Binding of erythropoietin (EPO) to its receptor (EPOR) on erythroid cells induces the activation of numerous signal transduction pathways, including the mitogen-activated protein kinase Jun-N-terminal kinase (JNK). In an effort to understand the regulation of EPO-induced proliferation and JNK activation, we have examined the role of potential autocrine factors in the proliferation of the murine erythroleukemia cell line HCD57. We report here that treatment of these cells with EPO induced the expression and secretion of tumor necrosis factor alpha (TNF-α). EPO-dependent proliferation was reduced by the addition of neutralizing antibodies to TNF-α, and exogenously added TNF-α induced proliferation of HCD57 cells. EPO also could induce TNF-α expression in BAF3 and DA3 myeloid cells ectopically expressing EPOR. Addition of TNF-α activated JNK in HCD57 cells, and the activity of JNK was partially inhibited by addition of a TNF-α neutralizing antibody. Primary human and murine erythroid progenitors expressed TNF-α in either an EPO-dependent or constitutive manner. However, TNF-α had an inhibitory effect on both immature primary human and murine cells, suggestive that the proliferative effects of TNF-α may be limited to erythroleukemic cells. This study suggests a novel role for autocrine TNF-α expression in the proliferation of erythroleukemia cells that is distinct from the effect of TNF-α in normal erythropoiesis.
Introduction
Erythropoietin (EPO) is the glycoprotein hormone necessary for the production of mature erythroid cells. Erythroid cells at the colony-forming units–erythroid (CFU-E) to proerythroblast stage of differentiation respond to EPO with proliferation, survival, and terminal differentiation. EPO affects these diverse cellular events via association with its receptor (EPOR) and subsequent activation of numerous signal transduction pathways, which then direct the appropriate cellular response. These pathways include Janus kinase/signal transducers and activators of transcription (JAK2/STAT5),1-4 PI3-kinase,5,6 the adaptor protein SHC,7-9 Src homology 2 domain–containing inositol 5-phosphatase (SHIP),10,11 and the MAP kinase pathways Jun N-terminal kinase (JNK)12,13 extracellular signal related-kinase (ERK)14,15 and p38.16,17 The ERK pathway is primarily associated with the proliferation of erythroid cell lines.12,18,19 The kinetics of the activation of these signals upon activation of the EPO receptor differ, however. In the EPO-dependent murine erythroleukemia cell line HCD57, the JAK2/STAT5, ERK, and PI3-kinase pathways are activated maximally within 5 minutes of EPO binding to its receptor, followed by a decrease in the signal to a lower basal level that is maintained as long as EPO is present. JNK and p38, by contrast, are activated 1 to 4 hours after EPO addition and reach maximum activation 24 hours after EPO addition.12 Whereas much is known about the early signals generated from EPO binding to its receptor, these long-lasting signals that result from EPO treatment are not well understood. One possible mechanism may be that the initial hormone triggers the autocrine or paracrine release of a new factor that maintains the cells.
Tumor necrosis factor alpha (TNF-α) is a cytokine produced by a variety of cell types, including macrophages, monocytes, lymphoid cells, and fibroblasts, usually in response to inflammation or infection.20 The TNF-α signal is mediated via 2 distinct receptors, TNF-α receptor-1 (TNFR1 p55) and TNF-α receptor 2 (TNFR2 p75).21 The extracellular domains of these receptors are closely related to those of CD30, CD40, CD27, and Fas, which are all members of the TNF-α superfamily. Although TNF-α is usually considered an inflammatory cytokine, inducing fever, shock, and apoptosis, TNF-α also has been shown to promote proliferation of human leukemia cells22 and differentiation of macrophages23 in vitro. TNF-α has been shown to stimulate proliferation of both lymphoid22 and nonlymphoid24 cells, as well as some cancers, including chronic lymphoid leukemia25,26 and ovarian cancer.27 This stimulation may be direct activation of the TNF-α receptor or secondary effects resulting from TNF-α–dependent secretion of an intermediate factor such as granulocyte-macrophage colony-stimulating factor.28 TNF-α has been shown to inhibit erythropoiesis,29-31 although it has been demonstrated that the inhibitory effects of TNF-α were likely mediated by β-interferon produced by macrophages in response to TNF-α and not due to direct actions of TNF-α.32,33TNF-α also has been reported to promote proliferation of CD34+ human hematopoietic cells.34 The direct action of TNF-α on erythroid cells therefore remains unclear.
Our laboratory uses HCD57 cells as a model cell system for the study of erythroid proliferation, survival, and apoptosis. These cells depend on EPO for survival and proliferation but do not differentiate in the presence of EPO. In an effort to elucidate the long-term EPO-induced signals of these erythroleukemia cells, we searched for potential autocrine factors that might promote proliferation of these cells. In this report, we will show that whereas TNF-α appears to inhibit both human and murine primary cell erythropoiesis, EPO can induce the production and secretion of TNF-α to promote proliferation in HCD57 cells, and TNF-α may mediate this proliferative signal by activation of JNK.
Experimental procedures
Reagents
Murine tumor necrosis factor-α (TNF-α), MTT reagent (3-(4,5-dimethyl-2-thiozol)-2,5-diphenyl-2H-tetrazolium bromide), and inhibitors PD98059, SB203850, U0126, and LY294002 were purchased from Calbiochem (La Jolla, CA). Recombinant stem cell factor was purchased from Intergen (Purchase, NY). Phosphospecific antibodies against JNKs (Thr183/Tyr185), ERKs (Thr/Tyr204), protein kinase B/AKT (Ser473), c-jun (Ser 63/Ser73), and p38 (Thr 180/Tyr182) were obtained from Cell Signaling Technologies (Beverly, MA). The goat polyclonal antibody recognizing both phosphorylated and nonphosphorylated forms of JNK1 (C-17) was obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). The neutralizing goat anti–mouse TNF-α antibody and isotype control were obtained from R&D Systems (Minneapolis, MN).
