Introduction
Hematopoietic cell transplantation (HCT) can be curative for selected malignant and nonmalignant diseases.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 However, utilization and success of HCT are limited by several obstacles, primarily related to the importance of donor-recipient genetic match for favorable outcomes. While in most settings, best results are offered by human leukocyte antigen (HLA)14,15 –identical sibling transplantations, more than two thirds of patients awaiting HCT lack a suitable related donor. HCT with unrelated donor (URD) grafts are more frequently associated with severe graft-versus-host disease (GVHD16 ) or graft rejection.17, 18, 19 T-cell depletion of URD grafts has substantially reduced the risk of GVHD and early treatment-related mortality (TRM), but has not resulted in a concurrent survival benefit.20, 21, 22, 23, 24
Despite the development of large international volunteer donor registries, still less than 50% of URD searches result in identification and availability of a suitably matched bone marrow (BM) graft.25 Donor searches for recipients not of Northern-European descent result in even lower success.25 These limitations have given impetus for the identification of alternative sources of hematopoietic stem cells (HSCs).
In the last decade, HCT using umbilical cord blood (UCB) grafts has increasingly been utilized, particularly for pediatric patients.26, 27, 28, 29, 30, 31, 32, 33, 34, 35 To date, it is estimated that more than 2000 unrelated umbilical cord blood transplantations (UCBTs) have been performed.26, 27, 28, 29, 30,33,35 The data indicate that UCB is a viable alternative source of HSCs and, in certain situations, may have advantages over URD marrow grafts. The aim of this review is to enumerate the advantages and disadvantages of each stem cell source and explore the issues involved in the selection process of marrow versus UCB grafts for specific patient populations.
Challenges to unrelated donor marrow transplantation
As of 2002, more than 7 million registered volunteer marrow donors exist in more than 40 registries worldwide.36 The National Marrow Donor Program (NMDP37 ), the single largest registry, now has more than 4 million potential donors listed (R. King, NMDP, oral communication, May 2002). Still, URD marrow is not readily available for all patients.
NMDP data indicate that a median of 4 months is required to complete searches that result in a transplantation.36 Others have reported similar38,39 or considerably longer search times.40,41 An undefined number of patients succumb to their disease while awaiting identification of a suitable HLA-matched donor.42 In addition, about half of the initiated searches fail to provide a suitable donor marrow graft, even more so for ethnic/racial minority patients. Moreover, even if a donor is identified based on HLA-match criteria, not all donors are available when they are needed. Reasons for donor attrition include inability to contact the donor as a result of geographic movement or name change, loss of motivation over time, disqualification because of age or medical status, temporary unavailability, and death. The NMDP reports that about 30% of registered donors identified as potential matches are not available for further evaluation at the time of request.36
These challenges in the donor search process are being addressed at multiple levels. Traditionally, potential donors have been serologically typed for HLA-A and HLA-B loci at recruitment. Registry studies indicate that HLA-A–, HLA-B–, and HLA-DR–typed donors are more likely to be available when needed and constitute the more often utilized pool.25,43 This has given impetus to more extensively type volunteers at the time of recruitment. As reported recently, between 16% to 52% of registered donors are HLA-A–, HLA-B–, and HLA-DR–typed in the various registries worldwide.36 Another step has been to target ethnic minorities in an effort to increase availability of rare haplotypes, although this has met with mixed success.36 The total number of donors recruited to registries has expanded rapidly in the last decade. However, the benefit of increasing registry size has perhaps reached its maximal impact. It is predicted that continuing focus on expanding the pool of registered donors, without attention to ethnicity, is likely to provide relatively little benefit.44, 45, 46
With almost 35 years of human bone marrow transplantation (BMT) experience, several large studies have described outcomes with HLA-matched and -mismatched URD marrow transplantations. Table 1 summarizes results of selected large URD BMT series.
