Abstract
The conventional description of platelet interactions with collagen-coated surfaces in vitro, based on serial static measurements, is that platelets first adhere and spread to form a monolayer and then recruit additional layers of platelets. To obtain dynamic information, we studied gravity-driven platelet deposition in vitro on purified type 1 collagen by video phase-contrast microscopy at 22°C. With untreated human and wild-type mouse platelets, soon after the initial adhesion of a small number of “vanguard” platelets, “follower” platelets attached to the spread-out vanguard platelets. Follower platelets then adhered to and spread onto nearby collagen or over the vanguard platelets. Thus, thrombi formed as a concerted process rather than as sequential processes. Treatment of human platelets with monoclonal antibody (mAb) 7E3 (anti–GPIIb/IIIa (αIIbβ3) + αVβ3) or tirofiban (anti–GPIIb/IIIa) did not prevent platelet adhesion but nearly eliminated the deposition of follower platelets onto vanguard platelets and platelet thrombi. Similar results were obtained with Glanzmann thrombasthenia platelets. Wild-type mouse platelets in the presence of mAb 1B5 (anti–GPIIb/IIIa) and platelets from β3-null mice behaved like human platelets in the presence of 7E3 or tirofiban. Deposition patterns of untreated human and wild-type mouse platelets were consistent with random distributions under a Poisson model, but those obtained with 7E3- and tirofiban-treated human platelets, 1B5-treated mouse platelets, or β3-null platelets demonstrated a more uniform deposition than predicted. Thus, in this model system, absence or blockade of GPIIb/IIIa receptors interferes with thrombus formation and alters the pattern of platelet deposition.
Introduction
Platelet adhesion and aggregation play important roles in physiologic and pathologic phenomena. Thus, platelet adhesion and aggregation after trauma to a blood vessel are crucial to stop hemorrhage and restore hemostasis, whereas adhesion and aggregation on a damaged atherosclerotic plaque can result in thrombosis, vaso-occlusion, and distal infarction. Depending on the surface, platelet adhesion is mediated by one or more surface receptors with affinity for one or more tissue components, including collagen, von Willebrand factor, fibrinogen, fibronectin, and perhaps vitronectin, laminin, and thrombospondin.1
Collagen is consistently a highly thrombogenic surface coating in model systems of platelet adhesion when judged by the rate of platelet deposition, the ability to support the formation of multiple layers of platelets, the strength of the adhesion, or the ability to support fibrin formation.2-5 Platelet-collagen interactions are complex, with the presence of multiple platelet receptors for collagen leading to differences in behavior as a function of collagen type; collagen architecture (monomeric or fibrillar); availability of soluble adhesive glycoproteins such as von Willebrand factor, fibronectin, and fibrinogen; the presence of divalent cations and their concentrations; and shear forces.6-22 Nonetheless, the central paradigm is that platelet interactions with collagen occur in a sequential manner in which platelet adhesion, platelet activation and spreading, and platelet-platelet interactions follow one another in series.
To understand better the process of platelet adhesion to type 1 collagen, the factors that contribute to the uniquely high thrombogenicity of collagen, and the role of the platelet glycoprotein (GP) IIb/IIIa (αIIbβ3) receptor in the process, we have studied the deposition of human platelets onto collagen-coated surfaces at unit gravity using time-lapsed videomicroscopy and electron microscopy. The effects of monoclonal antibody 7E3 (anti–GPIIb/IIIa + αVβ3) and the nonpeptide GPIIb/IIIa-specific inhibitor, tirofiban, on human platelet deposition was also assessed. In parallel, we analyzed adhesion to collagen of platelets from wild-type mice, platelets from wild-type mice treated with a hamster antibody to GPIIb/IIIa (1B5) that inhibits ligand binding,23 and platelets from mice deficient in the β3 integrin subunit; the latter lack GPIIb/IIIa and αVβ3.24 We found that platelet-collagen adhesion/thrombus formation was a concerted process rather than strictly sequential processes and that platelets could adhere to collagen after attaching to an already adherent platelet. Moreover, blockade of GPIIb/IIIa receptors not only prevented deposition of additional platelets onto adherent platelets and lateral cohesion between platelets, it altered the pattern of platelet deposition so as to make it deviate from the random distribution predicted by the Poisson distribution. Thus, blockade or absence of platelet GPIIb/IIIa receptors profoundly alters the nature of collagen-mediated adhesion/thrombus formation, despite the ability of such platelets to continue to adhere directly to collagen.
Patients, materials, and methods
Human and mouse GPIIb/IIIa antagonists
Collagen
Type 1 lathyritic skin collagen was purified from Sprague-Dawley rats fed 0.2% β-aminopropionitrile by differential salt precipitation and then lyophilized as previously described.9 It was reconstituted in 0.05% acetic acid (HAc) before it was used to coat the surface. In some experiments, purified type 1 rat tail collagen (Collaborative Biomedical Products, Bedford, MA) in 0.02 N HAc was used in place of the type 1 rat skin collagen. Preliminary experiments demonstrated similar results with both collagen preparations.