Cell culture
Murine HCD57 cells were cultured in Iscove modified Dulbecco medium (IMDM) (Invitrogen, Carlsbad, CA), 25% fetal calf serum (FCS) (Hyclone, Logan, UT), and 10 μg/mL gentamicin (Invitrogen) at 37°C in a 5% CO2 environment and maintained in 1 U/mL EPO media (EPOGEN, Amgen, Thousand Oaks, CA). Murine DA3-EPOR and BAF3-EPOR cells were cultured in RPMI media, 10% FCS, 10 μg/mL gentamicin, and 100 μg/mL geneticin (Invitrogen). Normal human colony-forming cells were purified in the laboratory of Dr Amittha Wickrema at the University of Illinois at Chicago. The human erythroid progenitors highly enriched for CFU-E were purified by a previously published method.35 Human CD34+cells were isolated as previously published,36 and 6.7 × 104 CD34+ cells were cultured in triplicate in IMDM, 30% FCS, 2 U/mL EPO in the presence or absence of 0.2, 2.0, or 20 μg neutralizing rabbit anti–human TNF-α antibody (Calbiochem) per milliliter or 2 μg/mL rabbit IgG negative control for 7 days at 37°C in a moist CO2 environment. For isolation of murine bone marrow cells, TNF-α homozygous (−/−) and control (wild type) mice (C57BL/6 × 129 genetic background) (Jackson Laboratories, Bar Harbor, ME) were humanely killed at 6 to 8 weeks of age by CO2 asphyxiation, and femurs were removed. Bone marrow was extracted in 5 mL of IMDM, 10% FCS medium, using a 23-gauge needle. Cells were enumerated with trypan blue and plated at the desired density.
RNAse protection analysis
For RNAse protection analysis of HCD57, DA3-EPOR, and BAF3-EPOR cells, 5 × 106 cells per time point were deprived of EPO for 18 hours in the following manner: cells were washed 3 times in 10 mL media per 5 × 106 cells in IMDM media with no serum or growth factors and incubated in complete media without EPO for 18 hours prior to stimulation with either 1 U/mL EPO, 10 ng/mL stem cell factor (SCF), or 10 ng/mL TNF-α for the times indicated in the figure legends. For the inhibitor studies, the cells were pretreated with the indicated concentrations of inhibitor for 2 hours or 0.1% dimethyl sulfoxide (DMSO) vehicle control for 2 hours prior to addition of EPO for 4 hours. For the human cells, following isolation of purified CFU-E as previously described,352 × 107 cells were washed 3 times to eliminate EPO. The cells were cultured in the same serum-free media without EPO, and 1 × 107 cells were collected at 1 and 6 hours after EPO withdrawal. Cells were harvested, and total RNA was isolated using the RNeasy RNA isolation kit (Qiagen, Valencia, CA). The radioactive RNA probe was transcribed from the mCK-3 (Figure 1A-C), mCK-3b (Figure 1D), or hCK-3 (for the human CFU-E) template sets (BD Pharmingen, San Diego, CA) using 32P-UTP and an in vitro transcription kit (BD Pharmingen) RNAse protection was carried out using the Riboquant RNase Protection kit (BD Pharmingen) using 10 micrograms (μg) of total RNA for the mouse RNA and 5 μg for the human CFU-E RNA, and 5.9 × 105 cpm of probe per sample. Protected fragments were resolved on a 6% polyacrylamide, 7M urea gel and visualized by autoradiography for 24 hours at −80°C with an intensifying screen.
EPO induces the expression of TNF-α in hematopoietic cell lines.
All panels represent RNAse protection analysis of total RNA isolated from the cell lines indicated. Arrows on left indicate the presence of protected fragments for lymphotoxin β (LTβ), TNF-α, interleukin-6 (IL-6), TGF-β2 (TGF-β), interferon gamma (IFN-γ), interferon-β (IFN-β), macrophage inhibitory factor-1 (MIF-1), and housekeeping genes L32 and glyceraldehyde phosphate dehydrogenase (GADPH). P indicates undigested probe. (A) HCD57 cells were deprived of EPO for 18 hours and then stimulated with nothing (lane 1) or EPO (lanes 2-7) for the times indicated. Mouse control RNA (lane 8) and yeast RNA (lane 9) were used as positive and negative controls for the RNAse protection, respectively. Lines on right of panel indicate location of undigested probe; arrows on left indicate protected fragments indicative of expression of factors and housekeeping genes. (B) HCD57 cells were deprived of EPO overnight and then stimulated with nothing (lane 1), EPO (lanes 2-4), SCF (lanes 5, 6), or TNF-α (lanes 7, 8) for the times indicated. C indicates positive control RNA; Y, yeast RNA. (C) HCD57 (lanes 1, 2), DA3-EPOR (lanes 3-6), or BAF3-EPOR (lanes 7-10) cells were cultured either continuously in EPO (c, lanes 3, 7) or in 10 ng/mL IL-3 overnight (lanes 4, 8), or deprived of EPO overnight and then stimulated with nothing (lanes 1, 5, 9) or EPO (lanes 2, 6, 10) for 4 hours. Y indicates yeast RNA (lane 11). (D) HCD57 cells were deprived of EPO for 18 hours and then pretreated with DMSO vehicle (lane 2), 5 and 50 μM PD98059 (lanes 4, 5), 1 or 10 μM U0126 (lanes 6, 7), 5 or 50 μM LY294002 (lanes 8, 9), or 2 and 20 μM SB203580 (lanes 10, 11) for 2 hours prior to addition of EPO for 4 hours (lanes 2-11). C indicates positive control RNA; Y, yeast RNA; P, undigested probe.
EPO induces the expression of TNF-α in hematopoietic cell lines.