Engraftment
In a recent report of more than 5000 URD marrow transplantations facilitated by the NMDP, primary graft failure occurred in 4%.47 However, with mismatched donor grafts, this risk increases significantly.38,48, 49, 50, 51, 52 In particular, disparity for HLA-C, or more than one allele-level mismatch of class I determinants, has been associated with increased risk of graft failure.53,54 Additional factors negatively influencing probability of marrow engraftment have been the use of T-cell–depleted grafts38,50,55,56 and diseases such as chronic myeloid leukemia (CML),40,41,57 Fanconi anemia,9,58 aplastic anemia,8,59,60 and inherited metabolic storage disorders.61,62 Improved platelet engraftment has been associated with absence of severe acute GVHD and possibly higher marrow nucleated cell dose.47,63,64
GVHD
Risk of GVHD is an important factor limiting HCT using an URD. With marrow transplantations, many studies have shown histoin-compatibility to be the greatest risk factor for the development of GVHD.52,54,63,65, 66, 67 Development of moderate to severe acute GVHD or extensive chronic GVHD is associated with diminished quality of life, as well as decreased overall survival, and is not regarded as desirable even in those with malignant disease where a graft-versus-leukemia (GVL68,69 ) effect is sought.68, 69, 70, 71 Grades II to IV GVHD is reported in 43% to 70% of phenotypically matched URD marrow transplantations, and in 63% to 95% of HLA one antigen-mismatched URD transplantations38,41,51,63,72, 73, 74, 75, 76 (Table 1). Chronic GVHD affects more than 55% of matched URD transplant recipients and as many as 80% of those receiving one antigen–mismatched URD HCTs.38,41,51,63,77 About 50% of patients with extensive chronic GVHD will die secondary to severe immune dysfunction.78
Attempts to decrease GVHD and early TRM by the use of T-cell–depleted grafts have produced encouraging results, including in settings of mismatched unrelated and haploidentical related donors.20,79, 80, 81, 82, 83, 84, 85 However, significant concerns remain with graft failure and poor immune reconstitution resulting in opportunistic infections, posttransplantation lymphoproliferative disease, and relapse.21, 22, 23, 24 A large randomized, multicenter unrelated donor marrow transplantation trial in the United States, comparing T-cell depletion with pharmocologic GVHD prophylaxis, has recently concluded accrual.86 Analysis of this trial will include determination of GVHD prophylaxis on 3-year disease-free survival (DFS).
Immune reconstitution
Recovery of immune function after BMT has been characterized by several investigators, with unmanipulated as well as T-cell–depleted marrow HCTs.87, 88, 89, 90, 91 Reconstitution of T cells occurs in 2 phases: an initial expansion of mature, previously antigen-exposed T cells infused with the donor graft, and a later thymus-dependent proliferation of T cells, probably derived from HSCs. Immune recovery is impaired by the development of severe GVHD, the risk of which is particularly high after URD transplantations and in adult recipients.72, 73, 74, 75, 76 T-cell depletion can decrease the risk of GVHD; however, it is still associated with delayed restoration of T-cell subsets and increased risk of infectious complications.21, 22, 23, 24,88,89
The immune response of the graft can also result in a GVL effect, potentially decreasing the risk of relapse in certain malignant disorders.68, 69, 70 With hematologic malignancies, both in pediatric and adult recipients, the biology of the disease and disease status at HCT have been critical factors determining risk of relapse after transplantation38, 39, 40, 41,51,57,63 (Table 1).
Survival
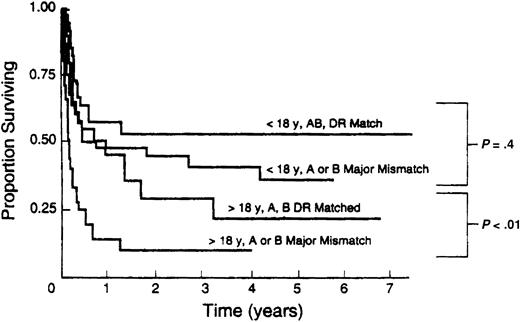
URD transplant recipients are at increased risk for GVHD, graft failure, infections, and decreased survival. BMT with HLA-mismatched grafts is associated with further increased morbidity and decreased survival, particularly in adult recipients48,49,63,65, 66, 67,92 (Figure 1). Moreover, as molecular techniques have developed, previously unrecognized mismatches at HLA-A, -B, and -DRB1 have been identified and shown to increase risk of graft rejection, GVHD, and mortality.53,54,67,93 Identification and study of additional transplantation antigens such as HLA-C53 and -DQB194 have suggested that outcome can be further optimized by higher levels of matching or matching across so-called ancestral haplotypes.95 However, with the extraordinary polymorphism of HLA alleles, such extensively matched URD transplantations will at best be limited to a minority of patients, particularly if allele-level matches at multiple loci are sought.
Association of HLA mismatch with survival after unrelated bone marrow transplantation. Results of 211 consecutive unrelated transplantations at the University of Minnesota between 1985 and 1992. Reproduced from Davies et al92 with permission.
Association of HLA mismatch with survival after unrelated bone marrow transplantation. Results of 211 consecutive unrelated transplantations at the University of Minnesota between 1985 and 1992. Reproduced from Davies et al92 with permission.
Better DFS and overall survival with URD transplantations have been achieved when performed early in the course of the disease, both for malignant and nonmalignant diseases such as immunodeficiencies, Fanconi anemia, and inborn errors of metabolism. Notably promising results have been achieved after HLA-A–, HLA-B–, and HLA-DRB1–matched URD marrow transplantations for early CML in patients younger than 35 to 40 years40,41,57,96 (Table 1), and specific immunodeficiency states in young children.97, 98, 99 While URD transplantations are associated with inferior transplantation outcomes and survival compared with matched-related donor BMT for better prognosis leukemia, the difference in survival is less evident with poor prognosis disease.51
Umbilical cord blood transplantation (UCBT) experience
The first HLA-matched sibling UCBT was performed by Gluckman et al100 in 1988 in a child with Fanconi anemia. Subsequently, reports documented the feasibility and efficacy of mismatched related and unrelated UCBT.28,101, 102, 103 By 1993, repositories of unrelated donor UCB were established in New York, Dusseldorf, and Milan.104,105 Currently, private and publicly funded cord blood banks worldwide store an estimated 70 000 cryopreserved HLA-A–, HLA-B–, and HLA-DRB1–typed units, mostly for the purpose of URD transplantations.102,104, 105, 106, 107
As limited HLA mismatch appears to be better tolerated with UCB grafts, units are identified for most patients even with the size of current repositories. A significant advantage of UCB is the rapidity with which an acceptable HLA-matched unit, once identified, can be acquired.26,107, 108, 109 As UCB is cryopreserved, acquisition of an HLA-matched unit is quick. In addition, UCB units are typically intermediate, or high resolution typed at HLA-A, -B, and -DRB1 loci at collection, further shortening search time. In one study, the median time for a UCB unit to be identified and available was 13.5 days.108 Therefore this HSC source is particularly appealing for patients who need to proceed urgently to HCT. In addition, rescheduling the date of infusion of a cryopreserved UCB unit to fit the recipient's needs is simple.
Total nucleated cell (TNC) dose of a UCB graft has proved to be a critical determinant of engraftment and survival with UCBT. Therefore, the fixed cell content of a UCB unit represents the major limiting factor, particularly for adult recipients. However, UCB has a higher frequency of progenitor cells compared with adult peripheral blood or bone marrow.110,111 Laboratory data also suggest that UCB-derived stem cells demonstrate an increased growth/engraftment potential as evidenced by larger size of colonies formed in cultures and efficient long-term, multilineage repopulation of immunodeficient mice by human UCB cells even in the absence of exogenous human cytokine support (in contrast to results with adult human BM grafts in the same mouse model).112, 113, 114 In human HCT, while the number of nucleated cells infused with an UCB graft can frequently be one-log less than that infused with a BM graft, these features perhaps explain its comparable engraftment potential. Outcomes of human UCBT from larger series are described in the remainder of this section and in Table 2.
Engraftment
As with marrow transplantations, several factors have been identified that predict successful engraftment with UCB grafts. The single most important factor influencing time to hematopoietic recovery appears to be the nucleated cell content of the graft relative to recipient size.28,29,31,32,35 The effect of HLA mismatch on engraftment is less clear (Table 2).
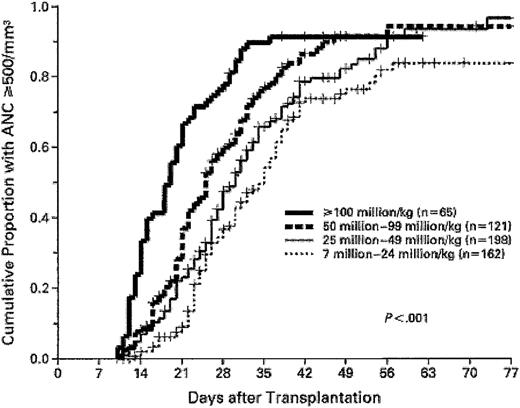
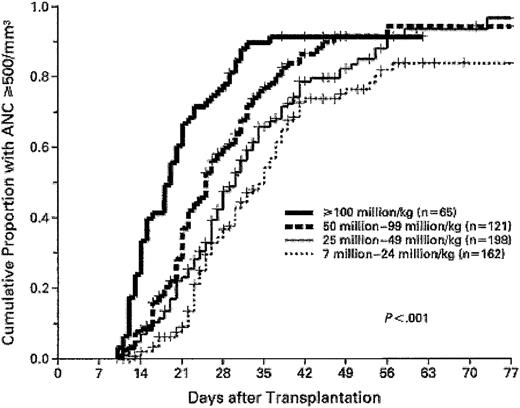
In a report by Gluckman et al28 (Table 2), a graft nucleated cell dose higher than 3.7 × 107/kg was associated with shorter time to neutrophil recovery (25 vs 35 days). Wagner et al35 (Table 2), in a series of 102 unrelated UCBTs, analyzed the influence of graft CD34+ cell dose and observed significantly inferior speed and probability of engraftment with CD34+ cell dose lower than 1.7 × 105/kg. Rubinstein et al29 showed that while a step-wise increasing graft nucleated cell dose progressively shortened the time to myeloid recovery, the final cumulative incidence of myeloid recovery was similar once the nucleated cell dose exceeded 2.5 × 107/kg, suggesting that a threshold number of nucleated cells are needed for engraftment (Figure 2). Analysis of HLA mismatch in this study29 (Table 2), and a more recent review update with 861 unrelated UCBTs,115 revealed a relationship between HLA match and engraftment. The median time to neutrophil recovery with 6 antigen–matched grafts was 23 days compared with 28 days with mismatched grafts (P = .0027).115 However, no association between engraftment characteristics and number of HLA mismatches (one vs more than one HLA mismatch) was observed.115
Association of umbilical cord blood nucleated cell dose with speed of myeloid engraftment. Higher graft nucleated cell doses result in shorter time to neutrophil recovery. However, the 3 highest cell doses have similar Kaplan-Meier probability of achieving engraftment. Reproduced from Rubinstein et al29 with permission, for English-language use only. Copyright 1998 Massachusetts Medical Society. All rights reserved.
Association of umbilical cord blood nucleated cell dose with speed of myeloid engraftment. Higher graft nucleated cell doses result in shorter time to neutrophil recovery. However, the 3 highest cell doses have similar Kaplan-Meier probability of achieving engraftment. Reproduced from Rubinstein et al29 with permission, for English-language use only. Copyright 1998 Massachusetts Medical Society. All rights reserved.
Consequent to these studies, a consensus is emerging that UCB grafts with higher cell doses should be selected wherever possible to optimize engraftment. Particularly poor results are seen after UCBT when the nucleated cell dose infused is lower than 1.5 × 107/kg.105,116,117 These data suggest that a minimum acceptable nucleated cell dose should be 1.5 × 107 nucleated cells/kg117 to reduce time to myeloid recovery and increase probability of engraftment.
GVHD
UCBT series in the URD setting has reported 33% to 44% and 11% to 22% incidences of grades II to IV and grades III to IV acute GVHD, respectively, and 0% to 25% incidence of chronic GVHD.28,29,31,35 These results are particularly notable since the majority of unrelated UCBTs were performed using 1 to 2 HLA–mismatched grafts. It could be argued that a majority of the recipient population was young in these reports; however, the incidence of severe GVHD in adults receiving mismatched UCB grafts has also been low.33
HLA mismatch has been the strongest risk factor for GVHD in recipients of marrow transplants. The association of histocompatibility with GVHD in the UCBT setting has only recently become apparent and remains less clear. Many UCBT series have failed to observe an influence of 0- to 3-antigen–mismatch on the risk of acute or chronic GVHD28,32,33,35 (Table 2). Eurocord studies, while observing association of HLA mismatch with risk of acute GVHD in the related UCBT setting, did not show an effect of HLA mismatch on risk of acute GVHD after unrelated UCBT.28,32 However, in a large series of unrelated UCBTs, subsequently updated to 861 patients, Rubinstein et al29 and Rubenstein and Stevens115 reported significantly higher rates of acute GVHD with mismatched grafts, although no direct association of HLA mismatch with chronic GVHD was observed.
Immune reconstitution
The goal of HCT is the establishment of donor cell immune tolerance to recipient self major histocompatibility complex (MHC) antigens, with reconstitution of effective immune surveillance as well as response to foreign antigens including tumors and pathogens. With UCB grafts, it has been hypothesized that with its lower and previously unstimulated T-cell content, the early, thymus-independent expansion of T lymphocytes may be impaired. However, preliminary analyses show that the recovery of immune-cell repertoire after UCBT is comparable with that after unmanipulated marrow HCT.30,118, 119, 120, 121, 122, 123 Natural killer (NK) cell numbers are reconstituted promptly, and the recovery of B lymphocytes and CD4+ T cells may actually be faster after UCBT.
The functional naiveté of neonatal lymphocytes and the lower risk of GVHD with UCB grafts have raised concern for a reduced GVL effect after UCBT.124 The exact relationship between GVHD and GVL is unclear,70,125,126 even though the data suggest that development of GVHD is associated with decreased risk of relapse.69,70,127,128
Initial experience with UCBT for leukemia suggests that similar to BMT, the biology of the disease and leukemia status at transplantation are the major determinants of outcome. Wagner et al,35 in a study of 102 UCBTs (median age, 7.4 years) from a single institution, reported a 37% cumulative incidence of relapse at 2 years, with age and malignancy risk group being the 2 factors associated with risk of relapse. Also, in a Eurocord report including 60 unrelated UCBTs in children (< 15 years) with acute leukemia, disease status at transplantation was the most important factor predicting outcome.32 The 2-year incidence of relapse and 2-year probability of DFS in the poor-risk and good-risk groups were 31% versus 75% and 7% versus 40%, respectively. Notably, cell dose and HLA mismatch of the graft did not influence DFS in the latter series.
Survival
Several studies have analyzed factors influencing survival after UCBT.27, 28, 29,31, 32, 33,35 Locatelli et al32 reported outcomes in pediatric acute leukemia from the Eurocord registry. Of 60 unrelated UCB grafts, 54 were 1 to 4 loci HLA disparate, with the majority being 1 to 2 loci HLA mismatched. In univariate or multivariate analyses, number of HLA mismatches did not influence survival. Similarly Gluckman et al,28 in a report of 65 unrelated UCBTs (0-3 antigen HLA mismatched) in pediatric recipients, and Laughlin et al,33 in a study of 68 adult recipients of 0 to 3 antigen HLA-mismatched UCBTs, showed no association of degree of HLA mismatch with overall survival in the unrelated setting. Of note, however, 2 larger series, by Wagner et al35 (102 UCBTs, single institution) and Rubinstein et al29 (562 UCBTs using grafts supplied by the New York Blood Center), have observed a significant association of HLA mismatch with survival after unrelated UCBT.
