 | 27 | |||||||
| Range | 0.3-13.8 | 3.5-10 | 118-750 | 4-61 | |||||||
Evans indicates Evans syndrome; post-BMT, patient given allogeneic bone marrow transplantation for treatment of type 1 mucopolysaccharidosis; Rheum arth, rheumatoid arthritis; SLE, systemic lupus erythematosus; —, not applicable.
All children had received 2 or more courses of immunosuppressive treatment. Eleven patients were given also a third immunosuppressive course, and splenectomy had been performed in 2 patients, in an attempt to control hemolysis. Details on immunosuppressive therapy before rituximab therapy are reported in Table2.
Therapies administered before rituximab treatment
| Patient no. . | First-line treatment . | Second-line treatment . | Interval from first- to second-line treatment, mo . | Third-line treatment . | Interval from second- to third-line treatment, mo . | Splenectomy . | Interval from diagnosis to splenectomy . |
|---|---|---|---|---|---|---|---|
| Responders | |||||||
| 1 | IV MPDN | Oral PDN | 0.5 | IV IgG | 0.5 | — | — |
| 2 | IV IgG | Oral PDN | 1.9 | — | — | — | — |
| 3 | IV IgG | Oral PDN | 0.3 | IV MPDN | 0.4 | ||
| 4 | Oral PDN + AZA | IV IgG | 18.0 | CY | 3.8 | Yes | 22 |
| 5 | Oral PDN | Cs-A | 0.3 | — | — | — | — |
| 6 | IV IgG | Oral PDN | 0.3 | IV MPDN | 7.6 | — | — |
| 7 | Oral PDN | IV IgG | 6.1 | Oral PDN | 5.2 | — | — |
| 8 | Oral PDN | IV IgG | 0.3 | Cs-A + AZA | 4.5 | — | — |
| 9 | IV MPDN | IV MPDN | 3.9 | IV IgG | 0.5 | — | — |
| 10 | IV MPDN | AZA | 0.3 | — | — | — | — |
| Responders who relapsed | |||||||
| 11 | IV MPDN | AZA | 0.3 | Cs-A + AZA | 1 | — | — |
| 12 | Oral PDN | IV IgG | 0.4 | AZA | 23 | — | — |
| 13 | IV IgG | IV MPDN | 15.4 | Cs-A + AZA | 30 | — | — |
| Nonresponders | |||||||
| 14 | IV MPDN | AZA | 0.8 | — | — | — | — |
| 15 | IV MPDN | IV IgG | 3.1 | Cs-A + AZA | 8 | Yes | 16 |
| Median | 0.5 | 4.5 | |||||
| Range | 0.3-18 | 0.4-30 |
| Patient no. . | First-line treatment . | Second-line treatment . | Interval from first- to second-line treatment, mo . | Third-line treatment . | Interval from second- to third-line treatment, mo . | Splenectomy . | Interval from diagnosis to splenectomy . |
|---|---|---|---|---|---|---|---|
| Responders | |||||||
| 1 | IV MPDN | Oral PDN | 0.5 | IV IgG | 0.5 | — | — |
| 2 | IV IgG | Oral PDN | 1.9 | — | — | — | — |
| 3 | IV IgG | Oral PDN | 0.3 | IV MPDN | 0.4 | ||
| 4 | Oral PDN + AZA | IV IgG | 18.0 | CY | 3.8 | Yes | 22 |
| 5 | Oral PDN | Cs-A | 0.3 | — | — | — | — |
| 6 | IV IgG | Oral PDN | 0.3 | IV MPDN | 7.6 | — | — |
| 7 | Oral PDN | IV IgG | 6.1 | Oral PDN | 5.2 | — | — |
| 8 | Oral PDN | IV IgG | 0.3 | Cs-A + AZA | 4.5 | — | — |
| 9 | IV MPDN | IV MPDN | 3.9 | IV IgG | 0.5 | — | — |
| 10 | IV MPDN | AZA | 0.3 | — | — | — | — |
| Responders who relapsed | |||||||
| 11 | IV MPDN | AZA | 0.3 | Cs-A + AZA | 1 | — | — |
| 12 | Oral PDN | IV IgG | 0.4 | AZA | 23 | — | — |
| 13 | IV IgG | IV MPDN | 15.4 | Cs-A + AZA | 30 | — | — |
| Nonresponders | |||||||
| 14 | IV MPDN | AZA | 0.8 | — | — | — | — |
| 15 | IV MPDN | IV IgG | 3.1 | Cs-A + AZA | 8 | Yes | 16 |
| Median | 0.5 | 4.5 | |||||
| Range | 0.3-18 | 0.4-30 |
IV MPDN indicates intravenous methylprednisolone; oral PDN, oral prednisone; IV IgG, intravenous immunoglobulin; AZA, azathioprine; Cs-A, cyclosporine A; CY, cyclophosphamide; —, not applicable.