All panels represent RNAse protection analysis of total RNA isolated from the cell lines indicated. Arrows on left indicate the presence of protected fragments for lymphotoxin β (LTβ), TNF-α, interleukin-6 (IL-6), TGF-β2 (TGF-β), interferon gamma (IFN-γ), interferon-β (IFN-β), macrophage inhibitory factor-1 (MIF-1), and housekeeping genes L32 and glyceraldehyde phosphate dehydrogenase (GADPH). P indicates undigested probe. (A) HCD57 cells were deprived of EPO for 18 hours and then stimulated with nothing (lane 1) or EPO (lanes 2-7) for the times indicated. Mouse control RNA (lane 8) and yeast RNA (lane 9) were used as positive and negative controls for the RNAse protection, respectively. Lines on right of panel indicate location of undigested probe; arrows on left indicate protected fragments indicative of expression of factors and housekeeping genes. (B) HCD57 cells were deprived of EPO overnight and then stimulated with nothing (lane 1), EPO (lanes 2-4), SCF (lanes 5, 6), or TNF-α (lanes 7, 8) for the times indicated. C indicates positive control RNA; Y, yeast RNA. (C) HCD57 (lanes 1, 2), DA3-EPOR (lanes 3-6), or BAF3-EPOR (lanes 7-10) cells were cultured either continuously in EPO (c, lanes 3, 7) or in 10 ng/mL IL-3 overnight (lanes 4, 8), or deprived of EPO overnight and then stimulated with nothing (lanes 1, 5, 9) or EPO (lanes 2, 6, 10) for 4 hours. Y indicates yeast RNA (lane 11). (D) HCD57 cells were deprived of EPO for 18 hours and then pretreated with DMSO vehicle (lane 2), 5 and 50 μM PD98059 (lanes 4, 5), 1 or 10 μM U0126 (lanes 6, 7), 5 or 50 μM LY294002 (lanes 8, 9), or 2 and 20 μM SB203580 (lanes 10, 11) for 2 hours prior to addition of EPO for 4 hours (lanes 2-11). C indicates positive control RNA; Y, yeast RNA; P, undigested probe.
Enzyme immunoassay
Triplicate samples of 1 × 105 HCD57 cells were cultured in 1 U/mL EPO or 10 nanograms (ng) SCF for 24, 48, 72, or 96 hours or with no growth factor for 96 hours as indicated in the figures. Culture media was filtered through a 0.45-mM filter and subjected to an enzyme immunoassay (EIA) using the TNF-α EIA kit from BD Pharmingen. TNF-α was quantitated against a standard curve of known concentrations of TNF-α.
MTT assay
HCD57 cells (1 × 105 in triplicate) were deprived of EPO as described above for 18 hours and then incubated with no additional growth factor, 1 U/mL EPO, 1 U/mL EPO with 0.01, 0.1, or 1.0 μg/mL anti–TNF-α neutralizing antibody, or 1, 10, 100, and 1000 ng/mL TNF-α alone for 48 hours. MTT in 1 × phosphate-buffered saline (PBS) was added to a final concentration of 5-μg/mL, and the cells were incubated for 4 hours at 37°C. The cells were then lysed with an equal volume of 0.2 N HCl in isopropanol, and the absorbance was read at 540 nM with a 630-nM reference filter.
Western blot analysis and in vitro kinase assay
For each time point, 5 × 106 cells were used. For Western blot analysis of JNK phosphorylation, the cells were washed to deprive them of EPO for 18 hours as indicated above. The cells were then stimulated with 1 U/mL EPO or 100, 10, or 1 ng/mL TNF-α for 2 hours at 37°C. The cells were lysed in 1 × sample buffer (0.05 M Tris [tris(hydroxymethyl)aminomethane], pH = 8, 2% sodium dodecyl sulfate, 0.1% bromophenol blue, 10% glycerol, 10% β-mercaptoethanol) and sonicated for 10 seconds each to shear the genomic DNA. Equal volumes (40 microliters [μL]) of sample were electrophoresed on an 8.5% sodium dodecyl sulfate–polyacrylamide gel (SDS-PAGE) and subjected to Western blot analysis with the phospho-specific antibodies JNK, ERK, and AKT, as previously described.12 Specific reactive proteins were detected using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ). The blot was stripped as previously described37 and reprobed with an antibody to JNK-1 to ensure equal loading of proteins. For the in vitro kinase assays, 5 × 106 cells per sample were incubated in EPO in the absence or presence of anti–TNF-α antibody or IgG control antibody for 18 hours at 37°C. TNF-α (100 ng/mL) was added to one sample containing anti–TNF-α (Figure 4B, lane 5) 2 hours prior to cell harvesting. Total cell extracts were immunoprecipitated as previously described with anti–JNK-135 and subjected to an in vitro kinase assay according to Cell Signaling Technologies' protocol for the SAPK/JNK in vitro kinase assay. Then 20 μL of the assay was electrophoresed on a 10% acrylamide SDS-PAGE gel and subjected to Western blot analysis using a phospho-cJun–specific antibody (1:1000 dilution) overnight at 4°C. Following exposure of the phospho-cJun, the blot was stripped and then probed with the anti-JNK1 antibody to ensure equal loading of proteins.
Flow cytometry analysis of CD34+ cells
Following incubation of the human CD34+ cells in the presence or absence of neutralizing TNF-α antibodies, the cells were collected, washed once in 1 × fluorescence-activated cell-sorter scanner (FACS) buffer (1 × PBS/5% FCS/0.1% sodium azide), resuspended in 10 μg/mL AB24G2 antibody (BD Pharmingen) to block FCγRII receptors, and incubated for 10 minutes at 4°C. Phycoerythrin (PE)–labeled anti–glycophorin A monoclonal antibody (clone GA-R2, BD Pharmingen) or PE-labeled mouse IgG isotype control (clone 27-35, BD Pharmingen) was then added to a final concentration of 10 μg/mL and incubated for 30 minutes at 4°C. The cells were washed twice with FACS buffer and resuspended in FACS buffer, and gycophorin-A–positive cells were detected using a FACSscan flow cytometer (Becton Dickinson, Franklin Lakes, NJ) gated on an FL-2 channel.