Based on the UCBT experience reported thus far, UCB cell dose has been the most critical factor in determining speed of engraftment and survival after UCBT. HLA disparity is also likely a major factor, although reports are contradictory.28,29,31,32,35,115,129 The challenge for transplantation centers in selecting the optimal stem cell source for a recipient is defining the cell dose below which clinical outcomes become significantly inferior. At the University of Minnesota, local experience suggests that 1.5 × 107 nucleated cells/kg or 1.7 × 105 CD34+ cells/kg define that threshold below which outcomes are significantly poor,35 and routine use of units below this threshold is now not permitted. While patients receiving lower cell doses can engraft, risk of graft failure is exceedingly high and time to neutrophil recovery is prolonged.28,33,35,116
Figure 3A-B shows data from the University of Minnesota, illustrating the impact of CD34+ cell dose on survival in recipients of 1 or 2 HLA–mismatched UCBTs. Survival is improved with the higher cell doses, suggesting that a higher cell dose can partially compensate for higher HLA disparity. Notably, a study from the New York Blood Center concluded that raising the nucleated cell dose by approximately 3 × 107 cells/kg may offset the negative effect of one HLA mismatch.130 As more data are collected, it will be possible to define with greater confidence an acceptable lower limit of cell dose for a given degree of HLA disparity.
Association of umbilical cord blood graft CD34+ cell dose with overall survival. University of Minnesota data: 102 consecutive unrelated UCBTs between 1994 and 2001, unpublished data. Survival by CD34+ cell dose (× 105/kg) for (A) 1-antigen mismatched UCBTs, and (B) 2-antigen mismatched UCBTs.
Association of umbilical cord blood graft CD34+ cell dose with overall survival. University of Minnesota data: 102 consecutive unrelated UCBTs between 1994 and 2001, unpublished data. Survival by CD34+ cell dose (× 105/kg) for (A) 1-antigen mismatched UCBTs, and (B) 2-antigen mismatched UCBTs.
Graft TNC dose has been used as a marker of graft “potency” in the majority of UCBT studies reported thus far; although, a few studies have also defined graft CD34+ cell dose as a predictor of speed of engraftment and survival with UCBT.33,35 The TNC count of any UCB unit includes a variable number of nucleated red cells. While TNC dose has consistently correlated with transplantation outcomes, and the nucleated red cell count has been shown to correlate with progenitor cell numbers,131 a progenitor assay such as colony-forming unit (CFU) or CD34+ content of a UCB graft may be a more precise predictor of engraftment and survival with any given UCB unit.132 In addition, CFU content (after thawing) is a measure of progenitor cell viability.
The contiguous integral segment of a cryopreserved UCB unit is representative of the whole product and can be utilized to accurately estimate CFU content and viability after thawing (D. A. Wall, oral communication, October 2002). Unfortunately, results of CFU assays are susceptible to minor variations in techniques and culture conditions used, and may not be accurately reproducible between different laboratories. While, CD34+ cell content of a UCB unit at harvest is not universally available at present, the authors' bias is toward CD34+ cell content to quantify graft potency. Studies directly comparing the relative precision of TNC versus CD34+ content to predict the various outcomes after UCBT are awaited.
BMT versus UCBT: comparative analysis
Engraftment
In a matched pair analysis34 comparing HLA-A–, -B–, and -DRB1–matched URD marrow transplantations using either methotrexate (BM-MTX) or T-cell depletion (BM-TCD) for GVHD prophylaxis, with 0 to 3 loci HLA–mismatched UCBT (Table 3), the speed of neutrophil recovery was significantly slower after UCBT when compared with the BM-MTX group (median, 29 vs 22 days; P = .03) or the BM-TCD group (median, 27 vs 14 days; P < .01). However by day 45, the overall myeloid engraftment rate was comparable in all groups. Time to platelet engraftment, while slower with UCBT, was not statistically different from the BM-MTX group, perhaps because of small patient numbers (median, 66 days vs 30 days: UCBT vs BM-MTX [P = .12]). However, at 6 months, there was no difference in platelet engraftment. In another registry report with larger numbers (Table 3), Rocha et al31 also observed significant delay in speed of both neutrophil and platelet engraftment after UCBT when compared with URD marrow transplantation in children with acute leukemia.
GVHD
Current data indicate that HLA mismatch may be better tolerated in the UCBT setting. A registry study (Table 3) comparing outcomes of HLA-identical sibling UCBT versus HLA-identical sibling BMT in pediatric recipients observed significantly lower incidence of acute and chronic GVHD in the UCBT group.133 This study is perhaps the clearest demonstration of a difference in biologic properties between the 2 stem cell sources, as interpretation of GVHD between unrelated UCBT and BMT is often complicated by different levels of HLA histocompatibility and other recipient heterogeneity.
There are 2 other reports that have compared GVHD frequencies in unrelated donor BM and UCB recipients. In a matched-pair analysis from a single institution (Table 3), risk of acute and chronic GVHD was similar when comparing outcomes in recipients of HLA-A–, HLA-B–, and HLA-DRB1–matched unmanipulated BM and mostly 1 to 2 antigen mismatched unrelated UCB transplants.34 In another registry study (Table 3), 0 to 3 antigen mismatched UCBT transplantations were associated with a significantly lower risk of acute and chronic GVHD compared with unmanipulated, mostly HLA-matched marrow transplantations.31 Taken together these data indicate that despite the greater degrees of HLA disparity accepted in UCBT, the risk of developing acute and chronic GVHD after 1 to 2 antigen HLA-mismatched unrelated UCBT27, 28, 29, 30, 31,34,35 is similar, or even lower, than that reported with HLA-matched bone marrow transplantation.38,54,63,65,134
The reason(s) for this lower risk of GVHD after UCBT is not clear. Functional and phenotypic immaturity of UCB lymphocytes135, 136, 137, 138, 139 and/or a reduced T-cell dose infused with UCB grafts35,105,140 may contribute to its reduced alloreactivity. Mature T cells present in the donor graft are the chief contributor to repopulating T cells in the first year after HCT.141 Large numbers of mature, antigen-specific T cells are infused in a BM graft and can initiate GVHD due to recognition of cross-reactive alloantigens.136 In contrast, characterization of the UCB αβ T-cell repertoire reveals a naive T-cell population unexposed to prior antigenic stimulation.136 It has been suggested that absence of clonal expansion in response to alloantigens by UCB T cells contributes to its enhanced tolerance.136
Relapse and GVL effect
Current experience comparing risk of relapse after UCBT versus BMT is limited. In an analysis of HLA-matched sibling transplantations (Table 3), the 3-year survival in patients with malignant diagnosis was comparable after UCBT or BMT (P = .69). Notably, in another study of HCT for acute leukemia in children by Rocha et al31 (Table 3), a larger proportion of recipients (18%-20%) in the unmanipulated BMT (UBMT) and UCBT groups had advanced-stage leukemia compared with the T-cell–depleted BMT (T-UBMT) group (9%). Interestingly, while in both T-UBMT and UCBT groups a lower incidence of acute and chronic GVHD was noted relative to the unmanipulated BM transplant recipients, the T-UBMT group, but not the UCBT group, had an increased risk of early relapse when compared with the UBMT group (P = .02).
Overall, there is no evidence thus far to suggest a higher risk of leukemia relapse after UCBT. The observations of (1) better donor cell immune tolerance of recipient MHC antigens, evidenced by acceptable risk of GVHD despite up to 2 HLA-mismatched grafts, and (2) preserved GVL effect after UCBT might be explained by intact UCB NK cell function combined with immaturity of umbilical B and T cells. In laboratory studies, NK-like cytotoxicity has not been linked with pathogenesis of GVHD, but has been shown to mediate a GVL effect.142, 143, 144
A recent study of HCT in mice and humans showed that infusion of donor-derived alloreactive NK cells not only provides a GVL effect, but may also protect against GVHD by targeting recipient antigen presenting cells.145 UCB contains levels of NK cells and inducible NK-like cytotoxic activity similar to those in adult peripheral blood,135,146,147 which might explain a preserved GVL effect. Prospective studies comparing similar patient populations receiving UCB or marrow graft are needed for reliable conclusions; although, it remains to be seen if such studies will be feasible or ever performed. Randomization will be difficult as UCB grafts are typically available much faster than URD marrow. Hence high-risk patients (eg, leukemia in tenuous remission) may be more likely to receive UCB transplants. Strict control of patient risk factors will be critical for any reliable comparison between UCB and marrow transplantations.
Survival
There are 3 retrospective reports that have compared survival between URD UCB and marrow HCT. A registry report comparing HLA-mismatched UCBT with unmanipulated marrow in children with leukemia (Table 3) noted a significantly higher early (< 100 days) TRM in the UCBT group; although, overall survival between 0 to 2 HLA-mismatched UCBT and mostly HLA-matched URD marrow transplantations was comparable.31 UCB recipients were more likely to have adverse prognostic factors in this study. Other groups have not reported increased early TRM with UCBT.
A second study from the International Bone Marrow Transplant registry (IBMTR), reported in abstract form, compared 296 UCBTs (94% with at least one mismatch at HLA-A, HLA-B, or HLA-DRB1) with 210 URD BMTs (62% matched at HLA-A, HLA-B, and HLA-DRB1) in children with hematologic malignancy.129 This study reported similar survival rates in recipients of BMT and 0 or 1 HLA-mismatched UCBT, with reduced GVHD in the UCB transplant recipients. In this study survival was reduced in recipients of 2 HLA-mismatched UCB transplants compared with recipients of better-matched UCB and BM transplant recipients. Also, a matched-pair case-control study (Table 3) showed comparable outcomes in recipients of HLA-A–, HLA-B–, and HLA-DRB1–matched marrow and mismatched UCB.34 Taken together, these results suggest that UCB with limited HLA mismatch is an acceptable alternative to marrow, at least in children.