Platelet preparation
Approval was obtained from the Institutional Review Board for these studies. Informed consent was provided according to the tenets of the Declaration of Helsinki. Human blood (8.5 mL) was drawn after informed consent into acid-citrate dextrose (ACD-A) (1.5 mL) and was centrifuged for 3.5 minutes at 700g at 22°C to prepare platelet-rich plasma (PRP). The PRP was mixed with 0.1-vol ACD and centrifuged at 1000g for 10 minutes at 22°C; the pellet was then resuspended in 1 mL HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–modified Tyrode buffer (138 mM NaCl, 10 mM HEPES, 12 mM NaHCO3, 2.7 mM KCl, 0.4 mM NaH2PO4, 0.1% glucose, 0.35% bovine serum albumin [BSA; Fraction V; Sigma, St Louis, MO], pH 7.4; HBMT) and was gel-filtered on a column of Sepharose-2B (Pharmacia, Piscataway, NJ) using HBMT for elution. Gel-filtered platelets (GFP) were pretreated with 0.01-vol buffer, antibody 7E3 (10 μg/mL), tirofiban (0.25 μg/mL), or control mouse immunoglobulin G (IgG) (10 μg/mL; Jackson ImmunoResearch Laboratories, West Grove, PA) for 15 minutes at 22°C. MgCl2 was added at 0.01 vol to a final concentration of 2 mM. In one experiment blood was drawn into ACD from a control and from a patient with Glanzmann thrombasthenia whose platelets contained no detectable GPIIb27 but did contain trace amounts of GPIIIa.28 PRP was prepared, washed twice, and resuspended in HBMT; adjustments were then made to 100 000 platelets/μL, MgCl2 (2 mM) was added, and the platelets were allowed to adhere to a collagen-coated microchamber for 1 hour at 22°C.
Alternatively, human blood was drawn into ACD and centrifuged as above. After removing the PRP, the buffy coat was removed and placed in another tube. A volume of buffer equal to the amount of PRP removed was then added to the buffy coat and to the residual red blood cells. Each tube was mixed and recentrifuged as above, and the resultant platelet-rich buffer (PRB) was removed. The PRP and PRB were pooled, and the suspension was incubated with buffer or 7E3; MgCl 2was then added as above.
Platelets from F2 wild-type mice of mixed C57Bl/6 and 129 lineages and β3 integrin-deficient mice of the same mixed lineage24were prepared from blood obtained by puncture of the retrobulbar venous plexus with a glass capillary tube using the PRP/PRB technique described above. Wild-type mouse platelets were also treated with monoclonal antibody (mAb) 1B5 (anti–GPIIb/IIIa) (30 μg/mL) for 15 minutes at 22°C.
Microchamber
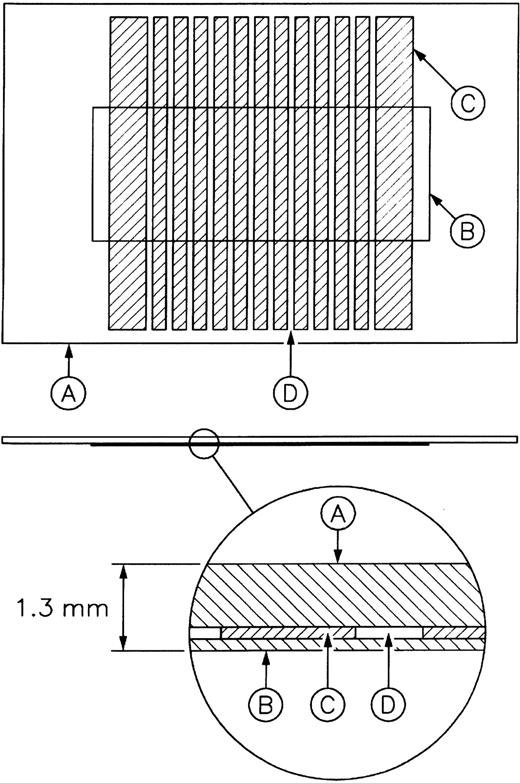
A 1.25-mm–thick microchamber was constructed from a glass slide coated with chlorinated organopolysiloxane (Sigmacote; Sigma), a silicone elastomer gasket (Sylgard 184; Dow Corning, Midland, MI), and a no. 1.5 glass coverslip coated first with Sigmacote and then with 1% polystyrene in chloroform using a spincoater. The gasket was designed to produce 12 separate channels of 3.4 μL (Figure1). The polystyrene-coated coverslip was then coated with collagen by filling the chambers by capillary action with a collagen solution (33 μg/mL in 0.05% HAc) and incubating for 1 hour at 37°C. Any residual uncoated surfaces in the channels were blocked by first rinsing and then incubating the chambers for 1 hour at 22°C in HBMT. Channels were washed with HBMT with or without 2 mM MgCl2. GFP or PRP/PRB (100 000 platelets/μL) were added to the channels by capillary action, and channels were sealed with silicone grease. Interactions between platelets and the collagen-coated surface were monitored at 22°C by microscopy, as detailed below.
Schematic diagram of the microchamber (12 channels).
(A) Glass slide. (B) Glass coverslip. (C) Silicone elastomer gasket. (D) Channel to hold sample.
Schematic diagram of the microchamber (12 channels).
(A) Glass slide. (B) Glass coverslip. (C) Silicone elastomer gasket. (D) Channel to hold sample.
Phase-contrast microscopy and recording
Phase-contrast microscopy was performed with an inverted Zeiss Axiovert microscope (Carl Zeiss, Thornwood, NY) with a computer-controlled mechanical stage (Ludl Electronic Products, Hawthorne, NY) using a × 100, 1.4 NA Zeiss Neofluar objective. Images were obtained automatically and concurrently for 2 hours from each channel at 2.4-minute intervals and were stored on a video disk recorder (TW-3038F; Panasonic, Secaucus, NJ). Each acquired image was an average of 2 video frames from the camera (Hamamatsu Newvicon, Bridgewater, NY).
