Abstract
Leukocyte arrest on vascular endothelium under disruptive shear flow is a multistep process that requires in situ integrin activation on the leukocyte surface by endothelium-displayed chemoattractants, primarily chemokines. A genetic deficiency of leukocyte adhesion to endothelium associated with defective β2 integrin expression or function (LAD-1) has been described. We now report a novel severe genetic disorder in this multistep process associated with functional defects in multiple leukocyte integrins, reflected in recurrent infections, profound leukocytosis, and a bleeding tendency. This syndrome is associated with an impaired ability of neutrophil and lymphocyte β1 and β2 integrins to generate high avidity to their endothelial ligands and arrest cells on vascular endothelium in response to endothelial chemoattractant signals. Patient leukocytes roll normally on endothelial selectins, express intact integrins and G protein–coupled chemokine receptors (GPCR), spread on integrin ligands, and migrate normally along a chemotactic gradient. Activation of β2 integrins in response to GPCR signals and intrinsic soluble ligand binding properties of the very late activation antigen-4 (VLA-4) integrin are also retained in patient leukocytes. Nevertheless, all integrins fail to generate firm adhesion to immobilized ligands in response to in situ GPCR-mediated activation by chemokines or chemoattractants, a result of a primary defect in integrin rearrangement at ligand-bearing contacts. This syndrome is the first example of a human integrin-activation deficiency associated with defective GPCR stimulation of integrin avidity at subsecond contacts, a key step in leukocyte arrest on vascular endothelium under shear flow.
Introduction
Circulating leukocytes must rapidly translate specific adhesive and stimulatory signals into firm adhesion to the endothelial lining of specific sites of inflammation or antigen presentation to extravasate the bloodstream at these sites.1 Leukocyte arrest at target endothelial sites is nearly exclusively mediated by integrin receptors, expressed on all circulating hematopoietic cells.2 Circulating leukocytes maintain their integrins in largely nonadhesive states to avoid nonspecific sticking to blood vessels. A unique feature of leukocyte integrins is that their adhesive capacity is dynamically regulated independent of their level of surface expression, in response to a variety of inside-out signaling events.3 The in situ activation of integrin avidity to endothelial ligands by endotheliumdisplayed activating signals serves as a key checkpoint for reversibly adhered rolling leukocytes to successfully arrest on target endothelial sites. Several recent studies indicated that activation of integrin avidity to endothelial ligands by endothelium-displayed chemoattractants (or chemokines) can take place within fractions of seconds and can promote both reversible rolling adhesions or immediate conversion of leukocyte rolling to firm arrest on vascular ligands.4,5 Chemokine triggering of integrin avidity involves enhanced integrin clustering alone or with concomitant induction of high-affinity recognition of ligand.5, 6, 7 Although implicating Gi protein signaling, very little is known about how chemokine activation of leukocyte-expressed chemokine receptors and their associated G protein machinery up-regulates integrin avidity to ligand at endothelial contacts.
The significance of vascular integrins and selectins in immune cell trafficking has been demonstrated by numerous studies in murine knock-out models.8 Clinical verification of the role of these adhesion receptors in human leukocyte trafficking has been provided in the past 2 decades by the identification of 2 kinds of rare adhesion deficiencies. The first, LAD-1, is the result of defective expression of CD18, the β2 subunit shared among the major leukocyte integrins, leukocyte function-associated antigen-1 (LFA-1), Mac-1, and p150,95.9 A second LAD syndrome, LAD-2, has been linked to defective expression of key fucosylated glycans, which comprise the carbohydrate ligands for both endothelial and leukocyte selectins.10 In addition, leukocyte adhesion deficiencies may arise from point mutations in normally expressed CD18 11 or from impaired activation of structurally intact integrins.12,13 We now describe a new LAD syndrome associated with recurrent infections, marked leukocytosis, and a severe bleeding tendency. The patient's leukocytes express normal integrin levels that are functionally intact in vitro in mediating adhesive tethers to respective endothelial ligands during dynamic contacts. However, LAD neutrophils and both LAD primary lymphocytes (peripheral blood lymphocytes [PBLs]) and Epstein-Barr virus (EBV)–transformed lymphoblasts fail to develop firm integrin-mediated adhesion to vascular endothelial surfaces or endothelial integrin ligands in response to rapid signals of surface-bound chemokines or chemoattractants. Consequently, LAD lymphocytes exhibit defective transendothelial migratory capacity triggered by an endothelial chemokine under physiological shear flow. This syndrome is a first example of a defect in the ability of vascular integrins on circulating leukocytes to rearrange with their endothelial ligands at adhesive contacts and rapidly arrest on target vascular endothelium in response to endothelial-displayed chemoattractants.
Materials and methods
Reagents and mAbs
Recombinant soluble 7-domain human vascular cell adhesion molecule-1, sVCAM-1,14 was the generous gift from Dr R. Lobb (Biogen, Cambridge, MA) and was stored in phosphate-buffered saline (PBS). Affinity-purified human intercellular adhesion molecule-1 (ICAM-1)15 was a gift from Dr T. Springer (Harvard University, Boston, MA). Recombinant ICAM-1–immunoglobulin G1 (IgG1) fusion protein as well as human SDF-1α, IP-10, and interleukin-2 (IL-2) were purchased from R&D Systems (Minneapolis, MN). Recombinant Mig was from Peprotech (Rocky Hills, NJ). Bovine serum albumin (BSA; fraction V), Ca2+- and Mg2+-free Hanks balanced salt solution (HBSS), EGTA (ethylene glycol tetraacetic acid), HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), fibrinogen, and Ficoll-Hypaque 1077 were obtained from Sigma Chemical (St Louis, MO). Human serum albumin (HSA; fraction V) and pertussis toxin were from Calbiochem (La Jolla, CA). The anti-α4 integrin subunit monoclonal antibody (mAb), (HP1/2),16 a gift from Dr R. Lobb; the anti–L-selectin (DREG-200),17 the anti-β2 integrin subunit mAb (TS1.18), the anti–LFA-1 (TS2.4), gifts from Dr T. Springer; and the β2 subunit activating mAb (KIM185), a gift from Dr I. Van Kooyk (VU Hospital, Amsterdam, the Netherlands) were all used as purified Ig. The anti–Mac-1 integrin mAbs, CBRM1/2 and CBRM1/5,18 were used as described. The β2 integrin stimulatory Fab, 240Q, and the Alexa-488–conjugated anti-β2 integrin neoepitope 327C mAb were previously described.19 The anti-CD44 mAb was purchased from Serotec (Kidlington, Oxford, United Kingdom). The anti-CD15a (SLex) was purchased from Becton Dickinson (Woburn, MA). The anti-CXCR4 mAb 12G5 was purchased from Pharmingen (San Diego, CA). The anti-CXCR4 mAb 6H8 20 was a gift from Dr F. Arenzana-Seisdedos (Institute Pasteur, Paris, France). The anti-CCR7 rat mAb (3D12) was a gift from Dr M. Lipp (Max Delbruck, Berlin, Germany). Approval was obtained from the Rambam Medical Center institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki. The human experimentation and ethics committees of the Weizmann Institute approved the use of human cells in accordance with the indicated procedures.
Isolation and culture of leukocytes and preparation of lymphoblasts
Human peripheral blood lymphocytes and neutrophils were isolated from citrate-anticoagulated whole blood as described.5,16 The isolated peripheral blood lymphocytes (PBLs) were more than 90% CD3+ and are therefore referred to as T lymphocytes. T lymphoblasts were derived from PBLs by stimulation with phytohemagglutinin (0.2 μg/mL; Sigma) and IL-2 (50 U/mL; R&D Systems) in the presence of irradiated allogeneic lymphocytes for 7 days. PBLs isolated from the LAD patient and from an age-matched healthy donor were transformed with EBV, and lymphoblastoid lines were derived and maintained in culture as previously described.21 Human umbilical cord endothelial cells (HUVECs) were isolated from umbilical cord veins and cultured as described.22,23
Fluorocytometry and confocal microscopy
Surface staining was performed and analyzed by FACScan as described.16 For analysis of neutrophil expression of the Mac-1 antigen (detected with CBRM1/2) and the Mac-1 activation neoepitope CBRM1/5, neutrophils were stimulated with agonists for 10 minutes at 37°C and immediately incubated with the appropriate primary mAb for 30 minutes at 4°C. Phycoerythrin (PE)–conjugated secondary Ab staining (Jackson ImmunoResearch Labs, West Grove, PA) and FACScan analysis were performed as described above. The β2 integrin activation on leukocytes was assayed by incubating intact or agonist-stimulated leukocytes with the neoepitopespecific 327C–Alexa-488 mAb19 for 10 minutes at 37°C. Very late activation antigen-4 (VLA-4) distribution on paraformaldehyde-fixed lymphoblasts was probed with the anti-α4 mAb HP1/2, followed by Alexa-488–labeled secondary mAb (Molecular Probes, Eugene, OR) as described.24 Samples were analyzed at 488 nm with a krypton/argon laser confocal microscope. Induction of ligand-induced binding site (LIBS) epitopes by soluble VLA-4 ligand mimetic peptide (Bio1211), a gift from Dr Lobb, was monitored as previously described.16
Western blot analysis
Western blot analysis was performed with antiphosphospecific extracellular signal-regulated kinase-1/2 (ERK1/2) mAb, polyclonal anti-ERK1/2 (both kind gifts from Dr Rony Seger, Weizmann Institute), and antiactin (Sigma) or with antiphosphospecific Akt mAb (Santa Cruz) and polyclonal anti-Akt (Santa Cruz) antibodies, as described.16
Preparation of adhesive substrates and HUVEC monolayers
Preparation of substrates for the laminar flow adhesion assays were performed as previously described.16,25 For adhesion experiments on resting or tumor necrosis factor-α (TNF-α)–activated endothelial cells (ECs), primary HUVECs (passage 2 or 3) were left intact or stimulated for 18 hours with heparin-free culture media supplemented with TNF-α (2 ng/mL, 50 U/mL; R&D Systems).25
Analysis of leukocyte tethering, accumulation, and resistance to detachment
All shear flow experiments were performed at 37°C. Neutrophils or PBLs were suspended in binding medium (cation-free HBSS containing 10 mM HEPES [pH 7.4] and 2 mg/mL BSA supplemented with Ca2+ and Mg2+ at 1 mM each) and immediately perfused through the chamber at controlled flow rates as described.5 All cellular interactions with the adhesive substrates were determined by manually tracking the motions of individual cells along 0.9 mm field as previously described.5 Transient tether lifetime was determined at a resolution of 0.02 seconds, and cell displacements were determined by computerized motion analysis.26 For analysis of integrin activation by chemokines or phorbol myristate ester (PMA; 100 ng/mL) at short stationary contacts, leukocytes were perfused into the flow chamber and allowed to settle onto the substrate for 1 minute. Flow was then initiated and increased stepwise every 5 seconds by a programmed set of flow rates. At the end of each 5-second interval increase in flow rate, the number of cells that remained bound was expressed relative to the number of cells originally settled on the substrate.
Analysis of lymphocyte migration under shear flow and shear-free conditions
Transendothelial migration assays were performed as previously described.25 Briefly, SDF-1α (100 ng/mL) in binding medium was overlaid for 5 minutes on TNF-α–stimulated HUVECs (18 hours, 2 ng/mL). Motion analysis was performed manually on all accumulated cells (ranging between 30 to 60 cells) from the initial site of cell tethering to the endothelial surface. Four distinct categories of interacting lymphocytes (detaching, arrested, locomoting over the EC without transmigration, or transmigrating) were defined as described.25 Chemokine-triggered PBL chemotaxis assay in modified Boyden chamber transwell filters was performed as described.27
Results
Patient
A male child, product of the first pregnancy to parents of Arab ethnic origin who were first cousins, was born at term through normal vaginal delivery. At birth multiple mulberry hematomas were observed all over his body, which resolved spontaneously. Initial laboratory tests showed a low hemoglobin, slightly reduced platelet count, and leukocytosis (results not shown). At the age of 5 days he developed periumbilical cellulitis and staphylococcal septicemia. Recovery with appropriate antibiotic therapy was slow but uneventful. The umbilical cord was shed at 4 weeks. During the subsequent months his clinical course was punctuated by severe mucosal bleeding that on several occasions necessitated blood transfusions and recurrent nonsuppurating skin infections. During this time interval, the platelet counts rose and were maintained above 150 000. The white cell count, however, remained constantly elevated and ranged from 30 000 to 60 000/mm3. Platelet aggregation studies demonstrated a grossly abnormal response to agonists (M.A., manuscript in preparation). On several occasions life-threatening hemorrhage was controlled by platelet transfusions. The incidence of infections was reduced by prophylactic antibiotic treatment but remained a significant clinical problem. Unfortunately, at the age of 6 years he died from disseminated fungal infection after a mismatched bone marrow transplantation. A younger brother who presented with the same clinical and hematologic phenotypes at birth died at 1 week of age from sepsis.
Auxiliary tests demonstrated normal expression of integrins on the patient's lymphocytes and neutrophils (Figures 1, 4, and 5), thus excluding LAD-1 syndrome. Similarly, LAD-2 was excluded by the normal expression of fucosylated marker CD15A comprising the sLex carbohydrate selectin ligant and normal blood group expression (Table 1). Flow cytometry studies showed normal distribution of T and B subsets. Patient T cells had reduced levels of the chemokine receptor CCR7 as well as of L-selectin and CD44 (Table 1). Reduced L-selectin and CCR7 were not, however, the cause of elevation in T-cell activation because the fraction of effector/memory CD45RO lymphocytes was in fact reduced from 60% of the total PBLs in healthy donors to 30% in patient lymphocytes (Table 1).
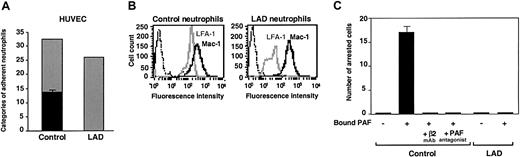
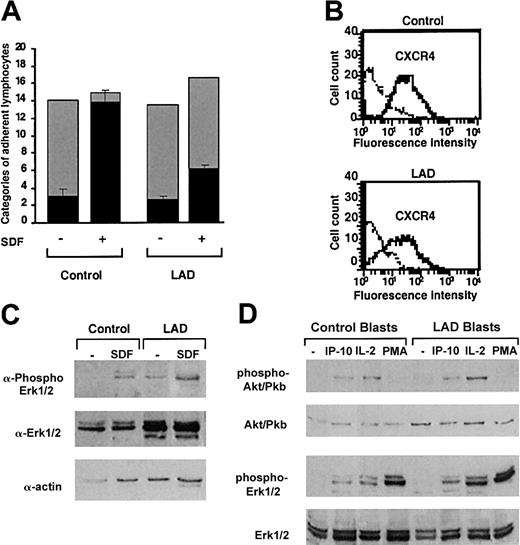
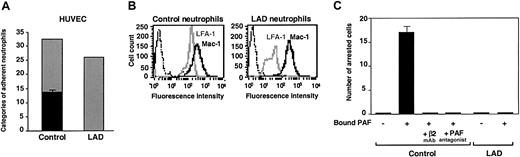
Firm adhesion but not capture and rolling are defective in LAD neutrophils. (A) Neutrophil accumulation and development of firm arrest on TNF-activated HUVECs under physiological shear flow. Leukocytes isolated from a healthy donor (control) or patient (LAD) were perfused for 1 minute at 0.75 dyne/cm2 over the HUVEC monolayers, and accumulated leukocytes were subjected to a shear stress of 5 dyne/cm2 for a 10-second period. The number of leukocytes remaining adherent that either continued to roll on the monolayer (▦) or came to full arrest (▪) was determined. The values of adherent categories depicted in the figure correspond to fractions of the original leukocyte flux perfused in immediate contact with the endothelial layer. (B) FACS (fluorescence-activated cell sorter) staining of LFA-1 (gray lines) and Mac-1 (solid lines) integrins on control and LAD neutrophils using the mAbs TS2.4 (anti-αL integrin subunit) and CBRM1/2 (anti-αM integrin subunit) as primary antibodies. Background staining is shown by the dotted line. (C) PAF-triggered neutrophil arrest on nonactivated HUVECs. Control or LAD neutrophils were perfused at 0.75 dyne/cm2 over unstimulated HUVECs overlaid with PAF (100 nM, 5 minutes of incubation). Arrested cells were determined as in panel A. Where indicated, neutrophils were pretreated with the β2 integrin–blocking mAb TS1.18 (20 μg/mL, 5 minutes, 4°C). Values in panels A and C are given as mean ± range of determinations in 2 fields of view. Because transient tethers comprised less than 10% of the total cell-capturing events, they were not included in the analysis. Results in panels A-C are representative of 3 independent experiments.
Firm adhesion but not capture and rolling are defective in LAD neutrophils. (A) Neutrophil accumulation and development of firm arrest on TNF-activated HUVECs under physiological shear flow. Leukocytes isolated from a healthy donor (control) or patient (LAD) were perfused for 1 minute at 0.75 dyne/cm2 over the HUVEC monolayers, and accumulated leukocytes were subjected to a shear stress of 5 dyne/cm2 for a 10-second period. The number of leukocytes remaining adherent that either continued to roll on the monolayer (▦) or came to full arrest (▪) was determined. The values of adherent categories depicted in the figure correspond to fractions of the original leukocyte flux perfused in immediate contact with the endothelial layer. (B) FACS (fluorescence-activated cell sorter) staining of LFA-1 (gray lines) and Mac-1 (solid lines) integrins on control and LAD neutrophils using the mAbs TS2.4 (anti-αL integrin subunit) and CBRM1/2 (anti-αM integrin subunit) as primary antibodies. Background staining is shown by the dotted line. (C) PAF-triggered neutrophil arrest on nonactivated HUVECs. Control or LAD neutrophils were perfused at 0.75 dyne/cm2 over unstimulated HUVECs overlaid with PAF (100 nM, 5 minutes of incubation). Arrested cells were determined as in panel A. Where indicated, neutrophils were pretreated with the β2 integrin–blocking mAb TS1.18 (20 μg/mL, 5 minutes, 4°C). Values in panels A and C are given as mean ± range of determinations in 2 fields of view. Because transient tethers comprised less than 10% of the total cell-capturing events, they were not included in the analysis. Results in panels A-C are representative of 3 independent experiments.
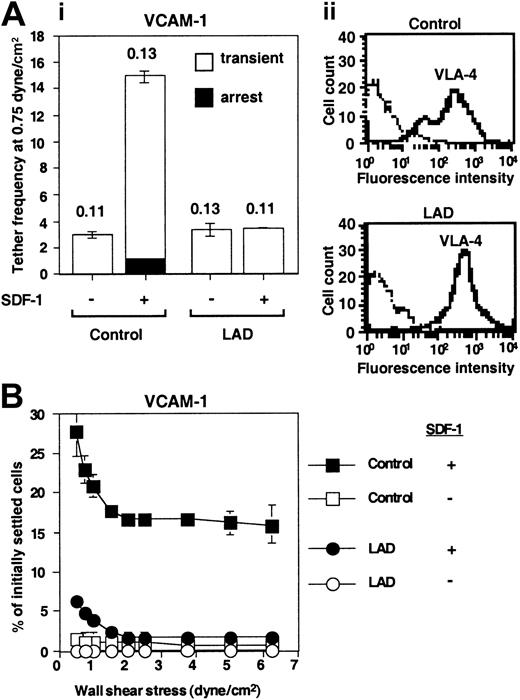
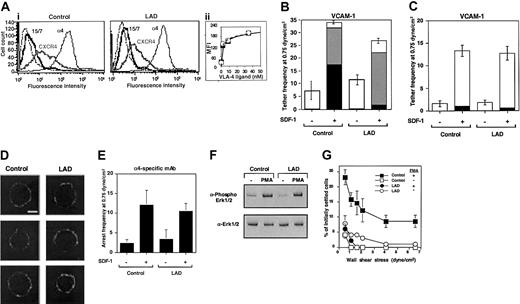
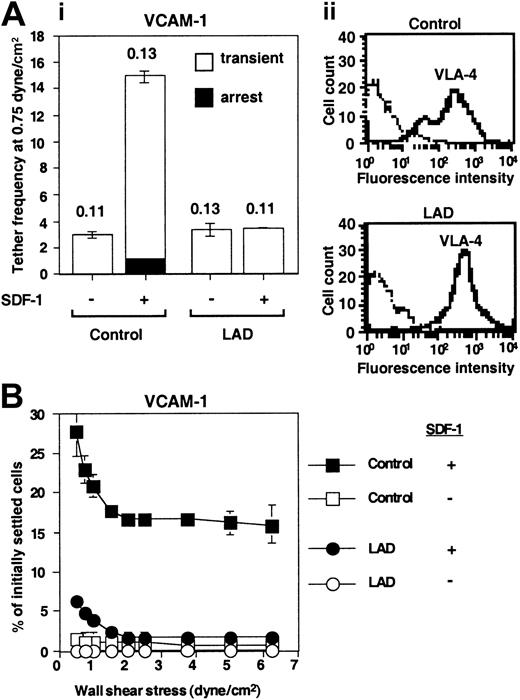
Defective triggering of VLA-4 avidity to VCAM-1 by chemokine in LAD PBLs. (A) SDF-1–augmented VLA-4–mediated capture and arrest of control and patient (LAD) PBLs to purified VCAM-1 under shear flow. (i) Frequency of PBL tethers to purified sVCAM-1 (2 μg/mL) with functional (+) or heat-inactivated (-) SDF-1 (2 μg/mL). The different tether categories were determined in 2 fields, and results are an average and range of each tether category. Mean duration of transient tethers is shown on top of bars. (ii) FACS staining of α4 integrins using the α4 subunit–specific mAb HP1/2. Background staining is indicated by the thin black lines. This mAb fully blocked all PBL adhesion to the different VCAM-1/SDF-1 substrates characterized in panels A-B (not shown). (B) SDF-1–triggered VLA-4–mediated adhesion of PBLs to VCAM-1 at rapid stationary contacts. Resistance to detachment by incremented shear forces developed by control or LAD patient PBLs settled for 1 minute at stasis on sVCAM-1 (2 μg/mL) coimmobilized with functional (+) or heat-inactivated (-) SDF-1 (2 μg/mL). Results are given as mean ± range of determinations in 2 fields of view. A representative experiment of 3 is shown.
Defective triggering of VLA-4 avidity to VCAM-1 by chemokine in LAD PBLs. (A) SDF-1–augmented VLA-4–mediated capture and arrest of control and patient (LAD) PBLs to purified VCAM-1 under shear flow. (i) Frequency of PBL tethers to purified sVCAM-1 (2 μg/mL) with functional (+) or heat-inactivated (-) SDF-1 (2 μg/mL). The different tether categories were determined in 2 fields, and results are an average and range of each tether category. Mean duration of transient tethers is shown on top of bars. (ii) FACS staining of α4 integrins using the α4 subunit–specific mAb HP1/2. Background staining is indicated by the thin black lines. This mAb fully blocked all PBL adhesion to the different VCAM-1/SDF-1 substrates characterized in panels A-B (not shown). (B) SDF-1–triggered VLA-4–mediated adhesion of PBLs to VCAM-1 at rapid stationary contacts. Resistance to detachment by incremented shear forces developed by control or LAD patient PBLs settled for 1 minute at stasis on sVCAM-1 (2 μg/mL) coimmobilized with functional (+) or heat-inactivated (-) SDF-1 (2 μg/mL). Results are given as mean ± range of determinations in 2 fields of view. A representative experiment of 3 is shown.
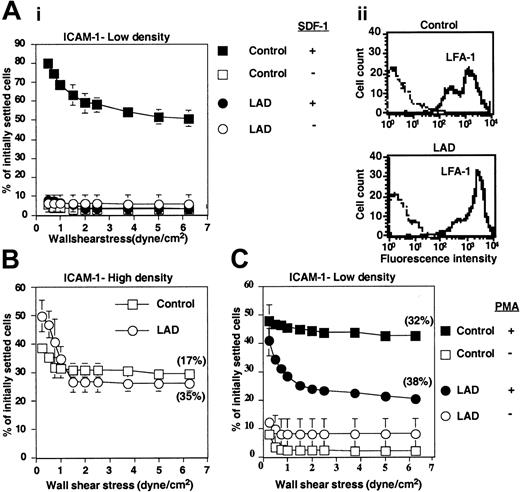
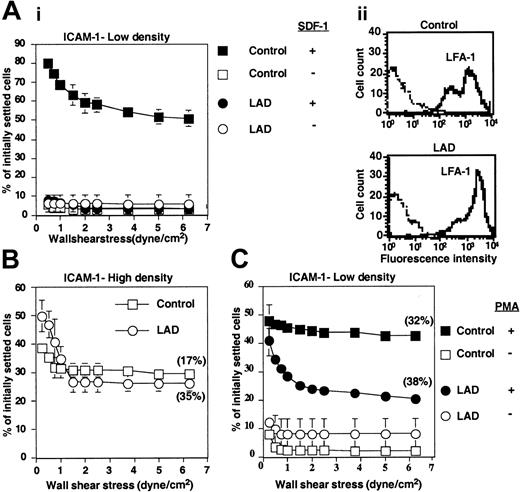
LAD PBLs express functional LFA-1 but fail to exhibit LFA-1–dependent adhesion to ICAM-1 in response to chemokine. (A) SDF-1–triggered LFA-1–mediated adhesion of PBLs to ICAM-1 at stationary contacts. (i) Resistance to detachment by incremented shear forces developed by control or LAD patient PBLs settled for 1 minute at stasis on ICAM-1 coimmobilized at 0.25 μg/mL with inactivated SDF-1 (-) or intact SDF-1 (+), each at 2 μg/mL. (ii) FACS staining of LFA-1 using the αL subunit–specific mAb TS2.4. (B) Spontaneous LFA-1–mediated adhesion to high-density ICAM-1 (0.75 μg/mL) developed upon 1-minute static contact of control (□) or LAD patient (○) PBLs. Level and strength of adhesion was determined by the relative resistance of the different PBLs to detachment by incremented shear stresses. The percentage of adherent cells remaining bound to ICAM-1 at a shear stress of 6.25 dyne/cm2 that underwent spreading on the ligand is shown in parentheses. Further details are outlined in “Materials and methods.” (C) PMA-triggered adhesion and resistance to detachment developed on ICAM-1–IgG (0.25 μg/mL overlaid on protein A) upon 1-minute static contact of control or LAD PBLs. The percentage of adherent cells that underwent spreading is shown in parentheses. Results in panels A-C are given as mean ± range of determinations in 2 fields of view, and the experiment shown is representative of 3 independent tests.
LAD PBLs express functional LFA-1 but fail to exhibit LFA-1–dependent adhesion to ICAM-1 in response to chemokine. (A) SDF-1–triggered LFA-1–mediated adhesion of PBLs to ICAM-1 at stationary contacts. (i) Resistance to detachment by incremented shear forces developed by control or LAD patient PBLs settled for 1 minute at stasis on ICAM-1 coimmobilized at 0.25 μg/mL with inactivated SDF-1 (-) or intact SDF-1 (+), each at 2 μg/mL. (ii) FACS staining of LFA-1 using the αL subunit–specific mAb TS2.4. (B) Spontaneous LFA-1–mediated adhesion to high-density ICAM-1 (0.75 μg/mL) developed upon 1-minute static contact of control (□) or LAD patient (○) PBLs. Level and strength of adhesion was determined by the relative resistance of the different PBLs to detachment by incremented shear stresses. The percentage of adherent cells remaining bound to ICAM-1 at a shear stress of 6.25 dyne/cm2 that underwent spreading on the ligand is shown in parentheses. Further details are outlined in “Materials and methods.” (C) PMA-triggered adhesion and resistance to detachment developed on ICAM-1–IgG (0.25 μg/mL overlaid on protein A) upon 1-minute static contact of control or LAD PBLs. The percentage of adherent cells that underwent spreading is shown in parentheses. Results in panels A-C are given as mean ± range of determinations in 2 fields of view, and the experiment shown is representative of 3 independent tests.
Lymphocyte recirculation through lymph nodes is necessary for maintaining their normal architecture and size.28 Consistent with defective homing of patient lymphocytes to peripheral lymph nodes, a process tightly regulated by lymphocyte L-selectin and CCR7,29,30 the patient had grossly reduced tonsillar tissue upon physical examination. The in vitro response of patient lymphocytes to various mitogens was, however, normal (data not shown). Furthermore, the expression levels of the major integrins on lymphocytes and neutrophils were largely conserved in the patient cells (see Figures 1, 2, 4, and 5), ruling out a LAD-1 syndrome. Patient leukocytes also did not appear to have a LAD-2–like fucosylation defect, because they expressed normal levels of the fucosylated marker CD15a, comprising the sLex carbohydrate selectin ligand (Table 1). Consistent with normal selectin ligand activity on LAD leukocytes, in vitro analysis also confirmed normal capturing and rolling of LAD neutrophils on purified endothelial selectins under physiological shear flow (data not shown).
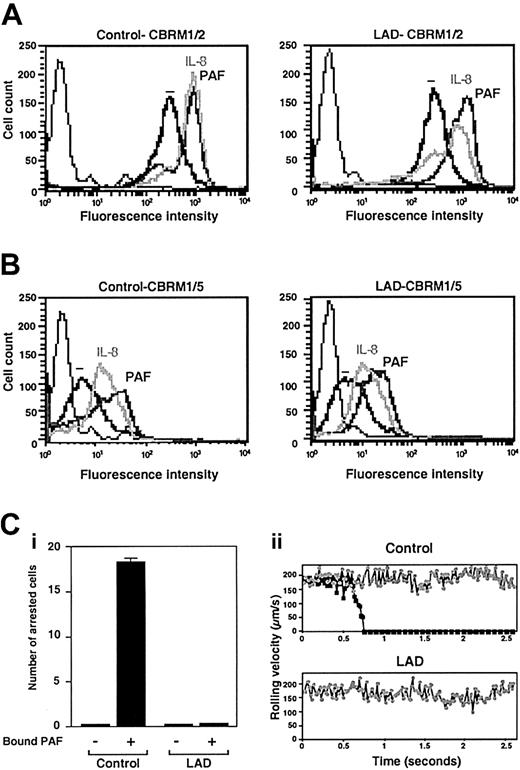
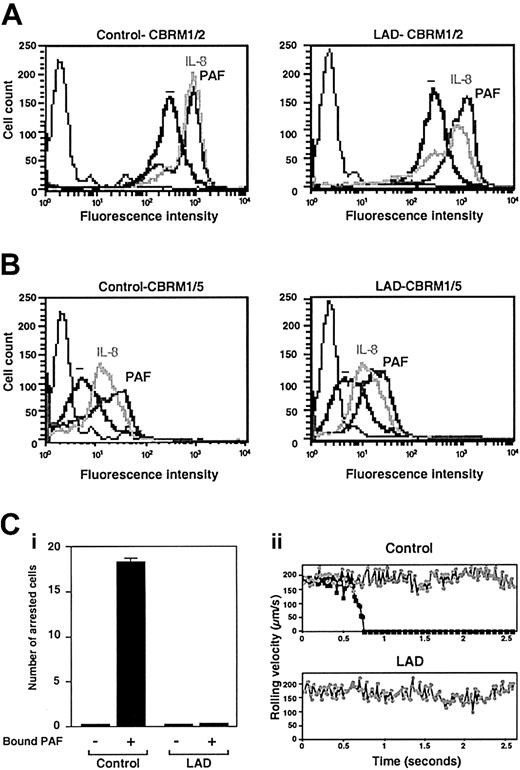
Normal chemoattractant activation of Mac-1 in LAD neutrophils is insufficient to trigger adhesion to Mac-1 ligand. (A) PAF- or IL-8–triggered up-regulation of Mac-1 on surface of control or LAD neutrophils. Cell surface Mac-1 on intact or agonist-treated neutrophils was assessed by FACS staining using the Mac-1 specific mAb CBRM1/2. (B) Induction of the activation neoepitope CBRM1/5 on surface Mac-1 of intact and chemoattractant-stimulated neutrophils. In panels A-B, neutrophils were stimulated with either 200 ng/mL IL-8 or 100 nM PAF as outlined in “Materials and methods.” Background staining is indicated by the thin black lines. (C) Arrest of PAF-stimulated neutrophils on fibrinogen (coated at 10 μg/mL) under low physiological shear flow. (i) Control or LAD neutrophils activated by PAF identically as in panels A-B were immediately perfused over fibrinogen at a shear stress of 0.5 dyne/cm.2 The number of leukocytes stably arrested on the substrate upon capture was determined. No transient tethers were observed in any of the indicated settings. Results are given as mean ± range of determinations in 2 fields of view. (ii) Instantaneous velocity profile (upper panel, ▪) of a PAF-stimulated control neutrophil tethered and arrested on the fibrinogen-coated substrate deduced from computerized cell tracking analysis.47 Velocity profile of a resting control neutrophil (⬢) is shown for comparison. Velocity profiles of both PAF-stimulated and resting neutrophils from the LAD patient (lower panel, ▪ and ⬢, respectively) show no velocity drops, suggesting lack of transient adhesive tethers. Profiles are shown for representative neutrophils.
Normal chemoattractant activation of Mac-1 in LAD neutrophils is insufficient to trigger adhesion to Mac-1 ligand. (A) PAF- or IL-8–triggered up-regulation of Mac-1 on surface of control or LAD neutrophils. Cell surface Mac-1 on intact or agonist-treated neutrophils was assessed by FACS staining using the Mac-1 specific mAb CBRM1/2. (B) Induction of the activation neoepitope CBRM1/5 on surface Mac-1 of intact and chemoattractant-stimulated neutrophils. In panels A-B, neutrophils were stimulated with either 200 ng/mL IL-8 or 100 nM PAF as outlined in “Materials and methods.” Background staining is indicated by the thin black lines. (C) Arrest of PAF-stimulated neutrophils on fibrinogen (coated at 10 μg/mL) under low physiological shear flow. (i) Control or LAD neutrophils activated by PAF identically as in panels A-B were immediately perfused over fibrinogen at a shear stress of 0.5 dyne/cm.2 The number of leukocytes stably arrested on the substrate upon capture was determined. No transient tethers were observed in any of the indicated settings. Results are given as mean ± range of determinations in 2 fields of view. (ii) Instantaneous velocity profile (upper panel, ▪) of a PAF-stimulated control neutrophil tethered and arrested on the fibrinogen-coated substrate deduced from computerized cell tracking analysis.47 Velocity profile of a resting control neutrophil (⬢) is shown for comparison. Velocity profiles of both PAF-stimulated and resting neutrophils from the LAD patient (lower panel, ▪ and ⬢, respectively) show no velocity drops, suggesting lack of transient adhesive tethers. Profiles are shown for representative neutrophils.
LAD neutrophils fail to trigger β2 integrin–mediated arrest on inflamed endothelial surfaces under flow
The preliminary results suggested that the LAD syndrome could be associated with impaired integrin avidity modulation at endothelial contact sites. To test this possibility, LAD neutrophils were perfused over a monolayer of TNF-stimulated HUVECs under physiological shear flow. This model of inflamed endothelium expresses high levels of E-selectin and multiple integrin ligands.5 Consistent with their normal expression of selectin ligands, LAD neutrophils were captured by the cytokine-activated HUVECs at comparable rates and initiated normal rolling on the endothelial monolayer (Figure 1A). Shortly after capture on the endothelial surface, normal neutrophils spontaneously arrested on the activated endothelium, in a β2 integrin–dependent manner (data not shown), a result of rapid β2 integrin activation by apically displayed chemoattractants, mainly PAF and IL-8.31 Notably, however, LAD neutrophils failed to arrest and continued to roll over the cytokineactivated EC monolayer (Figure 1A). This failure could not be explained solely by their reduced levels of the LFA-1 β2 integrin (Figure 1B), a major player in neutrophil arrest on vascular endothelium,32 because a second endothelial-binding β2 integrin, Mac-1, was highly expressed at normal levels on the LAD neutrophils (Figure 1B) and underwent normal surface upregulation and activation upon chemoattractant stimulation (Figure 2A-B). Furthermore, patient leukocytes failed to arrest on PAF-presenting nonstimulated HUVEC monolayers under flow (Figure 1C), whereas normal neutrophils arrested readily and rapidly on identical monolayers (Figure 1C and data not shown), a result of an in situ triggering of β2 integrin avidity to endothelial ligands by endothelial-presented PAF.33 Interestingly, both PAF and IL-8 triggered an activation Mac-1 neoepitope associated with a high-affinity integrin conformation18 to the same extent in both patient and normal neutrophils (Figure 2B). Additionally, β2 integrins on patient leukocytes could acquire activation neoepitopes in response to neutrophil or lymphocyte stimulation by phorbol ester or by the CD18 stimulatory mAb 240Q 19 (data not shown). Nevertheless, PAF-stimulated LAD neutrophils failed to even loosely tether to the Mac-1 ligand, fibrinogen (Figure 2C). Thus, despite the ability of patient β2 integrins to acquire high-affinity conformations in response to chemoattractants, these activated integrins were completely defective in their ability to develop firm adhesiveness to ligand at subsecond contacts under physiological flow conditions (Figures 1A,C and 2B-C).
LAD lymphocytes exhibit impaired chemokine-triggered avidity despite normal expression and intrinsic function of integrins and chemokine receptors
Normal and patient PBLs were next tested for their ability to interact with a monolayer of TNF-α–stimulated HUVECs under physiological shear flow. This EC model mediates the capture and rolling of PBLs through its abundant E-selectin.5,25 In agreement with their conserved expression of selectin ligands, patient lymphocytes established normal rolling on TNF-α–activated EC monolayers (Figure 3A). Furthermore, a small fraction of both normal and patient lymphocytes spontaneously arrested on the endothelial cells, a process mediated by chemokine-independent adhesiveness of both VLA-4 and LFA-1 to endothelial VCAM-1 and ICAM-1.5 Nevertheless, whereas the prototypic PBL chemokine, SDF-1 (CXCL12), overlaid on TNF-activated HUVECs could trigger robust integrin-dependent arrest of captured PBLs on the TNF-α–stimulated HUVEC monolayer, only a small subset of patient lymphocytes could arrest on the HUVECs in response to SDF-1 (Figure 3A). SDF-1–triggered integrin-dependent lymphocyte arrest on HUVECs involves chemokine activation of Gi protein machinery.4, 5, 6 This result therefore suggested that SDF-1–triggered integrin avidity to vascular endothelial ligands is impaired in patient lymphocytes. Patient lymphocytes had, however, normal expression levels and G protein signaling capacity of the key SDF-1 receptor, CXCR4 (Figure 3B-C). Similarly, T lymphoblasts generated from LAD patient or control PBLs underwent normal mitogen-activated protein (MAP) kinase activation in response to short exposure to IP-10 (Figure 3D), a prototypic chemokine to CXCR3 expressed by activated effector lymphocytes, although this chemokine failed to stimulate VLA-4–mediated adhesion to VCAM-1 (data not shown). We could not extend this observation to other major lymphocyte chemokines such as SLC or ELC (CCL21 and 19) in light of reduced expression of their receptor CCR7 in patient PBLs.
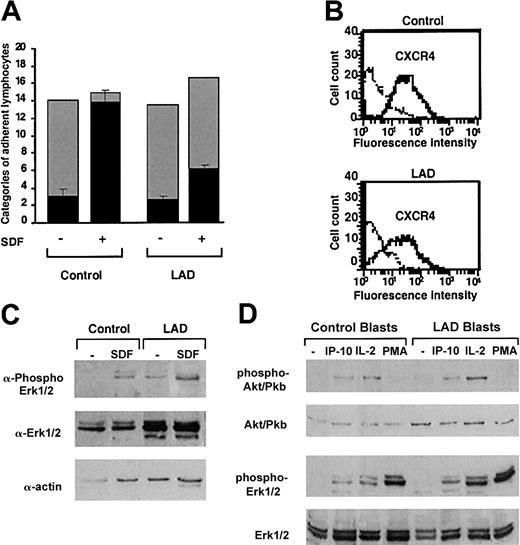
Normal GPCR signaling but defective chemokine-activated adhesion in LAD PBLs. (A) Effect of SDF-1 on normal and patient PBL rolling and arrest on TNF-activated HUVECs under physiological shear flow. Leukocytes isolated from a healthy donor (control) or patient (LAD) were perfused for 1 minute at 0.75 dyne/cm2 over HUVEC monolayers, and the number of accumulated leukocytes that either continued to roll (▦) or came to full arrest (▪) was determined. Transient tethers comprised less than 10% of the total cell-capturing events and were not included in the analysis. Results are given as mean ± range of determinations in 2 fields of view. (B) FACS staining of CXCR4 on control and LAD PBLs with the 12G5 mAb. Background staining is indicated by the thin black lines. (C) SDF-1 stimulation (100 nM, 30 seconds) of ERK1/2 phosphorylation in control or LAD PBLs. Immunoblotting with antiphosphospecific ERK1/2 (top panel) and anti-ERK (middle panel) is depicted. Cell lysates were separated on reducing 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Control actin immunoblotting (bottom panel) is shown. (D) IP-10, IL-2, and phorbol ester stimulation of Akt (Pkb) and ERK1/2 in T lymphoblasts. Blasts were stimulated with either IP-10 (100 nM, 30 seconds), IL-2 (1000 U/mL, 5 minutes), or PMA (100 ng/mL, 2 minutes) before lysis. Immunoblotting with antiphosphospecific Akt or ERK1/2 (first and third rows, respectively) and anti-Akt or ERK (second and fourth rows, respectively) is depicted. In panels C-D, lysates of agonist-stimulated lymphocytes were separated on reducing 10% SDS-PAGE.
Normal GPCR signaling but defective chemokine-activated adhesion in LAD PBLs. (A) Effect of SDF-1 on normal and patient PBL rolling and arrest on TNF-activated HUVECs under physiological shear flow. Leukocytes isolated from a healthy donor (control) or patient (LAD) were perfused for 1 minute at 0.75 dyne/cm2 over HUVEC monolayers, and the number of accumulated leukocytes that either continued to roll (▦) or came to full arrest (▪) was determined. Transient tethers comprised less than 10% of the total cell-capturing events and were not included in the analysis. Results are given as mean ± range of determinations in 2 fields of view. (B) FACS staining of CXCR4 on control and LAD PBLs with the 12G5 mAb. Background staining is indicated by the thin black lines. (C) SDF-1 stimulation (100 nM, 30 seconds) of ERK1/2 phosphorylation in control or LAD PBLs. Immunoblotting with antiphosphospecific ERK1/2 (top panel) and anti-ERK (middle panel) is depicted. Cell lysates were separated on reducing 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Control actin immunoblotting (bottom panel) is shown. (D) IP-10, IL-2, and phorbol ester stimulation of Akt (Pkb) and ERK1/2 in T lymphoblasts. Blasts were stimulated with either IP-10 (100 nM, 30 seconds), IL-2 (1000 U/mL, 5 minutes), or PMA (100 ng/mL, 2 minutes) before lysis. Immunoblotting with antiphosphospecific Akt or ERK1/2 (first and third rows, respectively) and anti-Akt or ERK (second and fourth rows, respectively) is depicted. In panels C-D, lysates of agonist-stimulated lymphocytes were separated on reducing 10% SDS-PAGE.
To substantiate these conclusions, we next compared both the spontaneous and SDF-1–stimulated integrin avidity developed by patient-derived PBLs to purified integrin ligands. Despite conserved VLA-4 expression levels (Figure 4A) and intact spontaneous VLA-4 adhesiveness to low-density VCAM-1 (Figure 4A), SDF-1–stimulated VLA-4 avidity, generated at subsecond contacts of PBLs tethered to VCAM-1 under shear flow, was entirely abrogated in patient lymphocytes (Figure 4A). Hence, SDF-1 did not augment VLA-4–dependent tethering to VCAM-1 in LAD PBLs and had no effect on the duration of the transient tethers spontaneously generated by intact LAD PBLs. A more prolonged contact of patient PBLs on VCAM-1 also did not restore their defective ability to develop high-avidity adhesion to VCAM-1 in response to SDF-1 stimulation, despite robust VLA-4 avidity stimulation by SDF-1 in normal lymphocytes (Figure 4B). Patient lymphocytes also failed to develop firm LFA-1–dependent adhesion to purified ICAM-1, induced by SDF-1 (Figure 5A), despite normal expression and spontaneous adhesiveness of their LFA-1 to high-density ICAM-1 (Figure 5Aii,5B). Chemokine stimulation of lymphocyte LFA-1 avidity to ICAM-1 at rapid stationary contacts does not implicate intact diacylglycerol (DAG)–dependent protein kinase C (PKC) (G.C. and R.A., unpublished results, 2002). However, phorbol ester–stimulated PKC-mediated enhancement of LFA-1 avidity to ICAM-1 was reduced by about 50% in patient lymphocytes compared with control lymphocytes (Figure 5C). Thus, in addition to a defective integrin activation by G protein–coupled chemokine receptor (GPCR) signaling, PKC-triggered activation of patient LFA-1 integrins was also partially impaired, although to a lesser extent than chemokine-triggered LFA-1–dependent adhesion (Figure 5A). The ability of LAD PBLs to spread on ICAM-1 remained, however, intact (Figure 5B-C), suggesting normal LFA-1 outside-in signaling and downstream actin remodeling in LAD lymphocytes. Taken together, SDF-1 activation of both VLA-4 and LFA-1 avidity to ligand under dynamic conditions of shear flow was severely impaired in LAD patient lymphocytes despite normal levels and intrinsic adhesiveness of both integrins and normal signaling capacity of the SDF-1 GPCR, CXCR4.
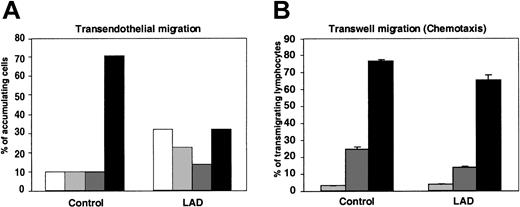
Transendothelial migration (TEM) of lymphocytes arrested on endothelium and subsequent chemotaxis are largely conserved in LAD cells
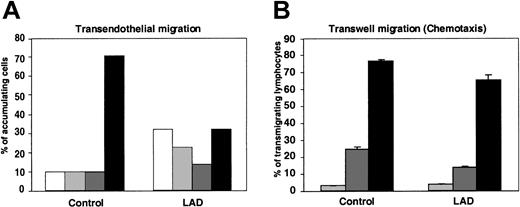
We have recently shown that lymphocyte TEM in the presence of physiological shear flow is promoted by apical endothelial chemokines and requires persistent integrin-mediated adhesion to the endothelial surface.25 In agreement with these results, within the fraction of LAD PBLs capable of arresting on TNF-activated HUVECs presenting apical SDF-1 after prolonged rolling periods, only half successfully transmigrated the EC barrier, compared with two thirds of normal arrested lymphocytes (Figure 6A). Reduced TEM potential of the LAD lymphocytes was mainly due to their inability to remain firmly adherent to the endothelial surface throughout the assay rather than to defective spreading, locomotion, or subsequent transmigration across the barrier (Figure 6A).
Chemokine-triggered transendothelial migration of patient PBLs is partially defective, but chemotaxis is conserved. (A) Migration of PBLs accumulated on TNF-activated HUVECs alone or overlaid with SDF-1 (100 ng/mL), subjected to physiological shear stress (5 dyne/cm2) for 20 minutes. The 4 indicated categories of lymphocytes accumulated on the HUVEC monolayer were defined as outlined in “Materials and methods.” White bars indicate detachment; light gray bars, arrest; dark gray bars, locomotion; black bars, TEM. Results represent the mean ± SEM of 3 independent experiments. (B) SDF-1–triggered PBL chemotaxis determined in a transwell filter assay. Control or LAD PBLs were allowed to migrate toward the indicated concentrations of SDF-1 placed in the lower chamber of a BSA-coated transwell. Light gray bars indicate no SDF-1; dark gray bars, 10 nM SDF-1; black bars, 50 nM SDF-1. Results are average ± range of duplicate wells.
Chemokine-triggered transendothelial migration of patient PBLs is partially defective, but chemotaxis is conserved. (A) Migration of PBLs accumulated on TNF-activated HUVECs alone or overlaid with SDF-1 (100 ng/mL), subjected to physiological shear stress (5 dyne/cm2) for 20 minutes. The 4 indicated categories of lymphocytes accumulated on the HUVEC monolayer were defined as outlined in “Materials and methods.” White bars indicate detachment; light gray bars, arrest; dark gray bars, locomotion; black bars, TEM. Results represent the mean ± SEM of 3 independent experiments. (B) SDF-1–triggered PBL chemotaxis determined in a transwell filter assay. Control or LAD PBLs were allowed to migrate toward the indicated concentrations of SDF-1 placed in the lower chamber of a BSA-coated transwell. Light gray bars indicate no SDF-1; dark gray bars, 10 nM SDF-1; black bars, 50 nM SDF-1. Results are average ± range of duplicate wells.
Consistent with this conserved inherent migratory ability of the LAD lymphocytes across endothelial barriers in response to chemokine signals (Figure 6A), the ability of LAD PBLs to migrate across transwell filters toward a chemotactic gradient of SDF-1, under conditions where integrin contribution is minimized (data not shown), was only slightly reduced relative to control PBLs (Figure 6B). These results indicate that impaired integrin activation by chemokines in LAD PBLs results in dramatic suppression of arrest but a relatively small defect in the ability of stably arrested lymphocytes to transmigrate through the endothelial barrier. Thus, rapid chemokine-stimulated integrin avidity at endothelial contacts could be functionally separated from the chemokine-stimulated migration and chemotaxis in the LAD lymphocytes, consistent with the conserved signaling capacity of the chemokine GPCR.
LAD VLA-4 fails to generate high avidity to ligand in response to distinct inside-out signals despite normal pre-existent clustering and affinity to soluble ligand
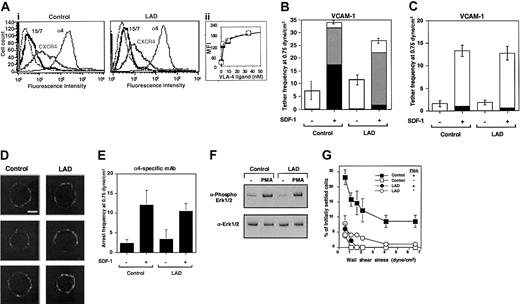
To gain further insight into the molecular basis of this integrin activation defect, we analyzed the adhesive properties of VLA-4 (α4β1) in EBV-transformed B lymphoblasts derived from the LAD patient PBLs and from age-matched control lymphocytes. LAD EBV blasts expressed normal α4β1 and CXCR4 levels as well as normal basal levels of the β1 integrin activation epitope, 15/7 34 (Figure 7A and data not shown). Furthermore, VLA-4 on LAD blasts underwent normal induction of this epitope in the presence of increasing concentrations of the monovalent VLA-4 ligand, Bio1211 (Figure 7A inset), suggesting that soluble ligand binding properties of VLA-4 are conserved in the LAD lymphocytes. In addition, SDF-1 did not elevate VLA-4 affinity to ligand in either control or LAD lymphoblasts (data not shown), as previously reported for PBLs.5 Nevertheless, LAD cells demonstrated a reduced ability to arrest on VCAM-1 coimmobilized with SDF-1 under shear flow relative to control cells, a step mediated by pre-existent high-affinity VLA-4 subsets on tethered lymphocytes.5 SDF-1 or Mig (CXCL9)–stimulated LAD cells also developed much weaker VLA-4–dependent adhesion to VCAM-1 at 1-minute stationary contacts (data not shown). However, VLA-4 on the LAD blasts efficiently responded to the chemokine signals and initiated weak rolling tethers on VCAM-1 (Figure 7B). Furthermore, the ability of SDF-1 to trigger functional VLA-4 tethers to low-density VCAM-1 in LAD blasts was indistinguishable from control cells, indicating normal subsecond GPCR signaling to integrins in LAD lymphoblasts (Figure 7C), complementing the fully intact GPCR signaling of primary LAD T cells and T-cell blasts (Figure 3). VLA-4 was also normally distributed in largely unclustered states on LAD and control cells prior to chemokine encounter (Figure 7D). Consistent with normal chemokine signaling to α4 integrins, SDF-1 coimmobilized with the α4 integrin specific mAb, HP1/2, which binds α4 integrins independent of their affinity to native ligands,16,35 enhanced α4-dependent LAD capture to immobilized HP1/2 with comparable efficiency to control cells (Figure 7E). This finding also suggested that the ability of VLA-4 to cluster with a high-affinity binding mAb when in situ activated by a chemokine signal was not impaired in LAD lymphoblasts. In addition to a defective stimulation of VLA-4 avidity by chemokine signaling, inside-out signaling triggered by phorbol ester–activated PKC, although normal in LAD cells (Figure 7F), failed to stimulate VLA-4 avidity to VCAM-1 in LAD lymphoblasts (Figure 7G). These results collectively suggest that even when VLA-4 responds normally to chemokine signals and promotes transient tethers to low density VCAM-1 (Figure 7C), both chemokine-facilitated and phorbol ester–stimulated avidity of VLA-4 to high-density VCAM-1 are severely impaired in LAD lymphocytes.
Chemokine-triggered adhesion strengthening of VLA-4 tethers is impaired in LAD lymphoblasts due to defective VLA-4 rearrangement despite conserved integrin clustering and affinity to soluble ligand. (Ai) FACS staining of α4 integrins on control and LAD-derived EBV-transformed B lymphoblasts with the α4 subunit–specific mAb HP1/2. Staining of the SDF-1 receptor CXCR4 was performed with the mAb 6H8. (ii) Induction of 15/7 LIBS epitope by soluble monovalent VLA-4 ligand (Bio1211). Dose-dependent induction of the epitope by increasing concentrations of the specific ligand is depicted for LAD cells (○) and control cells (▪). The 15/7 epitope staining, as detected with PE-antimouse IgG and analyzed by FACScan, is expressed in mean fluorescence intensity units (MFI). (B) Chemokine-triggered adhesion strengthening of VLA-4/VCAM-1 tethers at subsecond contacts is impaired in LAD EBV lymphoblasts. Immediate SDF-1–augmented VLA-4–mediated capture and arrest of control and patient cells on VCAM-1 under shear flow. The frequency of different types of cell tethers to purified sVCAM-1 (2 μg/mL) coimmobilized with functional (+) or heat-inactivated (-) SDF-1 (1 μg/mL) was determined as in Figure 4. Complete blocking of chemokine-triggered or spontaneous blast adhesion to VCAM-1 with α4 and β1 integrin mAbs but not with the α4β7-specific mAb suggested an exclusive role for VLA-4 in SDF-1–triggered lymphoblast tethering to VCAM-1 (not shown). □ indicates transient tethers; ▦, rolling; ▪, arrest. (C) Chemokine triggering of transient VLA-4–mediated tethers to low-density ligand is not defective in LAD blasts. The frequency of different types of cell tethers to purified sVCAM-1 (0.5 μg/mL) coimmobilized with functional (+) or heat-inactivated (-) SDF-1 (2 μg/mL) was determined as in Figure 4. □ indicates transient tethers; ▦, rolling; ▪, arrest. (D) VLA-4 is uniformly distributed both on normal and LAD lymphoblasts prior to contact with chemokine and ligand. Fluorescence microscopy of α4 integrins on normal and LAD EBV lymphoblasts. Fixed cells were incubated with HP1/2, stained with Alexa-488–conjugated secondary Ab, and analyzed by confocal microscopy as described in “Materials and methods.” White scale bar, 5 μm. In each experimental group, 3 representative cells are shown. (E) Capture of lymphoblasts on immobilized α4-specific mAb HP1/2 (0.2 μg/mL) triggered by coimmobilized SDF-1 (2 μg/mL; inactive, -; intact, +). All cell capture events resulted in immediate arrest in this experimental setting. No tethers were observed on SDF-1 alone or on control mAb anti-VCAM-1 (not shown). (F) Normal PMA stimulation (100 ng/mL, 2 minutes) of ERK1/2 phosphorylation in control and LAD EBV lymphoblasts. Immunoblotting with antiphosphospecific ERK1/2 (top panel) and anti-ERK (bottom panel) is depicted. Cell lysates were separated on reducing 10% SDS-PAGE. (G) Impaired PMA-triggered VLA-4–mediated adhesion of LAD-derived EBV-transformed B lymphoblasts to VCAM-1 at 1-minute stationary contacts. Resistance to detachment by incremented shear forces developed by cells, untreated or stimulated by PMA (100 ng/mL, 2 minutes), settled for 1 minute at stasis on sVCAM-1 (0.5 μg/mL). Results are given as mean ± range of determinations in 2 fields of view. PMA failed to stimulate VLA-4 avidity at subsecond contacts of both normal and LAD EBV lymphoblasts tethered to VCAM-1 under shear flow (not shown). Experiments depicted in panels B-C,E,G are each representative of 3 to 4 independent tests.
Chemokine-triggered adhesion strengthening of VLA-4 tethers is impaired in LAD lymphoblasts due to defective VLA-4 rearrangement despite conserved integrin clustering and affinity to soluble ligand. (Ai) FACS staining of α4 integrins on control and LAD-derived EBV-transformed B lymphoblasts with the α4 subunit–specific mAb HP1/2. Staining of the SDF-1 receptor CXCR4 was performed with the mAb 6H8. (ii) Induction of 15/7 LIBS epitope by soluble monovalent VLA-4 ligand (Bio1211). Dose-dependent induction of the epitope by increasing concentrations of the specific ligand is depicted for LAD cells (○) and control cells (▪). The 15/7 epitope staining, as detected with PE-antimouse IgG and analyzed by FACScan, is expressed in mean fluorescence intensity units (MFI). (B) Chemokine-triggered adhesion strengthening of VLA-4/VCAM-1 tethers at subsecond contacts is impaired in LAD EBV lymphoblasts. Immediate SDF-1–augmented VLA-4–mediated capture and arrest of control and patient cells on VCAM-1 under shear flow. The frequency of different types of cell tethers to purified sVCAM-1 (2 μg/mL) coimmobilized with functional (+) or heat-inactivated (-) SDF-1 (1 μg/mL) was determined as in Figure 4. Complete blocking of chemokine-triggered or spontaneous blast adhesion to VCAM-1 with α4 and β1 integrin mAbs but not with the α4β7-specific mAb suggested an exclusive role for VLA-4 in SDF-1–triggered lymphoblast tethering to VCAM-1 (not shown). □ indicates transient tethers; ▦, rolling; ▪, arrest. (C) Chemokine triggering of transient VLA-4–mediated tethers to low-density ligand is not defective in LAD blasts. The frequency of different types of cell tethers to purified sVCAM-1 (0.5 μg/mL) coimmobilized with functional (+) or heat-inactivated (-) SDF-1 (2 μg/mL) was determined as in Figure 4. □ indicates transient tethers; ▦, rolling; ▪, arrest. (D) VLA-4 is uniformly distributed both on normal and LAD lymphoblasts prior to contact with chemokine and ligand. Fluorescence microscopy of α4 integrins on normal and LAD EBV lymphoblasts. Fixed cells were incubated with HP1/2, stained with Alexa-488–conjugated secondary Ab, and analyzed by confocal microscopy as described in “Materials and methods.” White scale bar, 5 μm. In each experimental group, 3 representative cells are shown. (E) Capture of lymphoblasts on immobilized α4-specific mAb HP1/2 (0.2 μg/mL) triggered by coimmobilized SDF-1 (2 μg/mL; inactive, -; intact, +). All cell capture events resulted in immediate arrest in this experimental setting. No tethers were observed on SDF-1 alone or on control mAb anti-VCAM-1 (not shown). (F) Normal PMA stimulation (100 ng/mL, 2 minutes) of ERK1/2 phosphorylation in control and LAD EBV lymphoblasts. Immunoblotting with antiphosphospecific ERK1/2 (top panel) and anti-ERK (bottom panel) is depicted. Cell lysates were separated on reducing 10% SDS-PAGE. (G) Impaired PMA-triggered VLA-4–mediated adhesion of LAD-derived EBV-transformed B lymphoblasts to VCAM-1 at 1-minute stationary contacts. Resistance to detachment by incremented shear forces developed by cells, untreated or stimulated by PMA (100 ng/mL, 2 minutes), settled for 1 minute at stasis on sVCAM-1 (0.5 μg/mL). Results are given as mean ± range of determinations in 2 fields of view. PMA failed to stimulate VLA-4 avidity at subsecond contacts of both normal and LAD EBV lymphoblasts tethered to VCAM-1 under shear flow (not shown). Experiments depicted in panels B-C,E,G are each representative of 3 to 4 independent tests.
Discussion
Rapid stimulation of integrin adhesiveness to endothelial ligands is a hallmark of leukocyte recruitment to sites of inflammation and lymphocyte homing to lymph nodes.1,36 Although the molecular basis of this process is still poorly understood, the ability of leukocyte integrins to up-regulate their avidity has been linked to stimulatory signals transduced through specialized leukocyte GPCRs occupied by endothelial-displayed chemokines or chemoattractants at subsecond contacts.4,5,37 Integrin avidity is regulated by multiple cytoskeletal effectors that promote integrin affinity, clustering, and postligand integrin anchorage to the cytoskeleton.38,39 Thus far, no human or animal model with a specific defect in rapid GPCR-mediated integrin activation has been described. We now provide a first demonstration of a genetic defect in this key process, in which functionally intact integrins fail to undergo rapid stimulation of avidity to vascular ligand in response to a subsecond chemokinetriggered GPCR signal. This failure results in a marked leukocyte adhesion deficiency syndrome with severe clinical manifestations including profound leukocytosis and impaired leukocyte trafficking to sites of inflammation.
The 2 major genetic adhesion deficiencies described to date, LAD-1 and -2, although rare, have contributed key insights into the critical role of integrin and selectin adhesion receptors in leukocyte trafficking.40 The first syndrome, LAD-1, a result of defective expression of CD18,9,41 has been reported in more than 200 patients with more than 20 different mutations in the CD18 encoding gene.40 In addition, 2 reports described patients with a moderate LAD-1 phenotype, the result of point mutations that disrupted integrin function rather than expression.11,42 Three additional reports with some similarities to the present LAD case demonstrated severe LAD-1–like syndromes associated with normal expression of structurally intact integrins but defective activation of multiple integrin types, including platelet β3 integrin.12,13,19,43 However, in none of these LAD-1 variant cases was the ability of leukocyte integrins to develop high avidity to ligands in response to rapid chemokine-stimulation signals under physiological conditions of shear flow tested. The LAD-1 variant reported by Kuijpers and coworkers exhibited a major defect in the ability of β2 integrins on both neutrophils and B lymphoblasts to acquire high-affinity integrin activation states.12,19 In sharp contrast, the present LAD neutrophils and lymphocytes exhibited normal induction of multiple β2 integrin activation neoepitopes (Figure 2 and data not shown). The presently investigated LAD syndrome was also not associated with major impairment of spontaneous β2 integrin avidity to ICAM-1 or lymphocyte spreading triggered by LFA-1 occupancy (Figure 5B) but, rather, in a severe defect in a subsecond chemokine triggering of integrin-mediated arrest (Figure 5A). Furthermore, whereas artificial β2 integrin stimulation by the affinity-activating mAb, KIM185, completely rescued the presently described LAD defect (data not shown), different subsets of leukocytes in these earlier reports showed impaired β2 integrin–dependent adhesion in the presence of Mn2+ or the KIM185 mAb.13,43 In addition, whereas in 2 of these cases LAD variant leukocytes showed defective chemotaxis,12,13 the present LAD lymphocytes migrated normally toward SDF-1. Time-lapse microscopic analysis of LAD PBLs in SDF-1–containing collagen gels also revealed normal spreading and motility (data not shown). Interestingly, in one of the LAD cases, normal LFA-1–dependent lymphocyte spreading and motility was reported,43 reminiscent of the intact LFA-1–dependent T-cell spreading noted here. This LAD case reported, however, abnormally augmented constitutive clustering of both VLA-4 and LFA-1 integrins on the surface of T cells,43 contrasting the normal integrin clustering observed in the present study (Figure 7D). In conclusion, it is likely that although these 3 earlier LAD cases may share with the present case some similar dysfunctions of integrin activation, they likely each represent distinct molecular defects in inside-out integrin activation. Validation of subsecond integrin activation deficiencies by chemokine signals in each one of these earlier LAD cases remains to be tested and may reveal interesting hierarchies of integrin avidity modulation defects at rapid adhesive contacts.
The molecular defect underlying this specialized LAD phenotype—that is, functionally intact integrins that fail to undergo rapid stimulation of avidity and adhesion in response to a subsecond chemokine-triggered GPCR signal—is still obscure. Our previous findings suggested that subsecond induction of VLA-4 avidity to VCAM-1 by immobilized chemokines involves rapidly triggered, cytoskeletally stabilized integrin clustering rather than de novo induction of high affinity.5 To analyze the molecular basis of the LAD defect in this specific integrin activation process, we took several approaches to follow the course of VLA-4 avidity stimulation by SDF-1 in control and LAD EBV lymphoblasts. At a first level we observed normal preformed integrin distribution and monovalent ligand binding properties of VLA-4 in LAD blasts (Figure 7A,D). At the second level, we observed normal integrin clustering by cell capture to immobilized mAb specific to the VLA-4 integrin (Figure 7E). At a third level, we observed intact chemokine-mediated VLA-4 tethering to low-density VCAM-1, suggesting that intrinsic integrin response to subsecond GPCR signals is retained in this cellular system (Figure 7C) despite defective avidity induction on high-density VCAM-1 (Figure 7B and data not shown). Because we do not detect alterations in soluble ligand binding of VLA-4 by chemokine (data not shown and Grabovsky et al5 ), we postulate that in normal lymphocytes, VLA-4 molecules occupied by ligand (possibly a pre-existent subset with preformed high-affinity state5 ) rearrange at subsecond contacts to derive high-avidity binding and immediately arrest tethered lymphocytes. This chemokine-stimulated integrin rearrangement step is defective in LAD cells. In addition, distinct integrin stimulating inside-out signals, triggered by global PKC activation of PMA-treated lymphocytes, also resulted in impaired integrin avidity stimulation, although these processes operate at a much slower time scale than chemokines (Figures 5 and 7). In addition to abrogation of high-avidity VLA-4 stimulation in LAD-derived EBV lymphoblasts, low-avidity chemokine-stimulated VLA-4 tethers were also impaired in LAD primary T lymphocytes, suggesting that the severity of the integrin-rerarrangement defect may vary between different types and activation states of leukocytes. Consistent with a severe defect in integrin avidity generation in primary LAD leukocytes, even when β2 integrins in LAD neutrophils acquired activation neoepitopes associated with high-affinity ligand binding18,19 in response to chemoattractant stimuli, they completely failed to generate low or high avidity to ligand-containing adhesive sites in response to these stimuli (Figure 2). Thus, LAD β2 integrins failed to generate high-avidity contacts even when conformationally stimulated by GPCR signals, possibly due to failure to rearrange these stimulated integrins at sites of ligand occupancy.
The effectors that translate subsecond chemokine-induced signals into integrin rearrangements resulting in immediate avidity up-regulation are downstream targets of Gi-protein signaling.37,44 The identity and mode of activity of these effectors are still largely unknown. Different cytoskeletal associations have been proposed to regulate the avidity of distinct integrins.39 For instance, inhibition of LFA-1 release from cytoskeletal constraints was suggested to perturb stimulation of avidity at subsecond contacts.6 However, release of VLA-4 from the cytoskeleton completely abrogates its ability to up-regulate avidity in response to chemokine signals under shear flow.24 Notably, immediate targets for G protein activation such as phosphatidylinositol-3 kinase (PI-3K), phospholipase C (PLC), and DAG-dependent PKC, Src, or focal adhesion kinases3 were found to be dispensible for chemokine stimulation of VLA-4 avidity in T cells (E. Winter and R.A., unpublished results). Furthermore, although the guanosine triphosphatases (GTPases) RhoA and Rac have been suggested to play a key role in chemokine-stimulated integrin avidity,45,46 subsecond SDF-1–stimulated VLA-4 avidity was insensitive to attenuation of chemokine-triggered Rho or Rac activation (M.S.-E., manuscript in preparation). It is therefore possible that an as yet unidentified cytoskeletal adaptor is utilized by VLA-4, LFA-1, Mac-1, and other immune cell and platelet integrins to rapidly generate high avidity at adhesive contacts in response to in situ GPCR signals. Interestingly, the patient is a child of first cousins, most probably heterozygote carriers of the defective gene. The recessive nature of the genetic defect underlying the LAD phenotype rules out that the defect is due to a dominant suppressor abnormally expressed or functionally mutated in LAD leukocytes. Notably, partial suppression of chemokine activation of integrin avidity was also observed in PBLs derived from the patient's parents (data not shown). This observation suggests that the LAD disorder may be a result of a loss or a mutation in the cytoskeletal adaptor shared by leukocyte and platelet integrins, the function of which is rescued by a normal allele in the heterozygote patients. Identification of this integrin effector will lead to a better understanding of the most upstream adhesive events, triggered by GPCR agonists at specific endothelial sites, controlling the arrest and subsequent emigration of circulating leukocytes and hematopoietic progenitors at these sites to target extravascular tissues.
Prepublished online as Blood First Edition Paper, February 20, 2003; DOI 10.1182/blood-2002-11-3427.
Supported in part by the Israel Science Foundation founded by the Israel Academy of Sciences and Humanities and by the Minerva Foundation (R.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Drs R. Lobb, T. Springer, C. Beals, Y. van Kooyk, and M. Lipp for gifts of reagents. We also thank Dr Susanne Franitza for analysis of PBL motility and Dr S. Schwarzbaum for editorial assistance.
R.A. is the Incumbent of the Tauro Career Development Chair in Biomedical Research.