Outcomes in adult recipients
BMT for acute leukemia in adults, from donors other than HLA-identical siblings, is associated with a high risk of GVHD and treatment failure.38,51,148,149 UCBT may have an advantage in adult recipients because of potentially decreased risk of GVHD. However, as cell dose is associated with survival, this considerably limits the pool of eligible UCB grafts for adult recipients.
Laughlin et al33 reported the first major adult UCBT series from 5 US centers. The recipients (N = 68) weighed a median of 69.2 kg (range, 40.9-115.5 kg), and 54 had a hematologic cancer, of which 50 were considered intermediate/high risk. Of the grafts, 97% were 1 to 3 antigen HLA–mismatched with their recipient. The median nucleated cell dose infused was 2.1 × 107 cells/kg of body weight (range, 1-6.3 × 107 cells/kg). Engraftment frequency was similar to pediatric series with an estimated probability of myeloid recovery by day 42 of 90% (median time to engraftment, 28 days). Time to neutrophil count higher than 0.5 × 109/L was associated with nucleated cell dose (< 1.87 × 107 vs > 1.87 × 107; P = .003). Despite a high frequency of HLA-mismatched grafts, the probabilities of grades II to IV and grades III to IV GVHD were 60% and 20%, respectively. Of 68 recipients, 19 (18 disease-free [26%]) were alive at 22 months of follow-up. Speed of myeloid recovery and DFS were linked with higher nucleated cell and CD34+ cell dose. There was no significant association between the kinetics of myeloid recovery, graft failure, or acute GVHD with extent of HLA mismatch. Of note, the risk of severe acute and chronic GVHD was lower than typically reported after URD marrow transplantations.38,40,57,150
In another report151 of 22 adult patients weighing 41 to 85 kg, who received unrelated UCBT with TNC dose ranging from 1.01 to 4.96 × 107/kg, all 20 patients who survived longer than 30 days showed myeloid engraftment at a median of 22 days. One patient who received the lowest cell dose experienced secondary graft failure. Also, in a separate Eurocord report of 42 adult recipients of UCBT,116 median time to neutrophil recovery (> 0.5 × 109/L) was 35 days, and no patient who received less than 1.0 × 107 nucleated cells/kg survived. These results combined once again underscore the importance of graft cell dose relative to recipient size for optimal results after UCBT and support a minimum nucleated cell dose limit of 1.5 × 107 nucleated cells/kg.
Several strategies to increase the nucleated/CD34+ cell dose of the graft are being investigated, including ex vivo expansion of UCB hematopoietic cells.152,153 The hematopoietic cells in UCB have an immense capacity to expand ex vivo in the presence of an appropriate cytokine cocktail.111,154,155 However, while the yield of more mature hematopoietic progenitor cells is extensive, it is not yet clear if actual expansion of the pluripotent, long-term repopulating HSCs is possible or required.111,154,155 It will be important however, to demonstrate that HSCs are at least maintained, and safety studies are currently under way.
Alternative approaches to optimize outcomes are being explored, such as multiunit UCB transplantation,156 UCBT combined with infusion of mesenchymal stem cells, or UCBT after a nonmyeloablative preparative regimen.157 Barker et al158 reported short-term outcomes in 8 adults (median weight, 81 kg) with advanced hematologic malignancy using 2 unrelated 1 to 2 HLA-mismatched UCB grafts. Initials results are encouraging; however, further study is required to show if double UCBT is associated with improved engraftment and survival compared with single UCBT in larger-sized recipients.
Conclusions and recommendations
As noted earlier, there are a number of logistic and biologic differences between the 2 stem cell sources, which may make one more advantageous over the other. A comparison of issues involved in the selection of BM versus UCB grafts is outlined in Table 4.
For URD BMT, suitable donors have previously been accepted as HLA matched at HLA-A and HLA-B loci by serology, and at HLA-DRB1 by molecular techniques, with one mismatch allowed by some centers in recipients younger than 36 years.49,63,92,159,160 Additionally, allele-level matching at HLA-A, HLA-B, and HLA-C is increasingly being sought, in conjunction with matching at additional class II loci. This practice may, however, further restrict availability of suitably matched donors. Cell dose is generally not a limiting factor in identifying a marrow donor, although data indicate improved survival when higher cell doses are obtained.4,161, 162, 163
For UCBT, cell dose is the most critical determinant of outcome, and currently, 4 to 6 antigen HLA-matched grafts are considered acceptable. While the minimum acceptable UCB graft cell dose is yet to be unanimously agreed upon, a minimum threshold of 1.5 × 107 nucleated cells/kg or 1.7 × 105 CD34+ cells/kg has been suggested.35,117 Among 0 to 2 antigen HLA-mismatched grafts, current data suggest that for the same cell dose, survival is superior with better-matched grafts; although, the negative effect of HLA mismatch can be at least partially overcome by a higher cell dose.35,130 Hence, higher cell doses are even more important with HLA-mismatched grafts. While it has become obvious that both HLA match and cell dose must be considered in the algorithm for UCB graft selection, future studies are awaited to delineate their relative importance.