Ten patients had received a median of 4 packed red blood cell (RBC) transfusions before MoAb treatment (range, 2-11 transfusions). Four patients were transfusion dependent at time of beginning of rituximab treatment.
Treatment with rituximab
Rituximab (kindly provided by Roche, Milan, Italy) was administered intravenously at a dosage of 375 mg/m2/dose, as a 5-hour infusion. Three children received 2 weekly doses, whereas 10 patients received 3 doses and the remaining 2 were treated with 4 doses of rituximab. Before each infusion, all children received premedication with methylprednisolone and diphenhydramine.
In 2 patients, no concomitant therapy was administered, whereas the remaining 13 children were receiving steroids, alone (9 cases) or in combination with cyclosporine A (2 cases) or with cyclosporine A and azathioprine (2 cases), at time of rituximab treatment.
After completing the treatment, all children received intravenous substitutive therapy with a commercial immunoglobulin preparation (400 mg/kg every 3 weeks for 6 months) to prevent therapy-induced hypogammaglobulinemia.
Definitions
Treatment response was defined as at least a 1.5 g/dL increase of hemoglobin concentration (Hb) together with a 50% reduction of absolute reticulocyte count, observed within 2 months from MoAb administration. In all patients, a complete blood and reticulocyte count was performed twice weekly for the first 2 months or until a clinical response, meeting the criteria, was achieved. Subsequently, patients were followed once weekly or as clinically indicated.
Surface marker analysis
The MoAbs used in this study for characterizing phenotype of circulating T and B cells were: anti–Leu-4 (CD3), anti–Leu-3a (CD4), anti–Leu-2a (CD8), anti–Leu-12 (CD19), anti–Leu-16 (CD20), anti-human IgM, antihuman IgG, and antihuman IgA (Becton Dickinson, Mountain View, CA). Appropriate isotype-matched controls were included. Phenotypic analysis of cell populations was performed by means of indirect immunofluorescence with fluorescein-conjugated Fab2 goat anti-mouse or by direct immunofluorescence in 2-color analysis using directly labeled antibodies on a FACScan flow cytometer (Becton Dickinson). Results obtained from these patients were compared with those observed in age-matched healthy children.19
Data analysis and presentation
Data were analyzed as of October 1, 2002. Results were expressed as median and range or as absolute number and percentage, as appropriate or as otherwise stated.
Normal distribution of pretreatment and posttreatment hematologic values was evaluated with the Shapiro-Wilk W test. Pretreatment and posttreatment hematologic values were compared using the Wilcoxon matched-pairs test and the Student t test for dependent samples, as appropriate. P < .05 was considered statistically significant and expressed in detail; P from .05 to .1, even though not statistically significant, was shown in detail, whereas P > .1 was expressed as nonsignificant (NS). The SAS package (SAS Institute, Cary, NC) was used for the analysis of the data.
Results
The median follow-up for the 15 patients is 14 months (range, 7-28 months). All patients completed the therapeutic program.
Treatment safety and infectious complications
The treatment was generally well tolerated. Only 3 children presented moderate side effects during the infusions: fever in 2 children and upper airway edema in the other. In all, side effects promptly resolved with appropriate therapy (oral acetaminophen for the first 2 cases, and intravenous hydrocortisone plus inhalatory salbutamol and budesonide for the third case).