Quantitative assessment of the pattern of platelet deposition and statistical analysis
An observer masked to the platelet treatment viewed the time-lapse recordings. Using frame-by-frame analysis, the location of each new single platelet that developed stable adhesion (defined as the frame in which Brownian motion ceased and the platelet image was unobstructed) to collagen or to an already adherent platelet was recorded using Adobe Photoshop (Adobe Systems, San Jose, CA) and Equilibrium DeBabelizer Pro (Equilibrium Technologies, San Rafael, CA) software. A circle was drawn around the platelet image, and the coordinates of the center of the circle were used as the platelet position. Each subsequent frame was analyzed to document the position of each additional platelet for the 2-hour duration. After adhesion was completed, the distribution of accumulated positions was compared to that expected from a Poisson distribution. For each surface, minimum and maximum loci on the x-axis and y-axis were defined so as to identify a central area of length 600 pixels on the x-axis and height 450 pixels on the y-axis. Each area was then divided into 300 30 × 30 pixel-square grids, and the number of grids containing 0, 1, 2, or 3 or more platelets was tallied. If the positions of the platelets on the area were random, they would have conformed to the expectations under a Poisson distribution. The parameter of the putative Poisson variable was estimated as Λ = x/300, where x = total number of platelets in the designated area. The expected number of grids containing y platelets (y = 0,1,2…) was obtained from the Poisson distribution as 300(exp−Λ)(Λy/y!). Observed numbers were compared with expected numbers using a χ2goodness-of-fit test. The entire analysis was repeated using 1200 15 × 15 pixel-square grids. These calculations were implemented using SAS software for the PC and by hand calculations for the goodness-of-fit tests.
Scanning electron microscopy
Platelet adhesion to collagen-coated polystyrene microtiter wells (CoStar, Cambridge, MA) was conducted essentially as described. After platelets were allowed to adhere for 1 hour at 22°C, the wells were washed twice with 100 μL HBMT/MgCl2 and then with phosphate-buffered saline (PBS; 137 mM NaCl, 1.5 mM KH2PO4, 8 mM Na2HPO4, 2.7 mM KCl, pH 7.4). Platelets were fixed by adding 100 μL 1.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.4, and incubating the mixture overnight at 4°C. Wells were then washed with cacodylate and postfixed with 1% osmium tetroxide in cacodylate for 30 minutes. Wells were dehydrated in graded steps of ethanol and then critical point dried with liquid CO2. Platelets were sputter coated with gold-palladium to 10 nm. Samples were examined in a Hitachi S530 scanning electron microscope (Tokyo, Japan).
Differential interference microscopy and immunofluorescence
In some experiments, the coverslip containing adherent platelets was incubated at 22°C for 30 minutes with 1 μg/mL biotinylated murine antibody to P-selectin (G1/G1-4; Ancell, Bayport, MN) and then, after rinsing, for 30 minutes with 10 μg/mL fluorescein isothiocyanate–labeled avidin (UltraAvidin Leinco Technologies, St Louis, MO). After fixing with 4% paraformaldehyde and mounting in 50% glycerol in PBS, the coverslips were visualized with a × 100 objective by differential interference microscopy and immunofluorescence (BX60 microscope; Olympus, Melville, NY), Spot RT camera (Diagnostic Instruments, Sterling Heights, MI), or Olympus PM20 photomicroscopy system.
Transmission electron microscopy
The hydrophobic side of Thermanox coverslips (Ted Pella, Redding, CA) was fitted with a silicone gasket to create microtiter wells. Platelet adhesion to the collagen-coated surface was conducted as described. After platelets were incubated for 1 hour, the wells were washed twice with 100 μL HBMT/MgCl2 and then were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate, pH 7.4, for 1 hour at 22°C. Wells were then washed with cacodylate buffer and postfixed with 1% osmium tetroxide for 15 minutes. The wells were dehydrated in graded steps of ethanol and polymerized with increasing grades of Epon to 100% for 60 minutes. Sections of 0.14 μm were cut perpendicular to the plane of the coverslip with a diamond knife and a Reichert-Jung Ultra Cut Microtome (Vienna, Austria). Specimens were stained with 2% aqueous uranyl acetate + 100% ethanol (1:1) for 5 minutes and then were washed with distilled water. Thereafter, specimens were stained with Reynold lead citrate for 3 minutes and washed with distilled water. Samples were then examined in a Hitachi H7000 transmission electron microscopy (TEM) microscope.
Results
Human platelet adhesion to collagen
Videomicroscopy (see the Supplemental Videos link at the top of the online article for VHS video segment A, which plays at 3 frames per second) revealed that initially a small number of platelets, which we term vanguard platelets, adhered directly to the collagen-coated surface and rapidly began to spread and lose the distinctive mound-shaped structure typical of the granulomere. Then, though additional vanguard platelets also adhered directly to the collagen, other platelets in suspension (follower platelets) became tethered to vanguard platelets by way of filopodia. We could not unequivocally determine, however, whether the filopodia were extending from the vanguard platelets, the follower platelets, or both. Follower platelets remained tethered to the vanguard platelets by way of filopodia for variable periods of time, after which they adhered to nearby collagen directly and spread or began to spread over the vanguard platelets to which they attached. This process resulted in the development of a sheetlike underlayer(s) of platelets and the growth of platelet thrombi, as islands, on top of the sheet of platelets. Ultimately, platelets spreading from different islands contacted each other, and the platelet borders became indistinct when viewed en face. Once adjoining islands made contact, there appeared to be movement of the contents of the platelets toward each other, with subsequent continued active ruffling of the edges of the interacting platelets.