Colony-forming cell assays
To assess murine CFU-E and burst-forming unit–erythroid (BFU-E) colony formation, 6 × 105 total murine bone marrow cells were added to 3 mL methylcellulose media (Methocult M3334, Stem Cell Technologies, Vancouver, BC, Canada) containing 3 units EPO/mL but no other cytokines in the presence or absence of 1 or 10 ng/mL TNF-α. Of these cells, 1.1 mL were plated in duplicate onto 30-mM plates and cultured at 37°C in a moist CO2 environment. CFU-Es were counted 2 days after the start of the experiment, and BFU-Es were counted 8 days after the start of the experiment.
Results
As an initial screen to detect possible autocrine secretion of growth factors in HCD57 cells, we tested for the presence of likely candidate cytokines using RNAse protection analysis (RPA) templates that detect the mRNA expression of numerous cytokines. RPA of total RNA isolated from HCD57 cells cultured in the absence or presence of EPO revealed that TNF-α mRNA was expressed in the presence of EPO but expression was greatly reduced when EPO was removed (Figure1A). EPO induced the expression of TNF-α within 15 minutes of EPO addition (Figure 1B, lane 2). TNF-α expression reached a maximum 4 hours after EPO addition and was maintained over further incubation for 24 or 48 hours (Figure 1A, lanes 2-4). Other growth factors also were expressed (lymphotoxin B, interferon-γ, and TGF-β), but their expression was EPO independent. SCF also induced the expression of TNF-α, but this induction was weaker than the EPO-induced TNF-α expression. TNF-α did not affect its own expression (Figure 1B, lanes 7 and 8). To determine if the ability of EPO to activate TNF-α expression was unique to HCD57 cells, TNF-α expression was next tested in 2 cell lines ectopically expressing EPOR: DA3-EPOR and BAF3-EPOR cells. These cell lines are normally dependent on interleukin-3 (IL-3) for proliferation and survival, and EPOR can replace the IL-3 receptor in their proliferative and antiapoptotic properties.38 39 Both DA3-EPOR and BAF3-EPOR cells expressed TNF-α mRNA in response to EPO treatment (Figure 1C, lanes 6 and 10), indicating that EPOR has the capacity to signal TNF-α mRNA expression in other cells. IL-3 induced expression of TNF-α in DA3-EPOR cells (Figure 1C, lane 4) but not in BAF3-EPOR cells (Figure 1C, lane 8). DA3-EPOR and BAF3-EPOR cells also expressed interferon-β and IL-6, in addition to TGF-β and IFN-γ (Figure1C). Other human EPO-dependent cell lines tested (UT-7-EPO and TF-1) expressed TNF-α but did not express it in an EPO-dependent manner (data not shown).
The pathways upstream of EPO-induced TNF-α expression were next explored by treatment of HCD57 with inhibitors of known signal transduction pathways activated by EPO. Treatment with the PI3-kinase family inhibitor LY294002 inhibited EPO-induced TNF-α expression in HCD57 cells (Figure 1D, lanes 8 and 9); the map kinase kinase (MEK) inhibitors PD98059 and U0126 partially inhibited TNF-α expression in HCD57 cells (Figure 1D, lanes 4-7). The p38 inhibitor SB203580 had no significant effect on EPO-induced TNF-α activity at any concentration tested (Figure 1D, lanes 10 and 11). The inhibitors had no significant effects on the expression of other cytokines expressed, such as interferon-γ Therefore, EPO-induced expression of TNF-α is mediated in part by the activation of a PI3-kinase–related pathway or a non–PI3-kinase pathway inhibited by LY294002.
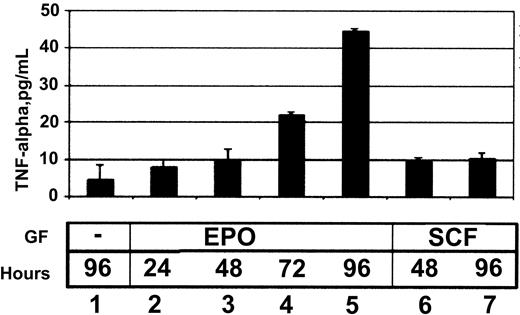
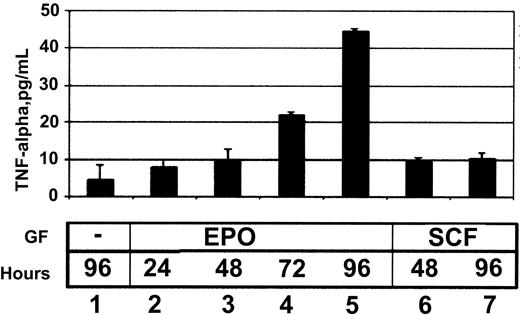
The ability of EPO to induce TNF-α protein secretion was then tested using an enzyme immunoassay. HCD57 cells secreted TNF-α into the media in response to EPO (Figure 2). SCF, which can promote proliferation but cannot promote survival of HCD57 cells, induced secretion of TNF-α within 48 hours of SCF addition (Figure 2, lane 8), but this amount did not increase further (Figure 2, lane 9).
EPO induces the secretion of TNF-α by HCD57 cells.
TNF-α enzyme immunoassay (EIA) of media harvested from HCD57 cells treated with no growth factors (lane 1), 1 U/mL EPO (lanes 2-5), or 10 ng/mL SCF (lanes 6, 7) for the number of hours indicated. TNF-α levels are measured in pg/mL compared with a standard curve using known quantities of TNF-α.
EPO induces the secretion of TNF-α by HCD57 cells.
TNF-α enzyme immunoassay (EIA) of media harvested from HCD57 cells treated with no growth factors (lane 1), 1 U/mL EPO (lanes 2-5), or 10 ng/mL SCF (lanes 6, 7) for the number of hours indicated. TNF-α levels are measured in pg/mL compared with a standard curve using known quantities of TNF-α.