One child developed primary varicella-zoster virus (VZV) infection 2 months after rituximab administration; the infection resolved with antiviral therapy without sequelae.
Treatment response
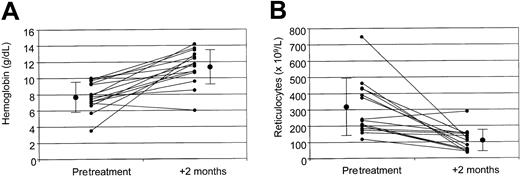
On the whole, 13 of the 15 patients enrolled (87%) responded to treatment, showing at least a 1.5 g/dL increase of Hb and a more than 50% reduction of absolute reticulocyte count. Two patients (13%), both affected by isolated AIHA with warm-reactive IgG autoantibodies, did not show any improvement after 3 doses of rituximab and were considered as nonresponders (Figure1A).
Hemoglobin and absolute reticulocyte counts.
Pretreatment and 2-months posttreatment levels of hemoglobin (A) and absolute reticulocyte count (B), as well as pretreatment and posttreatment mean values ± SD for the whole study population. The difference between pretreatment and posttreatment values is statistically significant (P < .001). Small circles indicate the value for each patient; large circles, median values for the entire study population.
Hemoglobin and absolute reticulocyte counts.
Pretreatment and 2-months posttreatment levels of hemoglobin (A) and absolute reticulocyte count (B), as well as pretreatment and posttreatment mean values ± SD for the whole study population. The difference between pretreatment and posttreatment values is statistically significant (P < .001). Small circles indicate the value for each patient; large circles, median values for the entire study population.
In responding patients, the 1.5 g/dL increase in Hb level was observed after a median of 12 days from the first MoAb administration (range, 5-72 days); the 50% reticulocyte reduction was detected after 21 days (range, 5-82 days; Table 3).
Details of treatment with rituximab and results
| Patient no. . | Interval from diagnosis to treatment, mo . | No. of rituximab doses infused . | Interval from treatment to response, d3-150 . | Duration of response, mo . | Relapse . | Second treatment . | Second remission . | Second relapse . | Third treatment . | Third remission . | Follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Responders | |||||||||||
| 1 | 2.5 | 3 | 37 | 20.4 | No | No | — | — | — | — | 20.4 |
| 2 | 3.6 | 4 | 72 | 13.7 | No | No | — | — | — | — | 13.7 |
| 3 | 2.1 | 3 | 7 | 27.6 | No | No | — | — | — | — | 27.6 |
| 4 | 98.5 | 3 | 7 | 10.6 | No | No | — | — | — | — | 10.6 |
| 5 | 3.4 | 3 | 14 | 15.6 | No | No | — | — | — | — | 15.6 |
| 6 | 16.6 | 3 | 27 | 14.9 | No | No | — | — | — | — | 14.9 |
| 7 | 16.9 | 2 | 5 | 13.1 | No | No | — | — | — | — | 13.1 |
| 8 | 24.7 | 3 | 24 | 11.1 | No | No | — | — | — | — | 11.1 |
| 9 | 5.1 | 4 | 10 | 12.6 | No | No | — | — | — | — | 12.6 |
| 10 | 15.6 | 2 | 5 | 9.5 | No | No | — | — | — | — | 9.6 |
| Responders who relapsed | |||||||||||
| 11 | 10.6 | 3 | 6 | 7.3 | Yes | Yes | Yes | — | — | — | 14.5 |
| 12 | 26 | 3 | 38 | 8.1 | Yes | Yes | Yes | Yes | Yes | Yes | 24.6 |
| 13 | 91.7 | 2 | 5 | 9.7 | Yes | Yes | Yes | — | — | — | 12.8 |
| Nonresponders | |||||||||||