The adhesion of platelets to collagen was not affected by the inclusion of 10 μg/mL control polyclonal murine IgG. In the presence of the antibody 7E3 or tirofiban, however, in addition to an approximately 30% decrease in total platelet deposition as a result of inhibiting the interactions between vanguard and follower platelets, there was a marked difference in platelet behavior (VHS video segment A). Platelets adhered to collagen, multiple filopodia extended from these platelets onto the nearby collagen surface, and the filopodia demonstrated dynamic movement, but spreading was more variable and overall appeared less extensive than in the absence of 7E3. When compared to the islandlike deposition pattern formed by untreated platelets, 7E3- and tirofiban-treated platelets developed more uniformly scattered patterns. The filopodia that extended out from the adherent platelets sometimes contacted other platelets, and some platelets partially spread over other platelets, but there was virtually no tethering of follower platelets, platelet thrombi, or evidence of platelets acting as an interacting mass (VHS video segment A). Thus, the platelets did not spread in the sheetlike manner that was observed in the absence of 7E3, and the platelet granulomeres remained more prominent than in the absence of 7E3. Even after 1 hour, the borders of the platelets continued to remain distinct. The similarity in results with 7E3 and tirofiban support the interpretation that the effect of 7E3 is primarily caused by its inhibition of GPIIb/IIIa. Microchamber studies were also conducted with the platelets of a patient with Glanzmann thrombasthenia whose platelets lacked immunodetectable GPIIb but had readily detectable trace amounts of GPIIIa.27,28 The pattern of platelet adhesion was indistinguishable from that of platelets in the presence of 7E3 (Figure2). Although we did not directly measure the patient's platelets for αVβ3, other Glanzmann thrombasthenia patients with a similar immunoblot pattern have been found to lack GPIIb/IIIa, but not αVβ3 receptors.29
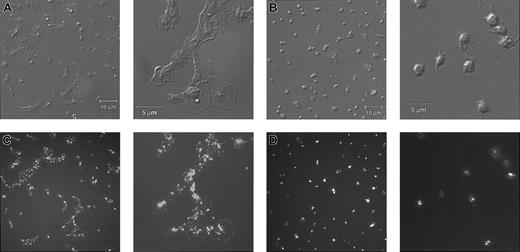
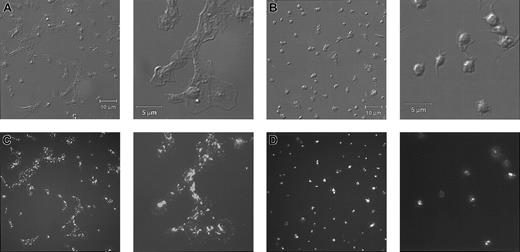
Platelets from a patient with Glanzmann thrombasthenia have decreased platelet spreading and platelet-platelet interactions on collagen.
Control platelets (A) and platelets from a patient with Glanzmann thrombasthenia (B) were anticoagulated with ACD, washed twice, and resuspended in HBMT buffer (100 000/μL). Platelets were added to channels in the collagen-coated microchamber, incubated for 60 minutes at 22°C, and analyzed by differential interference microscopy. Control platelets demonstrate platelet-platelet interactions and platelet spreading. The patient's platelets demonstrate few platelet-platelet interactions, a relatively uniformly scattered pattern of platelet deposition, and less platelet spreading. Scale bars represent 10 μm.
Platelets from a patient with Glanzmann thrombasthenia have decreased platelet spreading and platelet-platelet interactions on collagen.
Control platelets (A) and platelets from a patient with Glanzmann thrombasthenia (B) were anticoagulated with ACD, washed twice, and resuspended in HBMT buffer (100 000/μL). Platelets were added to channels in the collagen-coated microchamber, incubated for 60 minutes at 22°C, and analyzed by differential interference microscopy. Control platelets demonstrate platelet-platelet interactions and platelet spreading. The patient's platelets demonstrate few platelet-platelet interactions, a relatively uniformly scattered pattern of platelet deposition, and less platelet spreading. Scale bars represent 10 μm.
To quantitatively assess the patterns of platelet deposition, we compared the spatial distribution of adherent platelets in each experiment to that predicted by a Poisson distribution (Table1). In 3 separate experiments with control platelet preparations, values for the number of 30-pixel squares with 0, 1, 2, and 3 or more platelets were consistent with a Poisson distribution (P = .90, .73, and .81, respectively). In sharp contrast, all 3 platelet samples treated with 7E3 had decreased values relative to those predicted by a Poisson distribution for the number of squares with 0, 2, and 3 or more platelets, and increased values for the number of squares with one platelet. In each of the 3 experiments, the deviation of the actual distribution from that predicted by a Poisson distribution was significant (P < .0001; andP < .01). Similarly, the platelet sample treated with tirofiban produced a pattern that deviated significantly from the Poisson distribution in the same manner (P < .0001). Similar results were obtained using 15 × 15 pixel square grids (data not shown).
Scanning electron microscopic images of untreated human platelets after 1 hour of adhesion demonstrated a sheetlike underlayer(s) of extensively spread platelets and adherent, but unspread, follower platelets on top of the spread platelets (Figure 3A-B). In sharp contrast, the platelets treated with 7E3 did not form such a sheetlike underlayer and, even though platelets spread over each other, the borders between platelets were more distinct (Figure 3C-D). In addition, even though some platelets demonstrated a diminution in the prominence of their granulomeres, many retained their moundlike granulomeres to a greater extent than did those forming the sheetlike layer(s) in the untreated sample.