When URD HCT is indicated, initiating a simultaneous search of UCB and marrow donor registries is justifiable, as it will maximize the chance of finding an acceptable source of HSCs, particularly for children. When both a suitable UCB and marrow graft are available on preliminary search, the urgency for HCT and recipient's diagnosis may dictate the best stem cell source for the specific patient. The time required to acquire an identified UCB graft is significantly shorter. Also, since UCB grafts are banked, rescheduling the date of HSC infusion is simple. Hence, in situations in which the patient requires urgent transplantation, or when the patient's disease or medical status may require rescheduling the date of transplantation at short notice, an UCB graft has obvious advantages.
In comparison with BMT, there is relatively less experience with UCBT. However, sufficient data are becoming available to suggest at least comparable efficacy between HLA-matched marrow and UCB for pediatric patients with acute leukemia.28,29,31,32,34,35 Some investigators have also reported encouraging preliminary results after UCBT in young children (< 2.5 years) with leukemia, immunodeficiency, or inborn errors of metabolism.164,165 Of note, available UCB grafts for children this age typically deliver a high TNC dose.
Experience with UCBT for most nonmalignant disorders such as aplastic anemia, and for adults with hematologic malignancies, is more limited at present. In diagnoses in which HCT is associated with higher rates of graft rejection, or in which need for donor lymphocytes may arise after transplantation (eg, in chronic myeloid leukemia [CML]), marrow grafts may be a better option. Moreover, in certain diseases such as CML in chronic phase (< 40 years) or Wiskott-Aldrich syndrome in young children, disease-free survival after HLA-A–, HLA-B–, and HLA-DRB1–matched URD BMT is very good.40,57,96,98,99 For such patients, at present, UCB might be considered, but only if HLA-A–, HLA-B–, and HLA-DRB1–matched URD marrow is not available.
With certain inherited diseases, such as mucopolysaccharidoses or X-linked cerebral adrenoleukodystrophy, the disease manifestations can be rapidly progressive, potentially limiting the usefulness of HCT.11, 12, 13 Hence, the decision to wait for a marrow graft needs to be carefully weighed against the disease stage at presentation and the predicted rate of progression. In adult patients, while cell dose restricts the use of UCBT, results with URD BMT for acute leukemia are sufficiently poor to warrant investigation of UCB in an attempt to reduce nonrelapse mortality.38,51,148,149 A suggested approach to select an URD graft is shown in Figure 4.
Approach to unrelated donor search for HCT. HLA-mm indicates HLA mismatch; TNC, total nucleated cells; and *, see “Conclusions and Recommendations.”
Approach to unrelated donor search for HCT. HLA-mm indicates HLA mismatch; TNC, total nucleated cells; and *, see “Conclusions and Recommendations.”
Efforts to more rapidly identify and increase the availability of allele-level HLA-matched URD marrow grafts are ongoing by marrow donor registries worldwide. Alternatively, the majority of UCB searches are likely to identify a 0 to 2 antigen–mismatched graft for pediatric and many adult patients, even within the currently accessible UCB pool. With growing awareness and public interest, the number of UCB units being harvested and stored is rapidly expanding. Also, programs aimed at increasing the pool of uncommon haplotypes by recruiting ethnic/racial minority donors might meet with higher success for UCB “donors” compared with marrow donors. In the future, with a larger pool of stored UCB units, and with possibly increased representation of minority haplotypes, there will be an increased likelihood of identifying UCB units with 0 to 1 HLA mismatch. Unfortunately, currently a UCB search requires initiation of separate queries with individual cord blood banks, and different banks have diverse policies on methods of HLA typing and cell content assessment at harvest. This can make comparisons between different search reports difficult. The development of a unified system that will allow for a single search, with access to all available UCB units worldwide that have been stored by banks using uniform standards, will make the search process faster, more accurate, and comparisons of results after UCBT more reliable.
Prepublished online as Blood First Edition Paper, January 9, 2003; DOI 10.1182/blood-2002-08-2510.
Supported in part by Children's Cancer Research Fund (J.N.B., J.E.W., S.M.D.), and the National Institute of Health (P01 CA 21737 [J.E.W.], N01 HB 67139 [J.E.W.], and N01 HB 47095 [J.E.W., S.M.D.]).
S.M.D. and J.E.W. contributed equally to this paper.
The authors would like to acknowledge and thank the National Marrow Donor Program (NMDP) for providing registry data. We thank the staff at the University of Minnesota Blood and Marrow Transplant Program for their assistance in the investigation of the clinical role of UCBT. The authors also acknowledge Dr Norma K. C. Ramsay for her critical review of the manuscript and helpful suggestions.