| 14 | 3.2 | 3 | — | — | — | — | — | — | — | — | 7.2 |
| 15 | 48.1 | 3 | — | — | — | — | — | — | — | — | 16.7 |
| Median | 15.6 | 3 | 12 | 12.6 | 13.7 | ||||||
| Range | 2.1-98.5 | 2-4 | 5-72 | 7.3-27.6 | 7.2-27.6 |
| Patient no. . | Interval from diagnosis to treatment, mo . | No. of rituximab doses infused . | Interval from treatment to response, d3-150 . | Duration of response, mo . | Relapse . | Second treatment . | Second remission . | Second relapse . | Third treatment . | Third remission . | Follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Responders | |||||||||||
| 1 | 2.5 | 3 | 37 | 20.4 | No | No | — | — | — | — | 20.4 |
| 2 | 3.6 | 4 | 72 | 13.7 | No | No | — | — | — | — | 13.7 |
| 3 | 2.1 | 3 | 7 | 27.6 | No | No | — | — | — | — | 27.6 |
| 4 | 98.5 | 3 | 7 | 10.6 | No | No | — | — | — | — | 10.6 |
| 5 | 3.4 | 3 | 14 | 15.6 | No | No | — | — | — | — | 15.6 |
| 6 | 16.6 | 3 | 27 | 14.9 | No | No | — | — | — | — | 14.9 |
| 7 | 16.9 | 2 | 5 | 13.1 | No | No | — | — | — | — | 13.1 |
| 8 | 24.7 | 3 | 24 | 11.1 | No | No | — | — | — | — | 11.1 |
| 9 | 5.1 | 4 | 10 | 12.6 | No | No | — | — | — | — | 12.6 |
| 10 | 15.6 | 2 | 5 | 9.5 | No | No | — | — | — | — | 9.6 |
| Responders who relapsed | |||||||||||
| 11 | 10.6 | 3 | 6 | 7.3 | Yes | Yes | Yes | — | — | — | 14.5 |
| 12 | 26 | 3 | 38 | 8.1 | Yes | Yes | Yes | Yes | Yes | Yes | 24.6 |
| 13 | 91.7 | 2 | 5 | 9.7 | Yes | Yes | Yes | — | — | — | 12.8 |
| Nonresponders | |||||||||||
| 14 | 3.2 | 3 | — | — | — | — | — | — | — | — | 7.2 |
| 15 | 48.1 | 3 | — | — | — | — | — | — | — | — | 16.7 |
| Median | 15.6 | 3 | 12 | 12.6 | 13.7 | ||||||
| Range | 2.1-98.5 | 2-4 | 5-72 | 7.3-27.6 | 7.2-27.6 |
At least a 1.5 g/dL increase of hemoglobin. — indicates not applicable.
Considering the whole study population, median Hb level increased from a pretreatment value of 7.7 g/dL (range, 3.5-10.0 g/dL; mean, 7.7 ± 1.8 g/dL) to a 2-month posttreatment level of 11.8 g/dL (range, 6.0-14.2 g/dL; mean, 11.4 ± 2.1 g/dL; P < .001; Figure 1A). Median pretreatment and 2-month posttreatment absolute reticulocyte count was 236 × 109/L (range, 118-750 × 109/L; mean, 320 ± 175 × 109/L) and 109 × 109/L (range, 35-288 × 109/L; mean, 109 ± 67 × 109/L), respectively (P < .01; Figure 1B).
In the 13 responding patients the median increase in Hb level 2 months after completion of treatment was 4 g/dL (range, 1.5-9 g/dL; mean 4.2 ± 2.2 g/dL), and the median decrease in absolute reticulocyte count was −210 × 109/L (range, −22 to −600 × 109/L; mean, −217 ± 200 × 109/L).
DAT, positive in 14 of the 15 children before treatment, became negative in 6 of them (43%) at the evaluation performed 2 months after the first MoAb infusion, whereas indirect antiglobulin test (IAT) became negative in 2 of the 5 previously positive patients.
Transfusion-dependent patients did not require any more RBC transfusions 2 months after treatment discontinuation. The median time from the first dose of rituximab to the last RBC transfusion was 17 days (range, 4-31 days).