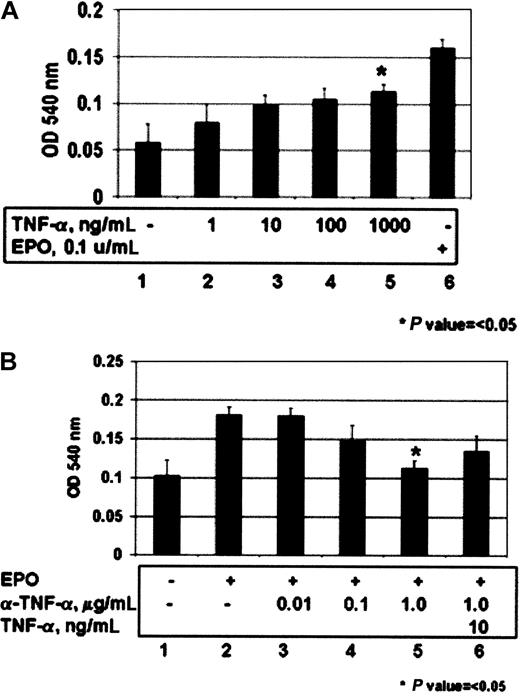
The role of TNF-α as an autocrine factor for proliferation in HCD57 cells was tested by assessing the ability of TNF-α to stimulate proliferation of these cells using a standard MTT dye reduction assay. Addition of exogenous TNF-α was able to induce proliferation in HCD57 cells in a dose-dependent manner. Likewise, treatment of HCD57 with neutralizing antibodies to TNF-α inhibited EPO-induced proliferation in a dose-dependent manner, and the inhibition could be partially reversed by the addition of excess TNF-α to the media (Figure 3B, lane 6).
TNF-α induces proliferation of HCD57 cells, and a neutralizing antibody to TNF-α inhibits EPO-induced proliferation of these cells.
Proliferation was measured using the MTT dye reduction assay as indicated in “Materials and methods.” (A) HCD57 (lanes 1-5) cells were deprived of EPO for 18 hours prior to addition of 1 (lane 2), 10 (lane 3), 100 (lane 4), or 1000 (lane 5) ng/mL TNF-α for 48 hours. (B) HCD57 cells were deprived of EPO overnight and then treated with EPO in the presence (lanes 3-6) or absence (lane 2) of neutralizing anti–TNF-α antibody for 48 hours. Indicated is μg/mL neutralizing antibody added. Excess TNF-α (10 ng/mL) was added to counteract the effect of the neutralizing antibody (lane 6).
TNF-α induces proliferation of HCD57 cells, and a neutralizing antibody to TNF-α inhibits EPO-induced proliferation of these cells.
Proliferation was measured using the MTT dye reduction assay as indicated in “Materials and methods.” (A) HCD57 (lanes 1-5) cells were deprived of EPO for 18 hours prior to addition of 1 (lane 2), 10 (lane 3), 100 (lane 4), or 1000 (lane 5) ng/mL TNF-α for 48 hours. (B) HCD57 cells were deprived of EPO overnight and then treated with EPO in the presence (lanes 3-6) or absence (lane 2) of neutralizing anti–TNF-α antibody for 48 hours. Indicated is μg/mL neutralizing antibody added. Excess TNF-α (10 ng/mL) was added to counteract the effect of the neutralizing antibody (lane 6).
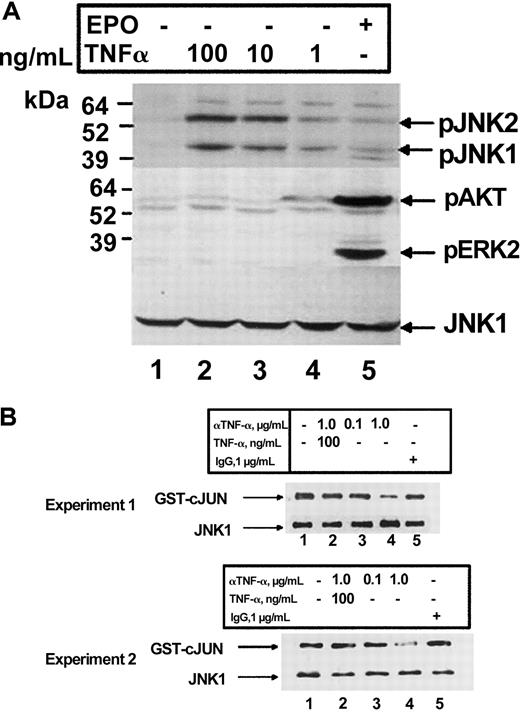
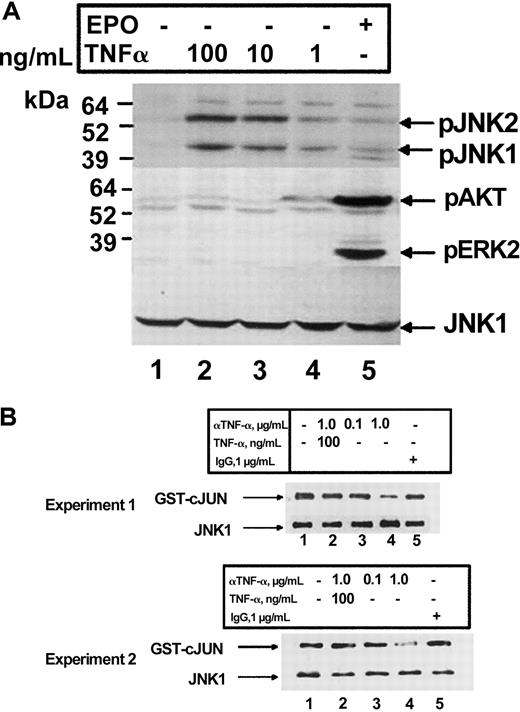
TNF-α has been shown previously to activate JNK and ERK, 2 kinases which have been shown to be important in the proliferation of erythroid cells.12 We therefore explored the ability of these kinases to be activated by TNF-α in HCD57 cells. We previously have reported that TNF-α induced the activation of JNK within 2 hours of cytokine addition in HCD57 cells.12 Treatment of HCD57 cells for 2 hours with increasing amounts of TNF-α resulted in a dose-dependent increase in phosphorylation of JNK, thus confirming and extending our previously published results (Figure4A). No phosphorylation of ERK or AKT in response to TNF-α treatment was detected. Furthermore, treatment of HCD57 cells with the TNF-α neutralizing antibody inhibited JNK activity (Figure 4B, lanes 3 and 4). This effect could be reversed by the addition of exogenous TNF-α (Figure 4B, lane 2) and was not seen with a rat IgG control antibody (Figure 4B, lane 5). Therefore, TNF-α may transduce its signal in erythroid cells via activation and activity of JNK.