Differential interference microscopy of normal human platelets revealed images similar to those obtained by scanning electron microscopy (Figure 4A). Immunofluorescence detection of the α-granule membrane protein P-selectin demonstrated staining throughout the sheetlike mass of platelets, including punctate staining near the edges of spread platelets and the localized staining of follower platelets on top of the sheetlike mass of platelets (Figure4C). In the presence of 7E3, the platelets remained more distinct and did not spread into sheetlike masses (Figure 4B). P-selectin immunofluorescence demonstrated greater retention of discrete α granules in the better-retained granulomere areas (Figure 4D).
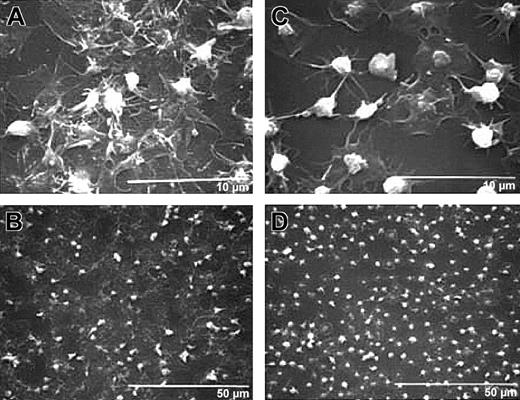
Treatment with antibody 7E3 decreases platelet spreading and development of a sheetlike mass.
Human platelets were anticoagulated with ACD, gel-filtered, and untreated or treated with 7E3 (10 μg/mL) for 15 minutes at 22°C. Platelets (120 000 μL; 50 μL) were added to the collagen-coated microtiter wells and incubated for 1 hour, and then the specimens were prepared for scanning electron microscopy. High-power (A) and low-power (B) views of untreated human platelets show extensive platelet spreading and additional layers of platelets on top of the spread platelets. High-power (C) and low-power (D) views show that 7E3-treated platelets extend filopodia and spread variably but do not form a sheetlike surface; they also retain their granulomeres to a greater extent than do the platelets making up the initial layer of untreated platelets.
Treatment with antibody 7E3 decreases platelet spreading and development of a sheetlike mass.
Human platelets were anticoagulated with ACD, gel-filtered, and untreated or treated with 7E3 (10 μg/mL) for 15 minutes at 22°C. Platelets (120 000 μL; 50 μL) were added to the collagen-coated microtiter wells and incubated for 1 hour, and then the specimens were prepared for scanning electron microscopy. High-power (A) and low-power (B) views of untreated human platelets show extensive platelet spreading and additional layers of platelets on top of the spread platelets. High-power (C) and low-power (D) views show that 7E3-treated platelets extend filopodia and spread variably but do not form a sheetlike surface; they also retain their granulomeres to a greater extent than do the platelets making up the initial layer of untreated platelets.
P-selectin staining of 7E3-treated platelets adherent to collagen is confined to the granulomere, whereas staining of control murine antibody–treated platelets is distributed throughout the sheetlike mass of platelets.
Differential interference contrast microscopy images of normal human platelets in the presence of control murine antibody (A) or 7E3 (B) deposited on collagen for 1 hour at 22°C. Panels on the left are at lower magnification and panels on the right are at higher magnification. Panels C and D contain P-selectin immunofluorescence images of panel A and panel B, respectively. P-selectin staining of the control antibody–treated platelets is distributed throughout the sheetlike mass, including punctate staining near the edges of the spread platelets, whereas the staining of the 7E3-treated platelets was more distinct and better localized to the granulomere areas.
P-selectin staining of 7E3-treated platelets adherent to collagen is confined to the granulomere, whereas staining of control murine antibody–treated platelets is distributed throughout the sheetlike mass of platelets.
Differential interference contrast microscopy images of normal human platelets in the presence of control murine antibody (A) or 7E3 (B) deposited on collagen for 1 hour at 22°C. Panels on the left are at lower magnification and panels on the right are at higher magnification. Panels C and D contain P-selectin immunofluorescence images of panel A and panel B, respectively. P-selectin staining of the control antibody–treated platelets is distributed throughout the sheetlike mass, including punctate staining near the edges of the spread platelets, whereas the staining of the 7E3-treated platelets was more distinct and better localized to the granulomere areas.
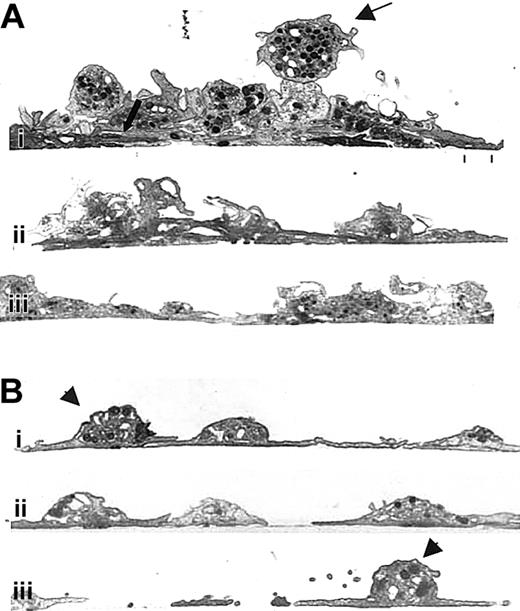
Transmission electron microscopy of cross-sections of untreated human platelets adhering to collagen (Figure 5Ai-iii) revealed that, in many areas, the sheetlike layer observed by scanning electron microscopy was actually made up of a stack of variably spread platelets whose membranes were intimately apposed (Figure 5Ai, thick arrow). Above these layers of platelets were adherent, partially spread platelets, and on the very top were follower platelets with readily detectable granulomeres (Figure 5Ai, upper thin arrow). In contrast, in the presence of 7E3 (Figure 5Bi-iii), fewer layers of platelets were observed. Even though platelet membranes were apposed or partially overlapped in some areas, the limits of the membrane of each platelet remained distinct. Moreover, a larger number of platelets directly adherent to the collagen retained their moundlike granulomeres (Figure 5Bi, iii, arrowheads).
Mouse platelet adhesion to collagen
Wild-type mouse platelets, though smaller than human platelets (approximately 4-5 fL vs 8-9 fL), adhered to collagen much like human platelets, though the loss of granulomere structure was not as pronounced (see VHS video segment B). Thus, vanguard platelets adhered and began to spread, and then follower platelets attached to the vanguard platelets. Follower platelets then adhered and spread, producing islands of platelet thrombi. Bridgelike filopodial extensions eventually formed between platelet islands, and this resulted in the movement of platelet material between islands and the formation of an interactive platelet mass. As with untreated human platelets, statistical analysis demonstrated that the pattern of platelet deposition conformed to that predicted by a Poisson distribution (Table 1).
β3-Null mouse platelets adhered to the collagen surface in a manner similar to that of human platelets in the presence of 7E3 or tirofiban. Thus, they adhered directly to the surface, extended filopodia, and formed a uniformly scattered pattern. The β3-null platelets did, however, appear to spread to a greater extent than did human platelets in the presence of 7E3. There was little or no tethering of follower platelets to vanguard platelets or deposition of follower platelets onto vanguard platelets. In addition, the granulomeres in most platelets remained more distinct than did those of the wild-type platelets. As with the human platelets treated with 7E3 or tirofiban, statistical analysis demonstrated that the pattern of platelet deposition deviated significantly from that predicted by a Poisson distribution (Table 1). Similarly, when wild-type mouse platelets were incubated with a hamster antibody to GPIIb/IIIa (1B5), the results were similar to those observed with the β3-null platelets (video segment B), and statistical analysis confirmed the similarity (Table 1).
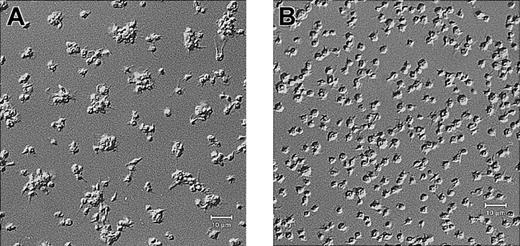
Scanning electron microscopy demonstrated that wild-type mouse platelets (Figure 6A-B), like untreated human platelets (Figure 3A-B), spread and formed a sheetlike mass in which the borders of the individual platelets could not be discerned. β3-null mouse platelets spread to a somewhat greater extent than did human platelets in the presence of 7E3 (compare Figure 3C-D to Figure 6C-D), but the borders between platelets were still more distinct than with wild-type platelets, and the platelets did not form an integrated mass (Figure 6C-D). Transmission electron micrographs revealed multiple vertical layers of wild-type platelets adhering to collagen, with the first layer of platelets spread extensively (data not shown). In contrast, the β3-null mouse platelets demonstrated much less layering, and even when platelets spread over each other and were in proximity with each other, the borders of their membranes remained distinct (data not shown).
Discussion
Studies of platelet adhesion to collagen and other adhesive proteins at select time points under static conditions, or under the influence of variable shear forces, have demonstrated that platelets not only adhere more rapidly to collagen-coated surfaces than other surfaces, they also form larger thrombi.2-4,8,14 In addition, such assays have helped to define the roles of platelet receptors that have been reported to participate in platelet adhesion to collagen or collagen-induced intracellular signaling, including α2β, GPV, GPVI, GPIV, and proteins of Mr 68 000/72 000, 65 000, and 47 000.7,14,17 19 Such studies have not, however, provided information on the cell-biologic dynamics of platelet adhesion and thrombus formation.
To overcome this limitation, we developed a high-resolution time-lapsed videomicroscopy system and studied adhesion of human and mouse platelets to collagen. Our data demonstrate that in contrast to the traditional description of platelet adhesion, platelet spreading, and platelet thrombus formation (ie, platelet-platelet interactions) as sequential processes, for at least some platelets (follower platelets), tethering through filopodia can precede adhesion to collagen and spreading over collagen or vanguard platelets. Release of granule contents from vanguard platelets could facilitate this process by providing high local concentrations of platelet activating agents (eg, adenosine diphosphate [ADP], thromboxane A2, serotonin) and adhesive glycoproteins (including fibrinogen and von Willebrand factor) that facilitate platelet-platelet interactions.30,31 Release of adhesive glycoproteins could also decorate nearby collagen, thus supporting GPIIb/IIIa-dependent platelet spreading. We propose that the unique thrombogenicity of collagen-coated surfaces results from the ability of one or more of the collagen receptors, probably primarily α2β1 and GPVI, to mediate attachment to collagen and initiate degranulation, shape change, and GPIIb/IIIa activation.20,30 31 This then allows GPIIb/IIIa to participate in platelet-platelet interactions and platelet spreading.
Quantitative analysis of the patterns of initial platelet deposition with untreated human and wild-type mouse platelets demonstrated that they conformed to that predicted by a Poisson distribution, indicating that the adhesion events occurred randomly. Thus, so-called platelet recruitment is not an active process of diverting platelets from where they would otherwise land randomly; rather, the ability to support stable platelet-platelet interactions allows the platelets to deposit randomly, even when that means landing on top of another platelet (Figure 7). Ultimately, the platelets develop into a sheetlike multilayered mass in which individual platelet borders are difficult to discern and in which there is active movement of platelet organelles and ruffling of platelet membranes.
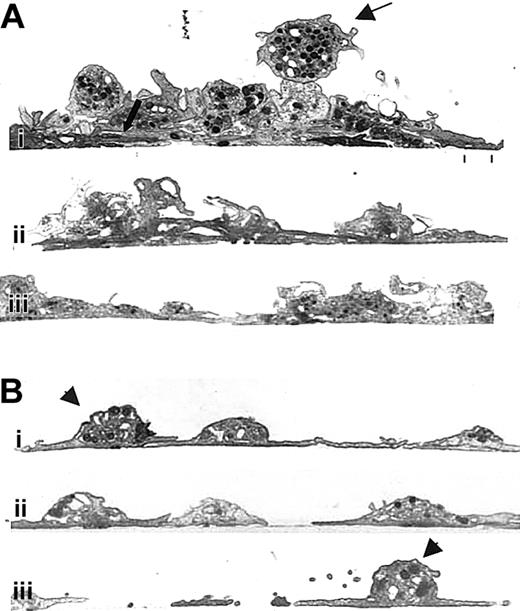
Unlike normal platelets, platelets treated with antibody 7E3 do not form multiple layers on a collagen-coated surface.
Human platelets were prepared and added to collagen-coated microtiter wells as in Figure 3. The specimens were then prepared for transmission electron microscopy after spreading for 1 hour at 22°C. (A) Three images from different regions (Ai, Aii, and Aiii) containing untreated human platelets showing an initial layer of spread platelets (lower thick arrow) and multiple additional layers of platelets with variably intact granulomeres and closely apposed membranes. The thin upper arrow points to a platelet that is tethered to the spread platelets below but retains an intact granulomere. (B) Three images from different regions containing 7E3-treated platelets (Bi, Bii, and Biii), which demonstrate primarily a single layer of platelets. Where one platelet partially spreads over another, the borders of the platelets remain distinct. Granulomeres of the 7E3-treated platelets making up the initial layer (Bi, Biii, arrowheads) are more distinct than those making up the initial layer of the untreated platelets shown in panel A.
Unlike normal platelets, platelets treated with antibody 7E3 do not form multiple layers on a collagen-coated surface.
Human platelets were prepared and added to collagen-coated microtiter wells as in Figure 3. The specimens were then prepared for transmission electron microscopy after spreading for 1 hour at 22°C. (A) Three images from different regions (Ai, Aii, and Aiii) containing untreated human platelets showing an initial layer of spread platelets (lower thick arrow) and multiple additional layers of platelets with variably intact granulomeres and closely apposed membranes. The thin upper arrow points to a platelet that is tethered to the spread platelets below but retains an intact granulomere. (B) Three images from different regions containing 7E3-treated platelets (Bi, Bii, and Biii), which demonstrate primarily a single layer of platelets. Where one platelet partially spreads over another, the borders of the platelets remain distinct. Granulomeres of the 7E3-treated platelets making up the initial layer (Bi, Biii, arrowheads) are more distinct than those making up the initial layer of the untreated platelets shown in panel A.
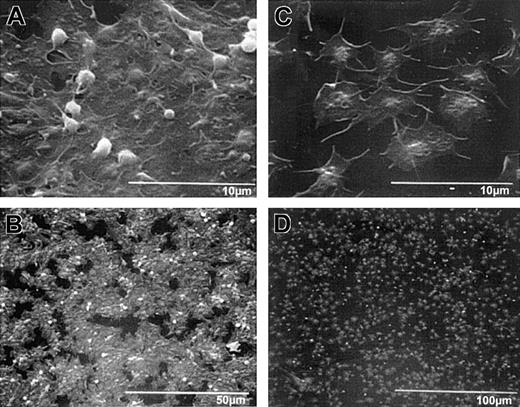
Scanning electron micrographs of wild-type and β3-null mouse platelets adherent to collagen demonstrate platelet morphologic features similar to those of human platelets without and with treatment with 7E3, respectively.
Wild-type and β3-null mouse blood was collected into ACD, and the resultant PRP/PRB mixtures were added to collagen-coated microtiter wells for 1 hour. Samples were then prepared for scanning electron microscopy. High-power (A) and low-power (B) views of wild-type platelets, and high-power (C) and low-power (D) views of β3-null platelet adhesion. Wild-type platelets in panel A show that the platelets have spread in a sheetlike formation; a few remaining follower platelets, with their granulomeres intact, are seen on top of the spread platelets. The extensive “island” formation is apparent at lower power (B). (C) The β3-null platelets do not spread as a sheet, though they appear to spread individually more than human platelets in the presence of 7E3. Note also Figure 3C.
Scanning electron micrographs of wild-type and β3-null mouse platelets adherent to collagen demonstrate platelet morphologic features similar to those of human platelets without and with treatment with 7E3, respectively.
Wild-type and β3-null mouse blood was collected into ACD, and the resultant PRP/PRB mixtures were added to collagen-coated microtiter wells for 1 hour. Samples were then prepared for scanning electron microscopy. High-power (A) and low-power (B) views of wild-type platelets, and high-power (C) and low-power (D) views of β3-null platelet adhesion. Wild-type platelets in panel A show that the platelets have spread in a sheetlike formation; a few remaining follower platelets, with their granulomeres intact, are seen on top of the spread platelets. The extensive “island” formation is apparent at lower power (B). (C) The β3-null platelets do not spread as a sheet, though they appear to spread individually more than human platelets in the presence of 7E3. Note also Figure 3C.
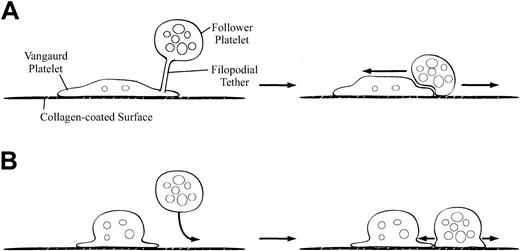
Hypothesis to explain how lack of functional GPIIb/IIIa receptors alters platelet deposition on collagen.
(A) In the presence of functional GPIIb/IIIa receptors, first the vanguard platelet adheres and spreads. A follower platelet that randomly comes to lie above (and potentially overlaps) the vanguard platelet can then tether to the vanguard platelet. Eventually the follower platelet adheres to, and spreads over, the vanguard platelet and the adjacent collagen. (B) In the absence of functional GPIIb/IIIa receptors, platelet-platelet interactions cannot occur; thus, a platelet that comes to lie above (and partially overlaps) an already adherent platelet moves laterally as it approaches the surface and adheres directly to the adjacent collagen.
Hypothesis to explain how lack of functional GPIIb/IIIa receptors alters platelet deposition on collagen.
(A) In the presence of functional GPIIb/IIIa receptors, first the vanguard platelet adheres and spreads. A follower platelet that randomly comes to lie above (and potentially overlaps) the vanguard platelet can then tether to the vanguard platelet. Eventually the follower platelet adheres to, and spreads over, the vanguard platelet and the adjacent collagen. (B) In the absence of functional GPIIb/IIIa receptors, platelet-platelet interactions cannot occur; thus, a platelet that comes to lie above (and partially overlaps) an already adherent platelet moves laterally as it approaches the surface and adheres directly to the adjacent collagen.
Studies with human platelets from a patient with Glanzmann thrombasthenia and human platelets in the presence of antibody 7E3 or tirofiban, as well as with wild-type mouse platelets in the presence of antibody 1B5 or platelets from mice deficient in β3 integrins, indicate that platelet tethering, thrombus formation, spreading, and formation of sheetlike masses all require functional GPIIb/IIIa receptors. Moreover, the absence of functional GPIIb/IIIa receptors also affects the pattern of deposition. Thus, in the absence of β3 receptors or in the presence of GPIIb/IIIa receptor antagonists, the pattern of deposition deviates significantly from that predicted by a Poisson distribution, suggesting that the failure of platelet-platelet interactions actually prevents follower platelets from depositing randomly. We propose that this reflects the inability of randomly deposited follower platelets to develop stable platelet-platelet interactions with the vanguard platelets that lie directly beneath them, resulting in lateral movement of follower platelets, away from already adherent platelets, as they approach the surface; the lateral movement results in the platelets adhering directly to the nearby collagen-coated surface (Figure 7). Furthermore, we propose that this mechanism accounts for the previously reported paradoxical finding that, compared with normal platelets, platelets from patients with Glanzmann thrombasthenia or normal platelets in the presence of GPIIb/IIIa antagonists produce greater surface coverage on collagen and increase the number of adherent single platelets.12,13,32 33
There are 2 major limitations to our study. First, we investigated adhesion and thrombus formation under static conditions at 22°C and did not use whole blood. Thus, it is possible that our observations are not relevant to in vivo conditions under flow. However, we and others have observed islands of platelet thrombi when platelets (in either buffer or whole blood) interact with a collagen-coated surface under flow in a parallel plate chamber at 37°C.19,32-35Moreover, our finding of decreased platelet spreading on collagen in the absence of β3 integrins or the presence of GPIIb/IIIa blockade is consistent with the results of a number of flow studies in which blood was passed over collagen-coated surfaces or subendothelial matrix at 37°C.36 37 Second, though we made observations about platelet granulomere morphology, we did not measure the release of either dense body or α-granule contents, and it is possible that retention of granulomere morphology does not correlate with release of granule contents. Direct analysis of granule release is required to assess the effects of the interventions we studied on degranulation.
Our studies also have potential significance for understanding the mechanism of action of abciximab, the Fab fragment of the murine/human chimeric derivative of antibody 7E3, which is used to prevent ischemic complications of percutaneous coronary interventions, and tirofiban. Thus, though these agents do not formally prevent platelet adhesion to collagen, they prevent platelet thrombus formation and alter the pattern of platelet deposition, decrease platelet spreading, and prevent the development of sheetlike masses of platelets.
We thank Dr Ronald Gordon and Norman Katz for their assistance with the scanning electron microscopy studies and Dr Scott Henderson and Valerie Williams for their assistance with the transmission electron microscopy studies, which were performed in the Mount Sinai School of Medicine Microscopy Center.
Supported by grant 19278 from the National Heart, Lung and Blood Institute and supported in part by General Clinical Research Center grant M01-RR00102 from the National Center for Research Resources at the National Institutes of Health.
B.S.C. is an inventor of abciximab and, in accordance with Federal law and the policies of the Research Foundation of the State University of New York, shares in royalties paid to the Foundation for sales of abciximab.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Barry S. Coller, Laboratory of Blood and Vascular Biology, The Rockefeller University, 1230 York Ave, New York, NY 10021; e-mail: collerb@rockefeller.edu.