A raise in platelet count, concomitant with Hb increase, was observed in patients with Evans syndrome; platelet number increased from a pretreatment median value of 27 × 109/L (range, 4-61 × 109/L; mean, 30 ± 27 × 109/L) to a value of 140 × 109/L (range, 64-150 × 109/L; mean, 118 ± 47 × 109/L) 2 months after start of treatment.
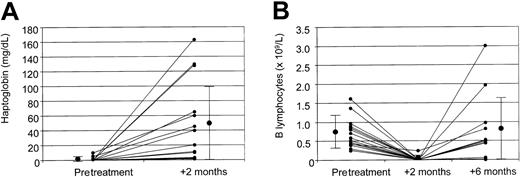
Figure 2A shows the raise of haptoglobin level after treatment, increasing from undetectable to 20 mg/dL (range, 0-160 mg/dL; mean, 44 ± 55 mg/dL) 2 months after start of treatment.
Haptoglobin and absolute B-lymphocyte counts.
Pretreatment and 2-month posttreatment levels of haptoglobin (A) and pretreatment, 2-month posttreatment, and 6-month posttreatment absolute B lymphocyte counts (B), as well as mean values ± SD for the whole study population. Small circles indicate the value for each patient; large circles, median values for the entire study population.
Haptoglobin and absolute B-lymphocyte counts.
Pretreatment and 2-month posttreatment levels of haptoglobin (A) and pretreatment, 2-month posttreatment, and 6-month posttreatment absolute B lymphocyte counts (B), as well as mean values ± SD for the whole study population. Small circles indicate the value for each patient; large circles, median values for the entire study population.
In all responding patients corticosteroids and concomitant immunosuppressive drugs were progressively tapered and stopped, in 10 of them at a median time of 12 weeks (range, 9-25 weeks). The 10 patients with sustained response are free from any other treatment.
Immunophenotype analysis
Pretreatment absolute number of B lymphocytes was 0.7 × 109/L (range, 0.25-1.6 × 109/L; normal values in our laboratory being 0.15-0.8 × 109/L), with the median percentage of B lymphocytes being 22% (range, 14%-39%; normal values in our laboratory being 5%-20%). After treatment, B lymphocytes became undetectable in all patients, without any difference between responders and nonresponders (Figure 2B). As expected, no significant modification in the levels of CD3+, CD4+, and CD8+ cells were documented.
B-lymphocyte count returned to normal 6 months after treatment in 10 of the 15 patients (67%), whereas it was still below the normal range in 5 (33%; Figure 2B). Possibly due to the relatively limited number of patients, no statistically significant correlation was observed between the B-lymphocyte number 6 months after treatment and the risk of recurrence of hemolysis in the 13 responding patients.
Disease status at last follow-up
Three responder patients (23%) experienced a recurrence of hemolysis at 7, 8, and 10 months after the first rituximab infusion, respectively. All these children received a second treatment course with rituximab and all achieved a second disease remission. One of these children subsequently received a third and a fourth course of rituximab, due to further relapses of hemolysis, with a new positive response after each treatment.
The remaining 10 patients (77% of the responders) are alive and free from immunosuppressive drugs at a median of 13 months after treatment (range, 10-28 months). At time of last follow-up, median Hb level in this subgroup of patients was 12.3 g/dL (range, 10-14.7 g/dL) and median reticulocyte count was 57 × 109/L (range, 21-73 × 109/L). Also total and direct bilirubin, as well as lactic dehydrogenase and haptoglobin, were within the normal ranges (data not shown). Nevertheless, DAT remains positive in 3 of these 10 patients (30%) and IAT is positive in 2 (20%).
Discussion
The anti-CD20 MoAb rituximab has been shown to be effective for the treatment of B-cell malignancies, in particular low-grade lymphomas.3,6,7,20 More recently, this new agent was described, in preliminary reports, as a possible promising treatment for patients with refractory AIHA.9-18,21 However, treatment of a rather limited number of patients was reported, with heterogeneous clinical features and a relatively short follow-up period. Only the study of Quartier et al9 evaluated the efficacy of rituximab on a relatively homogenous group of 6 children with AIHA.