TNF-α activates JNK in HCD57 cells.
(A) Western blot analysis of HCD57 cells deprived of EPO for 18 hours prior to treatment. Cells were treated with nothing (lane 1), 100, 10, or 1 ng/mL TNF-α (lanes 2-4), or EPO (1 U/mL) (lane 5), and whole cell lysates were probed for anti–phospho-JNK1/2 (top panel), anti–phospho AKT and anti–phospho ERK1/2 (middle panel), and anti-JNK1 (bottom panel). (B) In vitro kinase assay of JNK1 immunoprecipitates using glutathione-S–transferase (GST)–cJun as a substrate from HCD57 cells treated with EPO in either the absence (lane 1) or presence of 0.1 or 1.0 μg/mL anti–TNF-α antibody (lanes 3, 4), 1.0 μg/mL anti–TNF-α antibody plus 100 ng TNF-α (lane 2), or goat IgG control (lane 5). Shown are 2 separate experiments to indicate reproducibility of the result. Phosphorylated GST-cJun (top panel) and total JNK1 (bottom panel) are indicated.
TNF-α activates JNK in HCD57 cells.
(A) Western blot analysis of HCD57 cells deprived of EPO for 18 hours prior to treatment. Cells were treated with nothing (lane 1), 100, 10, or 1 ng/mL TNF-α (lanes 2-4), or EPO (1 U/mL) (lane 5), and whole cell lysates were probed for anti–phospho-JNK1/2 (top panel), anti–phospho AKT and anti–phospho ERK1/2 (middle panel), and anti-JNK1 (bottom panel). (B) In vitro kinase assay of JNK1 immunoprecipitates using glutathione-S–transferase (GST)–cJun as a substrate from HCD57 cells treated with EPO in either the absence (lane 1) or presence of 0.1 or 1.0 μg/mL anti–TNF-α antibody (lanes 3, 4), 1.0 μg/mL anti–TNF-α antibody plus 100 ng TNF-α (lane 2), or goat IgG control (lane 5). Shown are 2 separate experiments to indicate reproducibility of the result. Phosphorylated GST-cJun (top panel) and total JNK1 (bottom panel) are indicated.
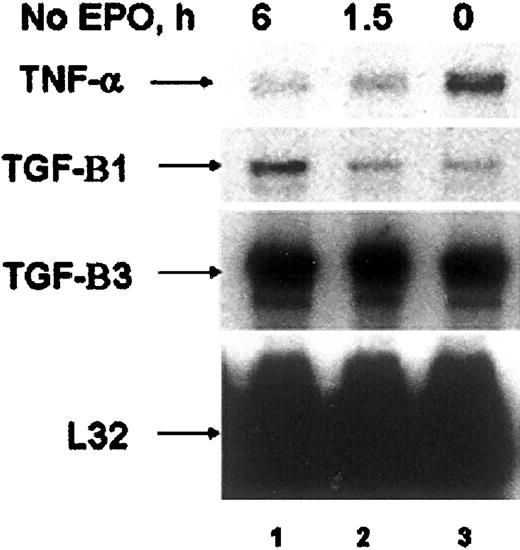
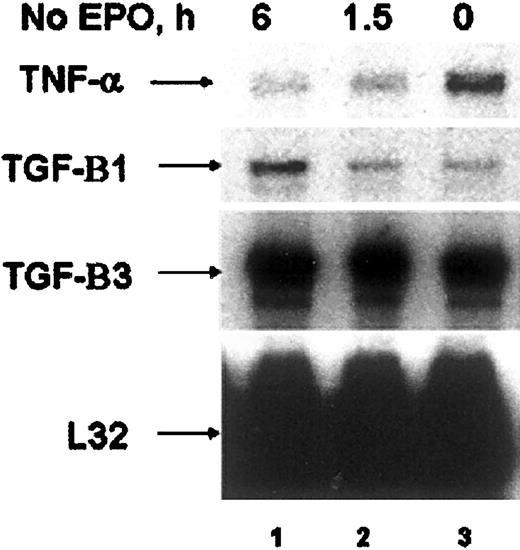
The EPO-induced expression of TNF-α in these cell lines led us to investigate whether primary erythroid cells might express TNF-α. We therefore investigated the ability of EPO to induce TNF-α in both human colony-forming cells (CFU-E)35 and CD34+human erythroid progenitors,36 and in primary murine erythroid progenitors purified from the spleens of mice infected with the anemia strain of the Friend spleen focus-forming virus (FVA cells).40 RNAse protection analysis of CFU-Es cultured in the presence of EPO revealed expression of TNF-α (Figure 5, lane 3). When EPO was removed, TNF-α expression decreased, whereas the expression of TGF-β increased (Figure 5, lane 1). CD34+ human cells and FVA cells also expressed TNF-α in the absence of EPO (data not shown). Addition of EPO to either the CD34+ or the FVA cells, however, failed to further induce TNF-α expression (data not shown).
TNF-α is expressed in primary human colony-forming cells.
RNAse protection analysis of human CFU-E cultured in the presence of EPO (lane 3) or deprived of EPO for 1.5 and 6 hours (lanes 1, 2). Cytokines expressed are indicated by arrows.
TNF-α is expressed in primary human colony-forming cells.
RNAse protection analysis of human CFU-E cultured in the presence of EPO (lane 3) or deprived of EPO for 1.5 and 6 hours (lanes 1, 2). Cytokines expressed are indicated by arrows.
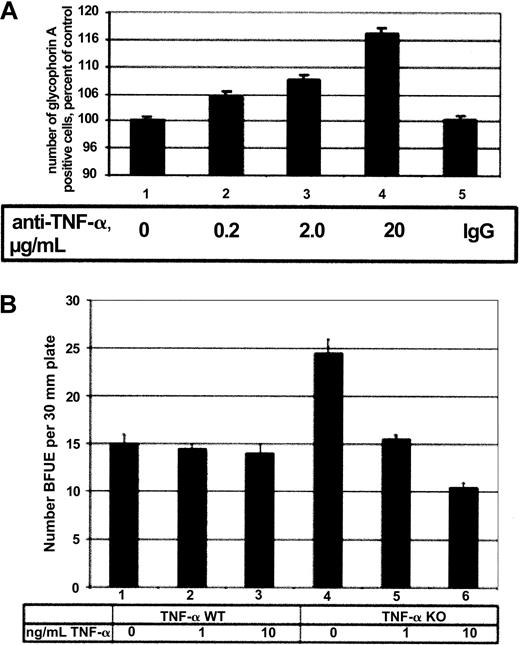
Because TNF-α traditionally has been considered an inhibitor of erythropoiesis,41 we wished to investigate the effect of TNF-α on primary cell erythropoiesis in both human and murine erythroid cells. First, human CD34+ cells were cultured in EPO for 7 days with neutralizing antibodies to TNF-α, and the number of mature erythroid cells was determined by immunostaining against the erythroid-specific protein glycophorin A (Figure6A). During the experiment, approximately 10% of total cells were found to express glycophorin A after culture (data not shown). The presence of neutralizing TNF-α antibody resulted in a dose-dependent increase in the number of glycophorin A–positive cells, indicating that TNF-α was inhibiting erythroid cell proliferation in this system. To investigate the effect of TNF-α on murine erythropoiesis, bone marrow cells were isolated from wild-type and TNF-α−/− mice, and these cells were assayed for CFU-Es and BFU-Es in either the absence or presence of TNF-α in semisolid media. TNF-α had no significant effect on the number of CFU-Es in these experiments (data not shown). Furthermore, the addition of TNF-α had no significant effect on the number of BFU-Es in TNF-α wild-type mice (Figure 6B, lanes 2 and 3). However, the addition of TNF-α to TNF-α–deficient bone marrow cells inhibited the formation of BFU-Es in a dose-dependent manner (Figure 6B, lanes 5 and 6). Taken together, these results suggest that TNF-α has an inhibitory effect on both human and murine normal erythropoiesis in vitro.
Effects of TNF-α on human and murine primary erythroid cells.
(A) Human CD34+ cells were cultured in EPO or in EPO with 0.2, 2.0, or 20.0 μg/mL neutralizing TNF-α antibody for 7 days and assessed for glycophorin A expression by flow cytometry. Increasing amounts of antibody resulted in an increase in the number of glycophorin A–positive cells (lanes 2-4), whereas the addition of control IgG had no effect (lane 5). (B) Total bone marrow isolated from TNF-α wild-type (WT) (lanes 1-3) and TNF-α−/− (lanes 4-6) mice was incubated in EPO alone (lanes 1, 4) or EPO with 1 or 10 ng/mL TNF-α (lanes 2, 3, 5, 6) in semisolid media. The number of BFU-Es was counted 8 days after the start of the experiment and is expressed as number of BFU-Es detected per 30-mM plate.
Effects of TNF-α on human and murine primary erythroid cells.
(A) Human CD34+ cells were cultured in EPO or in EPO with 0.2, 2.0, or 20.0 μg/mL neutralizing TNF-α antibody for 7 days and assessed for glycophorin A expression by flow cytometry. Increasing amounts of antibody resulted in an increase in the number of glycophorin A–positive cells (lanes 2-4), whereas the addition of control IgG had no effect (lane 5). (B) Total bone marrow isolated from TNF-α wild-type (WT) (lanes 1-3) and TNF-α−/− (lanes 4-6) mice was incubated in EPO alone (lanes 1, 4) or EPO with 1 or 10 ng/mL TNF-α (lanes 2, 3, 5, 6) in semisolid media. The number of BFU-Es was counted 8 days after the start of the experiment and is expressed as number of BFU-Es detected per 30-mM plate.
Discussion
TNF-α has been shown to be a potent inhibitor of hematopoiesis.30,41,42 TNF-α inhibition of erythropoiesis has been demonstrated in normal hematopoietic progenitors,43,44 and TNF-α expression and suppression of erythropoiesis has been associated with a number of hematopoietic disorders such as Fanconi anemia,45 myelodysplastic disease,46 aplastic anemia,47 and anemia due to chronic disease.32,48 49 The finding that HCD57 cells could not only express and secrete TNF-α in response to EPO, but also respond to TNF-α with enhanced proliferation is therefore intriguing. We also detected EPO-inducible TNF-α expression in DA3-EPOR and BAF3-EPOR cells, indicating that the ability of EPOR to transduce a TNF-α–inducing signal is not a unique property of HCD57 cells. The EPO-dependent expression of TNF-α may be mediated by PI3-kinase activation, since treatment with the PI3-kinase inhibitor LY294002 greatly inhibited TNF-α expression in both HCD57 cells. Treatment with the MEK inhibitors PD90859 and U0126 also partially inhibited TNF-α expression in HCD57 cells, suggesting a partial contribution of the ERK/MAP kinase pathway to TNF-α expression as well.
TNF-α activated the JNK pathway in a dose-dependent manner in HCD57 cells (Figure 4). The fact that proliferation, JNK activation, and JNK activity were only partially inhibited by the neutralizing antibody to TNF-α suggests that additional EPO-dependent pathways also contribute to these processes. Alternatively, there may be internal TNF-α that is not secreted, so that the neutralizing antibody would have no effects on these internal TNF-α–activated events.
Our finding that TNF-α had the capacity to induce proliferation of erythroleukemia cells led us to investigate the expression of TNF-α in primary erythroid cells and the effect of TNF-α on erythropoiesis. We detected TNF-α expression in both human and murine primary erythroid cells. This expression, however, was not reliably shown to be EPO dependent. The expression of TNF-α in FVA primary proerythroblasts may be constitutive due to activation of signaling pathways by Friend virus infection, because the virus activates the MAP kinase pathway but not other EPO-dependent signals.50TNF-α expression also has recently been reported in CD34+hematopoietic cells and BFU-E cells that we now confirm in CD34+ human cells.51 The decrease in TNF-α expression upon EPO withdrawal from the human CFU-E for 6 hours suggests that EPO-dependent TNF-α expression may occur in these cells. We cannot rule out that the decrease in TNF-α expression was due to a general down-regulation of transcription that occurs when the cells undergo apoptosis due to EPO withdrawal; however, the increase in TGF-β expression upon EPO withdrawal (Figure 5, lane 1) suggests that not all messages are down-regulated upon EPO withdrawal. This result strongly suggests that the TNF-α in the purified primary cells does not arise from contaminating nonerythroid cells but from the erythroid cells themselves.
Our results in both human CD34+ cells treated with neutralizing antibodies to TNF-α and in murine bone marrow cells isolated from TNF-α–deficient mice treated with TNF-α indicate that in these systems, TNF-α inhibits either the proliferation or differentiation and/or induces apoptosis of maturing erythroid cells. Therefore, the EPO-dependent TNF-α secretion and proliferation from HCD57 cells may be a result of the transformation of the cells and may not be an inherent property of primary erythroid progenitors. This still does not explain, however, how HCD57 cells can proliferate in response to TNF-α and activate JNK in response to TNF-α, whereas differentiation of erythroid progenitors is inhibited by TNF-α. TNF-α has been reported to induce apoptosis through sustained activation of JNK.52 However, in many systems, TNF-α–induced JNK activation does not induce apoptosis and has even been reported to be cytoprotective.53 It also has been demonstrated that TNF-α can be a synergistic inducer of proliferation in immature CD34+/CD38 cells34 and may induce proliferation of multipotent hematopoietic progenitors while inhibiting the development of committed progenitors.54 HCD57 erythroleukemia cells are arrested at an early stage in erythroid development, as evidenced by the fact that forced expression of the activator protein-1 (AP1) transcription factor JunB induced the expression of some mature erythroid markers such as β-globin and spectrin-α and required at least 48 hours before these markers were seen.55 It is possible, therefore, that committed erythroid cells must reach a certain stage at which they are insensitive to inhibition by TNF-α. Alternatively, there may be a loss of a proapoptotic signal usually stimulated by JNK that is absent in HCD57 cells. There also may be a gain of an antiapoptotic TNF-α–induced signal (such as NF-κB56,57) or an antiapoptotic signal unrelated to TNF-α (such as Bcl-x(L)58) that suppresses TNF-α–induced apoptosis. Therefore, it might be possible to render these cells sensitive to TNF-α–induced killing or reduced proliferation by identifying and suppressing these pathways.
TNF-α also has been shown to induce the proliferation of myeloid leukemia cells lines,22 but this effect has not been demonstrated for an erythroleukemia cell line. It is possible that the HCD57 cell line has acquired, as a part of its leukemic phenotype, the characteristics of other myeloid lineages; however, the expression of mature erythroid proteins by the induction of JunB or hemin indicate that it has retained the characteristics of an immature erythroid cell.55,59 The acquisition of EPO-dependent TNF-α expression may benefit erythroleukemic cells by gaining the ability to induce its own proliferation and/or by killing cells in the bone marrow, spleen, or blood that might compete with the leukemic cells for resources. Inhibition of this TNF-α autocrine loop may therefore provide a means to specifically inhibit the leukemic cell proliferation. It is interesting that TGF-β is very strongly expressed in HCD57, DA3-EPOR, and BAF3-EPOR (Figure 1), in human primary colony-forming cells (Figure 6), and in FVA cells. Recent studies have indicated that TGF-β may drive the differentiation of erythroid progenitors and EPO-dependent erythroid cell lines; autocrine TGF-β may therefore play a role in this process.60 61
In conclusion, this study demonstrates that some erythroid cell lines have the capacity to proliferate in response to TNF-α and that EPO-activated EPOR has the capacity to induce the synthesis and secretion of TNF-α in some cells but not others. TNF-α appears to induce proliferation by modulation of the JNK pathway. Inhibition of this TNF-α–dependent modulation of JNK slowed the proliferation of these leukemic cells. The identification of this autocrine loop suggests the possibility that anti–TNF-α strategies may be useful in inhibiting the proliferation or survival of these leukemias in a clinical setting. The study also suggests the possible existence of a negative feedback of TNF-α expressed by mouse and human CFU-Es or proerythroblasts that act on immature erythroid progenitors to suppress maturation of these cells.
The authors would like to thank Dr Maurice Bondurant for his assistance with RNAse protection analysis of primary murine erythroid progenitors. D.L.B is a research scientist at the National Cancer Institute of Canada.
Prepublished online as Blood First Edition Paper, August 29, 2002; DOI 10.1182/blood-2001-11-0084.
Supported by grants R01DK39781 (S.T.S.), R01HL65906 (S.T.S.), RO1 AI43433 (J.J.R.), RO1CA91839 (J.J.R.), R01CA88906 (P.D.), and R01DK52825 (P.D.) from the National Institutes of Health; grant 9804806U from the American Heart Association (S.M.J.-H.); Intramural Research Grant (IRG)-100036 from the American Cancer Society (S.M.J.-H.); grant 98-0148 (P.D.) from the Department of Defense; the Department of Veterans Affairs (E.N.D.); and Canadian Institute for Health Research (D.L.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen T. Sawyer, Department of Pharmacology/Toxicology, PO Box 980613, Richmond, VA 23298; e-mail:ssawyer@hsc.vcu.edu.