In our trial, the efficacy and safety of rituximab was prospectively evaluated in the largest series of children with AIHA reported so far. Whereas in the paper by Quartier et al9 all patients achieved sustained remission, in our cohort rituximab was not effective in 2 patients and 3 more children had relapses. Remarkably, our study population comprised a significant number of very young patients, the median age at diagnosis being 2 years. In children with onset of AIHA before the age of 2 years the course of disease is commonly protracted and immunosuppressive treatment is not consistently effective and carries a risk of death from infections.2 9 In our cohort of patients, only 1 of the 8 children younger than 2 years of age was refractory to treatment with rituximab. On the other hand, 2 of the 3 relapses occurred in this subgroup of patients.
Rituximab could be safely readministered in the 3 patients with relapses, confirming the low immunogenicity of the MoAb due to its human component, which would not preclude retreatment.
As concerns the type of autoantibody responsible for the hemolysis, Finazzi, reviewing the data available in the literature, suggested that a better response could be achieved in patients with cold-agglutinin disease, as compared with warm-autoantibody AIHA.22 In our study population, only one child had cold-agglutinin disease; this child had a good response to the treatment, but had a relapse 7 months after the first course of rituximab.
In the study published by Quartier et al,9 all patients received at least 4 infusions of rituximab, which is the standard schedule of treatment used in patients with B-cell malignancies. However, because B cells have been reported to disappear from peripheral blood as soon as after 2 to 3 doses of rituximab,10 most of our patients were given 3 infusions of the MoAb, thus reducing costs.
One possible reason for concern regarding this form of treatment is represented by the prolonged impairment of antibody production, leading to an increased risk of viral and bacterial infections. Pure RBC aplasia due to parvovirus B19 infection has been reported after administration of rituximab,8,23 as well as acute viral hepatitis B24 and bacterial pneumonia.25 In our series of patients, we did not observe an increased risk of infectious complication. Only one child had primary VZV infection, 2 months after rituximab infusion. This infection, despite the impairment of humoral immunity, was successfully cured with administration of acyclovir.
Compared with the other immunosuppressive agents used for the treatment of antibody-mediated autoimmune disorders, rituximab presents the advantage of inducing selective B-cell immunosuppression, sparing cellular immunity mediated by T cells and natural killer (NK) cells. The specific impairment of antibody production can be easily corrected by prophylactic intravenous immunoglobulin administration, which allows maintenance of normal IgG levels for the whole period of B-cell depletion. For this reason, we gave replacement therapy with intravenous immunoglobulins. However, the need for prophylactic infusion of immunoglobulins has not been proved in a controlled study and most patients treated with rituximab for B-cell malignancies did not receive any replacement therapy.
The mechanism by which treatment with rituximab is effective in AIHA is still not completely defined. The simplest explanation is that the source of pathogenetic antibodies is removed. However, some studies on other autoimmune diseases did not show any correlation between the decline of autoantibody levels and response,26 27suggesting that additional mechanisms involving antigen presentation and help to T cells are involved.
In conclusion, our data indicate that rituximab is effective in reducing or even abolishing hemolysis in most pediatric patients with AIHA and that a sustained response can be achieved in the majority of cases. Moreover, the effects of prolonged therapy with steroids (growth impairment, fluid retention, avascular necrosis of bone) or other nonspecific immunosuppressive drugs (eg, life-threatening infections) are avoided. Recurrence of the hemolysis may occur, but a second treatment course is feasible and may be successful in controlling the disease.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/blood-2002-11-3547.
Supported in part by grants from AIRC (Associazione Italiana per la Ricerca sul Cancro), CNR (Consiglio Nazionale delle Ricerche), and IRCCS Policlinico San Matteo (F.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Franco Locatelli, Oncoematologia Pediatrica, IRCCS Policlinico San Matteo, P.le Golgi 2, I-27100 Pavia, Italy; e-mail: f.locatelli@smatteo.pv.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal