Immature and predendritic cells (pre-DCs) of human blood are the most readily accessible human DC sources available for study ex vivo. Murine homologues of human blood DCs have not been described. We report the isolation and characterization of 2 populations of precursor DCs in mouse blood. Mouse blood cells with the surface phenotype CD11cloCD11b−CD45RAhi closely resemble human plasmacytoid cells (or pre-DC2) by morphology and function. On stimulation with oligonucleotides containing CpG motifs (CpG), these cells make large amounts of type 1 interferons and rapidly develop into DCs that bear CD8, though they may be distinct from the CD8+ DCs in the unstimulated mouse. A second population of cells with the surface phenotype CD11c+CD11b+CD45RA− closely resembles the immediate precursors of pre-DC1, rapidly transforming into CD8− DCs after tumor necrosis factor-α (TNF-α) stimulation. These findings indicate the close relationship between human and mouse DCs, provided cells are obtained directly from equivalent source materials.
Introduction
In 1992, Inaba et al1 identified proliferating cells in mouse blood that differentiated into dendritic cells (DCs) after 7-day culture in granulocyte macrophage–colony-stimulating factor (GM-CSF). Soon after, 2 groups2,3 identified monocytelike DC precursors (pre-DCs) in human blood. The DCs generated in vitro from human blood monocytes after 7 days under the influence of GM-CSF and interleukin-4 (IL-4), followed by a maturation stimulus such as tumor necrosis factor-α (TNF-α), have since been used extensively in clinical immunotherapy trials. Human blood also contains more developed immature DCs (iDCs),4,5 which are already moderate stimulators of allogeneic T cells and which are able to rapidly develop into mature DCs in culture. These iDCs are CD11c+HLA+ and display a myeloid phenotype; hence, they are considered to derive from the monocyte pre-DCs. T cells stimulated with mature CD11c+ DCs show a helper 1 T cell (TH1)–skewed cytokine production profile6 7; hence, the DCs are termed DC1 and the precursors pre-DC1.
Another distinct DC lineage cell found in human blood is the CD11c−IL3R+CD45RA+ plasmacytoid cell. These are also present in various human lymphoid tissues,8-11 where they were first described as plasmacytoid T cells because they had plasma cell morphology and lacked B-lineage or myeloid markers but expressed CD4.11,12 They rapidly produce large amounts of type 1 interferon (IFN) on stimulation with viral, bacterial, or CD40-L stimulation13-18; therefore, they represent a first line of defense against microbial invasion. They do not initially stimulate naive allogeneic T cells, but upon culture with IL-3 or microbial stimuli they rapidly acquire DC morphology and up-regulate costimulator molecules and major histocompatibility complex class 2, and they become effective stimulators of T cells.11,15,16,18 The stimulated T cells are often skewed toward the TH2 cytokine production profile, so the DCs are termed DC2 and the precursors are termed pre-DC2.7
In contrast to these studies on human blood, little attention has been paid to the DC-lineage cells of mouse blood since the pioneering work of Inaba et al.1,19 Instead, most information on murine DCs derives from their direct isolation, in a relatively mature form, from lymphoid tissues. Multiple subtypes of mature mouse DCs have been described.20-22 However, the relationship of these directly isolated mouse DCs to human DCs generated from blood precursors in culture is unclear, and it has been argued that the products of human blood pre-DC1 and pre-DC2 are normally only produced on microbial stimulation and that their equivalents might not be represented among the DCs of uninfected laboratory mice.6However, in one case in which mature DCs were directly isolated using similar procedures from human thymus and mouse thymus, there was a close correlation of most surface markers and of cytokine production potential; an exception was CD8α, expressed on mouse but not human DCs.9
Accordingly, to provide a direct comparison with the human DC system, it was important to isolate and characterize the immediate DC precursors of mature DCs from mouse blood, despite the inconvenience and expense of this source. In the present study we have identified 2 populations in mouse blood that appear to be the equivalents of those in human blood. A CD11c+ iDC population is described that expresses myeloid markers and produces CD8α− DCs in culture; this likely corresponds to the human pre-DC1. A CD11cloCD11b−CD45RAhi population is described that rapidly produces IFN-α on stimulation in culture with oligonucleotides containing CpG motifs (CpG) and that concurrently develops into functional, mature CD8+ DCs; this appears to be the equivalent of the human plasmacytoid pre-DC2.
Materials and methods
Mice
Mice were bred under specific pathogen-free conditions in the Walter and Eliza Hall Institute (WEHI) animal breeding facility. Male and female C57BL/6J WEHI mice were used at 6 to 15 weeks of age. Blood was taken from pools of 20 to 50 mice. T cells used in allostimulatory–mixed-leukocyte reactions (allo-MLR) were from CBA CaH WEHI mice.
Immunofluorescence labeling of DCs
Before staining with specific antibodies, nonspecific binding was blocked by incubating the cells with 1 mg/mL rat immunoglobulin. Most of the monoclonal antibodies (mAbs), the fluorescent conjugates, and the multicolor labeling procedures have been specified previously.20 For presorting to eliminate natural killer (NK) cells, DX5 biotin (BD PharMingen, San Diego, CA) was used with streptavidin-Cy5 as the second-stage detection reagent. For sorting pre-DC populations, anti-CD11c (N418)–fluorescein isothiocyanate (FITC) conjugate and anti-CD45RA (clone 14.8)–phycoerythrin (PE)–conjugate (BD PharMingen) were used.
For surface phenotype analyses, the following mAb conjugates were used: anti-CD11c (N418)–Cy5 or FITC, anti–major histocompatibility complex (MHC) class 2 (N22 or M5/114)–FITC or Alexa 594 (the conjugation levels were deliberately less than maximal to ensure the strong staining for MHC class 2 on DCs at saturation did not cause inaccurate color compensation problems in other channels), anti-CD8α (YTS169.4)–Cy5, anti-CD4 (GK1.5)–Alexa 594, anti-CD40 (FGK45.5)–FITC, anti-CD205 (NLDC-145)–FITC, anti-CD11b (M-170)–FITC, CD45R (RA3-6B2)–FITC, Sca-1 (E13161-7)–FITC, CD24 (M1/69)–FITC, CD62L (MEL-14)–FITC, and CD38 (NIM-R5)–biotin. Propidium iodide (PI) was included at 1 μg/mL in the final wash after immunofluorescence staining to label dead cells.
FACS analysis and sorting of DC populations
For sorting DC and pre-DC populations, a MoFlo instrument (Cytomation, Fort Collins, CO) was used. Reanalysis was routinely performed, and populations were only used for further functional analyses when the purity was greater than 95%. Most analyses were performed on a FACStar Plus instrument (Becton Dickinson, San Jose, CA), as previously described,20 using up to 4 fluorescence channels for immunofluorescence staining (FL1 for FITC, FL2 for PE, FL3 for Cy5, and FL4 for Alexa 594), with the FL5 channel set to exclude PI-positive dead cells and autofluorescence cells. Care was taken during gating that any cells brightly fluorescent in FL3 and spilling over into FL5 were not gated out as dead cells.
Preparation of mouse spleen DCs
Spleen DCs were isolated exactly as described elsewhere.20
Preparation of pre-DCs from mouse blood
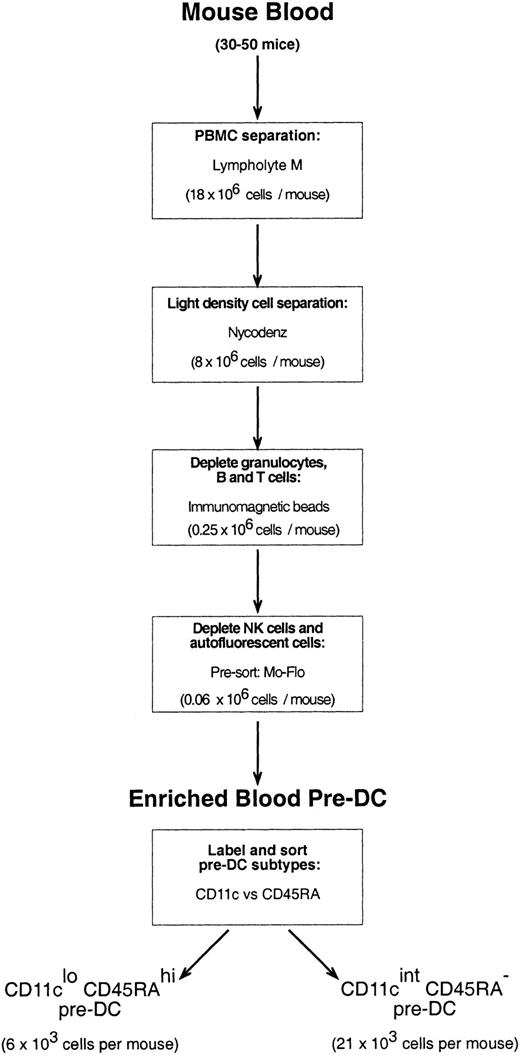
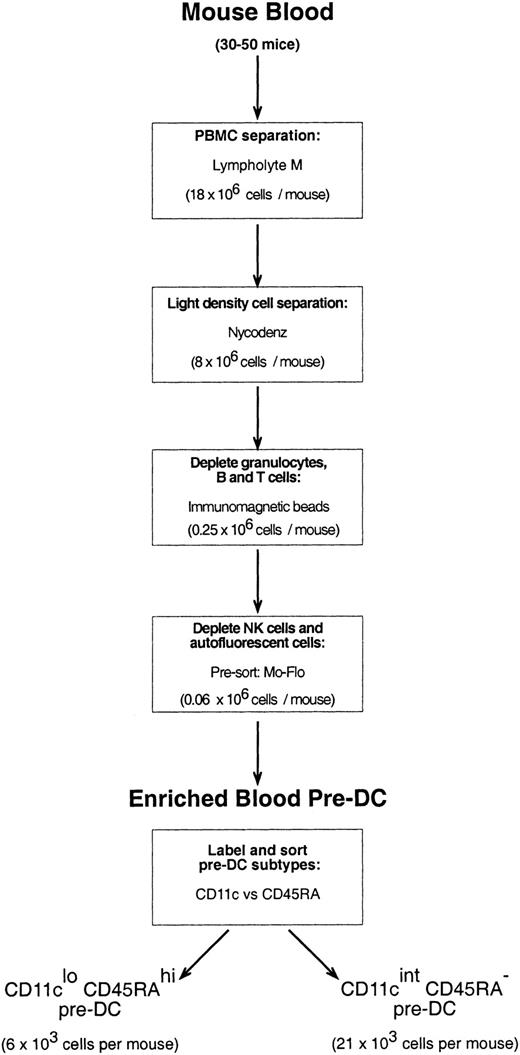
Mouse blood was collected from 20 to 50 mice through cardiac puncture into heparinized glass tubes. The blood was diluted 1:3 with a mouse osmolarity salt solution lacking divalent ions and containing 5 mM EDTA (ethylenediaminetetraacetic acid)–balanced salt solution (EDTA/BSS).23 Diluted blood samples were layered over Lympholyte M (density, 1.0875 ± 0.0005 g/cm3; Cedarlane Laboratories, Ontario, QC, Canada) and centrifuged at room temperature (RT) according to the manufacturer's instructions. The peripheral blood mononuclear cell (PBMC) fraction was then removed, washed in EDTA/BSS, and resuspended in Nycodenz medium (1.086 g/cm3at 4°C) mouse osmolarity (analytical grade powder; Nycomed Pharma AS, Oslo, Norway; made iso-osmotic in water, then diluted in EDTA/BSS). Cells suspended in Nycodenz medium were layered on an equal volume of Nycodenz medium and were subjected to further density separation (1700g, 15 minutes, 4°C) to further enrich the sample for DCs and pre-DCs. The light density cell fraction (now only approximately 30% of the PBMCs that were recovered from Lympholyte) was washed in EDTA/BSS, pelleted, and then incubated with a cocktail of optimally titrated monoclonal antibodies to deplete the preparation of CD19+ B cells (mAb, ID3), T cells (mAb, KT3-1.1), granulocytes (mAb, RB68C5), and residual red blood cells (mAb, TER-119). Cells binding mAb were depleted by antirat immunoglobulin magnetic beads (Dynabeads; Dynal, Oslo, Norway), as previously described for the purification of splenic DCs.20Approximately 50% of the resultant cells were either autofluorescent and had extremely high side scatter (these cells were most prevalent in male mice) or were positive for the pan-NK cell marker, DX5; neither of these cells included immediate precursors of DCs (data not shown). The antibody-depleted preparation was, therefore, labeled with DX5-biotin and streptavidin-Cy5 and DX5-positive cells and cells autofluorescent in the FITC and PE channels. Cells autofluorescent in the FITC and PE channels were eliminated by fast presorting on the MoFlo instrument, with the nonfluorescent cells providing a highly enriched source of pre-DCs. For final purification of pre-DC subtypes, the presorted cells were labeled with anti-CD11c–FITC and anti-CD45RA, and the 2 distinct CD11cloCD45Rahi and CD11cintCD45RA− populations were sorted using the MoFlo instrument. The procedure is summarized in the flow chart of Figure 1.
Electron microscopy
Cell pellets were fixed in 2.5% glutaraldehyde in phosphate-buffered saline (PBS) at 4°C overnight, washed in PBS, and postfixed in 1% osmium tetroxide in PBS for 1 hour. After they were washed in distilled water, the cell pellets were dehydrated in acetone and embedded in Procure-Araldite resin (ProSciTech; Thuringowa, Australia). During dehydration, the cells were stained en block with 2% uranyl acetate in 70% acetone. Semithin (1 μm) sections were cut through the maximum thickness of each pellet and stained with a solution of 1% methylene blue and 1% sodium tetraborate. Ultrathin sections were stained with a 5% aqueous solution of uranyl acetate for 10 minutes, washed with distilled water, and stained with Reynold lead citrate for 10 minutes. After staining, the sections were examined by a Philips 300 electron microscope operated at 60 kV.
Cytokines and stimulants of pre-DCs
Murine recombinant granulocyte macrophage–colony-stimulating factor (GM-CSF; used at 200 U/mL), murine recombinant interleukin-3 (rIL-3; used at 100 U/mL), murine recombinant tumor necrosis factor-α (rTNF-α; used at 100 U/mL), and murine rIL-4 (used at 100 U/mL) were gifts from Immunex (Seattle, WA). Recombinant rat IFN-γ (used at 20 ng/mL and bioactive with mouse cells) was purchased from PeproTech (Rocky Hill, NJ). Murine rIL-12 p70 was purchased from R&D Systems (Minneapolis, MN). Oligonucleotides containing a fully phosphorothioated CpG motif were synthesized by GeneWorks (Adelaide, Australia) according to a published sequence (CpG1668)24 and were used at 200 nM.
Differentiation and activation of pre-DCs in culture
The pre-DCs were incubated at 0.5 × 106 or 0.25 × 106 cells/mL in U-bottom wells of 96-well tissue-culture plates in a humidified 10% CO2 in air incubator at 37°C for 8 to 36 hours. Modified mouse osmolarity RPMI 1640 medium25 was used, together with the appropriate cytokines and stimulants.
Quantitation of cytokine production
Analysis of IL-12 production in culture supernatants was carried out using enzyme-linked immunosorbent assay (ELISA), as previously described.26 27
A bioassay for type 1 IFN was performed as described previously.26 In addition, IFN-α was assayed through 2-site ELISA, using as a capture antibody antimouse IFN-α (clone RMMA-1; PBL Biomedical Laboratories, New Brunswick, NJ) and as a detection antibody rabbit antimouse IFN-α polyclonal (PBL Biomedical Laboratories), followed by a F(ab′)2 fragment donkey–antirabbit–horseradish peroxidase (HRPO) conjugate (Jackson Immunoresearch Laboratories, West Grove, PA), with final readout as described for IL-12. The IFN-α standard used was recombinant mouse IFN-αA (PBL Biomedical Laboratories).
MLR cultures for T-cell stimulation capacity
CD4+ T cells were purified from pooled mesenteric, axillary, brachial, and inguinal lymph nodes of CBA/CaH mice as previously described.28 Pre-DC subpopulations, isolated and sorted as described above, were first cultured overnight as above in their own optimal stimulating medium—CD11cloCD45RAhi in 200 U/mL GM-CSF, 200 nM CpG or CD11cintCD45RA− in 200 U/mL GM-CSF, and 100 U/mL TNF-α—and in the optimal stimulating medium of the other population. Cultured pre-DCs were washed 3 times with EDTA/BSS containing 2% fetal calf serum (FCS), and the number of viable cells was determined. Freshly isolated or viable precultured pre-DCs (1 × 103) were added to U-bottom wells containing 20 × 103 T cells. All cells were suspended and cultured in modified RPMI 1640 medium described in “Preparation of pre-DCs from mouse blood.” Total culture volume was 100 μL. Replicate culture trays were incubated at 37°C in 10% CO2 in air for 3 to 6 days. At days 3, 4, 5, and 6, a culture tray was pulsed with 3H-thymidine, 1 μCi (0.037 MBq)/well, for 6 hours, then frozen. Trays were thawed, cells were harvested onto glass fiber filters, and thymidine incorporated was counted by liquid scintillation. All cultures at all times were grown at least in triplicate, and background controls, with T cells or pre-DCs only, were included at each time point.
Results
Low levels of mature DCs in mouse blood
Analysis of mouse blood PBMC preparations or of further enrichment stages revealed only extremely low levels of cells with a surface phenotype approaching that of the mature DCs found in lymphoid organs (MHC class 2hi, CD11c+). The numbers of these relatively mature DCs detected varied between preparations, but indications were that there were fewer than 1000 cells of near-mature phenotype in the blood of one mouse. The few that were detected were mainly CD11b+CD8−.
Detection and enrichment of pre-DCs in mouse blood
In view of the paucity of mature DCs, we tested whether mouse blood, like human blood, contained earlier stages of DC development. PBMCs from mouse blood were segregated in various ways, then tested for their ability to produce mature DCs in short-term culture. A range of cytokine and bacterial stimuli combinations was tested for the up-regulation of MHC class 2 and CD11c and for the acquisition of typical DC morphology. Precursors of such putative mature DCs were present in the PBMC fraction and were enriched in among the lighter density cells. However, they were absent from the PBMC fractions positive for certain markers of B cells (CD19), T cells (CD3), and erythroid cells. They were also absent from cells expressing very high levels of the myeloid markers CD11b and GR-1, which were extremely adherent or granular in appearance.
A large group of PBMCs coexpressed intermediate levels of DX5 and CD11c but, on culture, down-regulated CD11c and did not express MHC class 2 or acquire DC morphology; thus, it appeared these were NK-lineage cells and not pre-DCs. A large but variable group of autofluorescent cells, which had the potential to contaminate subsequent immunofluorescence sorting, was also found not to include pre-DCs. By positively selecting for the lighter density cells and excluding, by depletion and presorting steps, these inactive populations of lineage-positive or autofluorescent cells, the scheme of Figure 1 was devised to provide a highly enriched source of pre-DCs.
Two populations of pre-DCs in mouse blood
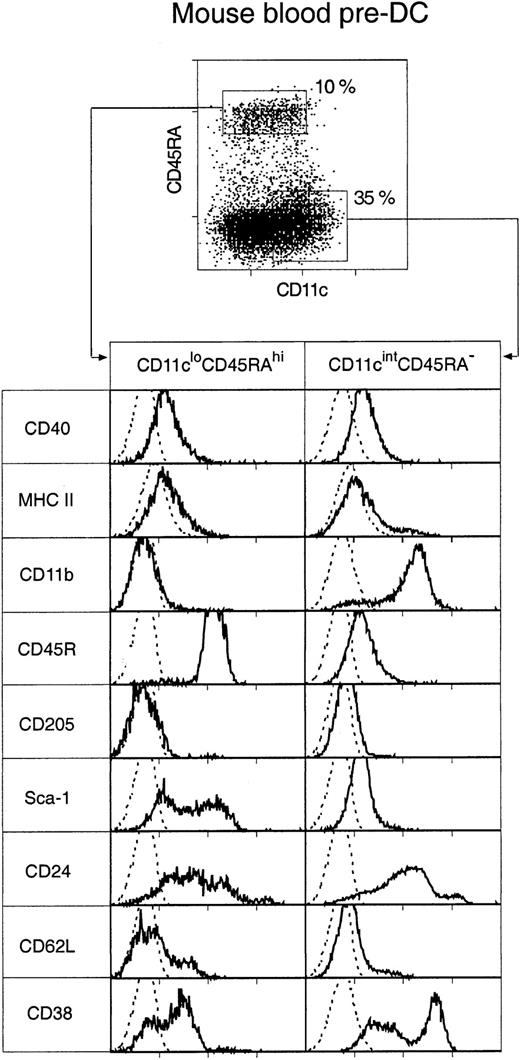
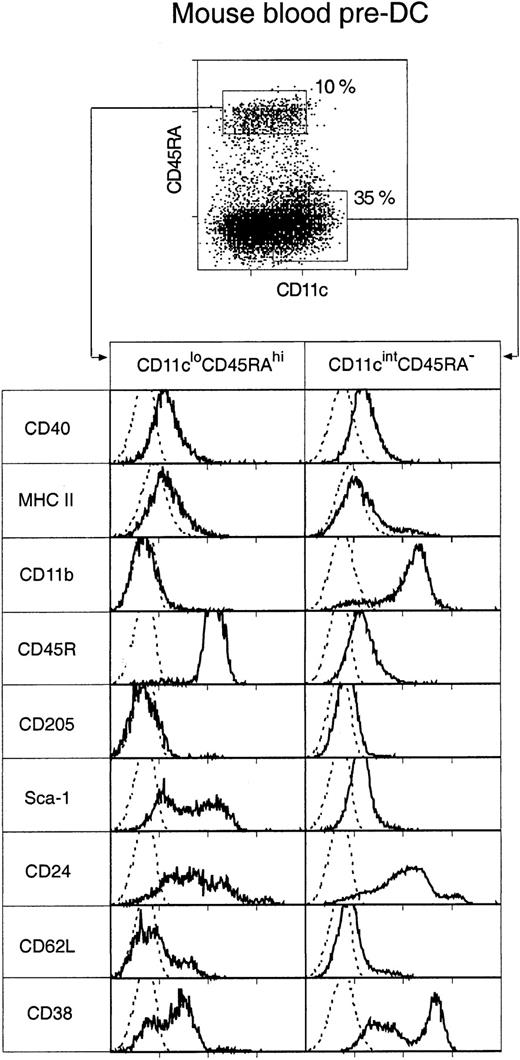
Two distinct populations of pre-DCs, both able to rapidly differentiate into DCs in culture, were discovered. These were cells of the phenotypes CD11cintCD45RA−CD11b+ and CD11cloCD45RA+CD11b− (Figure2), and they represented 35% and 10%, respectively, of the enriched pre-DC population or 0.07% and 0.025%, respectively, of mouse PBMCs. Both populations were medium-sized cells with medium-high forward and low side light-scatter profiles. Their surface phenotype, as freshly isolated, is shown in Figure 2.
Surface phenotype of mouse blood pre-DCs.
Mouse blood pre-DCs were enriched as described in “Materials and methods” and in Figure 1, including presorting to eliminate autofluorescent cells. The enriched preparation was then stained for surface expression of CD11c and CD45RA, together with MHC class 2 and one of the additional markers shown. Resultant fluorescence distribution data were gated on either the CD11cloCD45RAhi or the CD11cintCD45RA− pre-DC subgroups, as shown in the top dot plot. Distributions of the other markers are shown in the bottom panels. The broken line gives the background fluorescence with only the relevant stain omitted. Both pre-DC populations were negative for expression of CD4, CD8α, CD80, CD86, and IL-3R (data not shown). Results are representative of 2 to 5 such analyses.
Surface phenotype of mouse blood pre-DCs.
Mouse blood pre-DCs were enriched as described in “Materials and methods” and in Figure 1, including presorting to eliminate autofluorescent cells. The enriched preparation was then stained for surface expression of CD11c and CD45RA, together with MHC class 2 and one of the additional markers shown. Resultant fluorescence distribution data were gated on either the CD11cloCD45RAhi or the CD11cintCD45RA− pre-DC subgroups, as shown in the top dot plot. Distributions of the other markers are shown in the bottom panels. The broken line gives the background fluorescence with only the relevant stain omitted. Both pre-DC populations were negative for expression of CD4, CD8α, CD80, CD86, and IL-3R (data not shown). Results are representative of 2 to 5 such analyses.
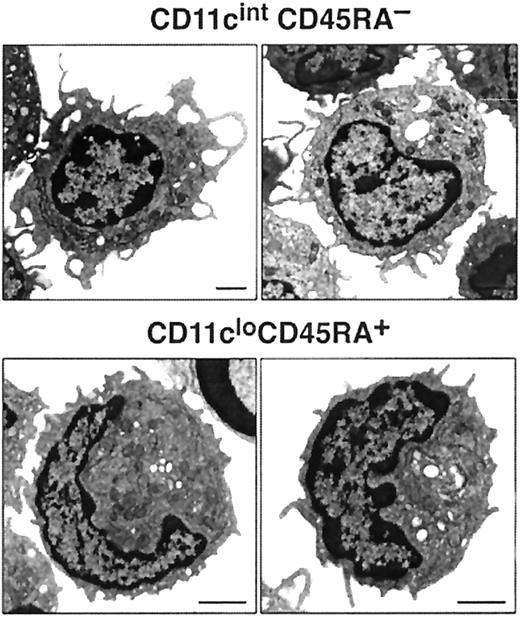
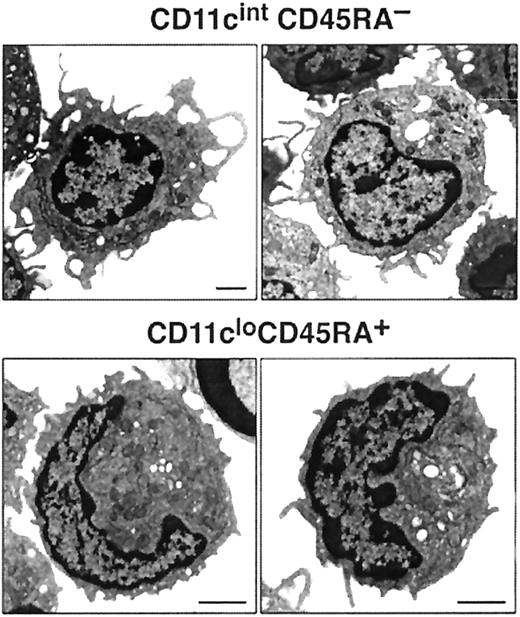
Most cells in both blood pre-DC cell populations expressed extremely low levels of MHC class 2, low levels of CD40, and undetectable levels of CD80 and CD86. The only exception to this was a small subgroup of cells within the CD11cintCD45RA−CD11b+subset—those expressing the highest levels of CD11c and higher levels of MHC class 2 and that appeared to correspond to the circulating, more mature DCs. Such partially mature DCs were excluded on the basis of higher CD11c expression from all subsequent sorts to assess pre-DC function. CD4 or CD8α was not detected on either of the pre-DC populations (data not shown). The population sorted as CD45RAhiCD11clo was distinct in also being CD11b− and CD45RAhiB220+ and including many Sca-1hi cells. Although this population resembled, in many respects, human CD11c− plasmacytoid pre-DCs, it did not express detectable levels of the IL-3 receptor (IL-3R) (data not shown). Electron micrographs of these cells also resembled those of human plasmacytoid pre-DCs11 because the CD11cloCD45RA+ cells were of plasmacytoid appearance, with many cells displaying extensive endoplasmic reticulum (Figure 3). The sorted CD45RA−CD11cint population was also distinct in expressing CD11b (though at an intermediate DC level, not at a high macrophage level) and in including many cells expressing high levels of CD38. Electron micrographs revealed the CD11cintCD45RA− cells as nonplasmacytoid cells that resembled immature DCs, with cells displaying small cytoplasmic protrusions (Figure 3).
Images of mouse blood pre-DCs.
Electron micrographs of CD11cintCD45RA− and CD11cloCD45RA+ cells. The bar represents 1 μm. Images are representative of more than 6 different fields of CD11cintCD45RA− and CD11cloCD45RA+ cells from 2 separate blood preparations.
Images of mouse blood pre-DCs.
Electron micrographs of CD11cintCD45RA− and CD11cloCD45RA+ cells. The bar represents 1 μm. Images are representative of more than 6 different fields of CD11cintCD45RA− and CD11cloCD45RA+ cells from 2 separate blood preparations.
Maturation of CD11cintCD45RA− pre-DCs in culture
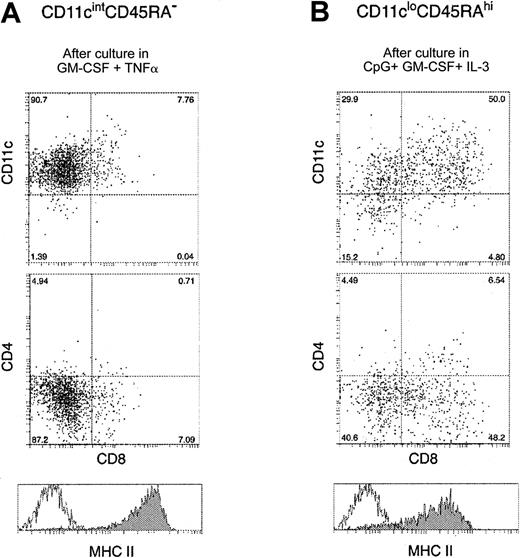
The CD11cintCD45RA− population up-regulated surface MHC class 2 and acquired DC morphology after as little as 8 hours culture, even in the absence of exogenous cytokines in the media (data not shown). Under these conditions, however, cell viability was poor. The presence of GM-CSF improved the overall recovery of viable cells to approximately 70% after overnight culture, and the additional presence of TNF-α produced maximal expression of MHC class 2 and CD40. However, the cells did not express CD4 or CD8, even if cultured in the presence of CpG. At this time, the cultured products of CD45RAhiCD11clo precursors closely resembled CD4−CD8− DCs isolated from mouse spleen and cultured for the same time period (data not shown). After 36 hours of culture, cell viability had dropped to 30% to 40%, but the remaining viable cells were all large cells with DC morphology, expressing high levels of CD11c and MHC class 2 (Figure4A). At this time approximately 10% of the cells showed low staining for CD8α, though most remained CD8− and all remained CD4−.
Surface phenotype of mouse blood pre-DCs after in vitro maturation.
Mouse blood pre-DC populations were incubated with their respective optimal stimuli, as described in “Results.” After 36-hour culture, the cells were recovered and stained with antibodies to CD11c, CD4, CD8, and MHC class 2. Panel A shows the surface staining of CD11cintCD45RA− mouse pre-DCs after incubation with GM-CSF and TNF-α. Panel B shows the surface staining of CD11cloCD45RAhi cells after incubation with GM-CSF, IL-3, and CpG. In both panels, the unfilled line within the MHC class 2 histogram gives the background fluorescence with only the MHC class 2 stain omitted. Data are from one experiment, representative of 5 to 10 separate experiments, each of which examined the pre-DCs obtained from the pooled blood of more than 20 mice.
Surface phenotype of mouse blood pre-DCs after in vitro maturation.
Mouse blood pre-DC populations were incubated with their respective optimal stimuli, as described in “Results.” After 36-hour culture, the cells were recovered and stained with antibodies to CD11c, CD4, CD8, and MHC class 2. Panel A shows the surface staining of CD11cintCD45RA− mouse pre-DCs after incubation with GM-CSF and TNF-α. Panel B shows the surface staining of CD11cloCD45RAhi cells after incubation with GM-CSF, IL-3, and CpG. In both panels, the unfilled line within the MHC class 2 histogram gives the background fluorescence with only the MHC class 2 stain omitted. Data are from one experiment, representative of 5 to 10 separate experiments, each of which examined the pre-DCs obtained from the pooled blood of more than 20 mice.
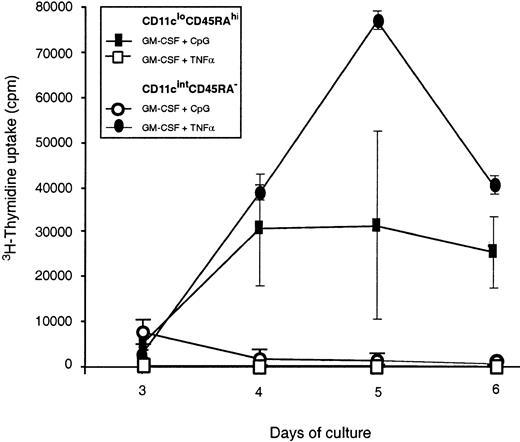
Functional maturation was checked by the ability to stimulate allogeneic mouse T cells in MLR culture. Freshly isolated CD45RAhiCD11clo pre-DCs did not efficiently stimulate the allogeneic T cells, even after 6 days of coculture, provided the small group of CD11chi near-mature DCs was gated out during sorting. However, if the CD11cintCD45RA− population was first matured by overnight culture with GM-CSF and TNF-α, it became an efficient stimulator of T cells (Figure 5), with similar efficiency to freshly isolated CD4−CD8− splenic DCs. Coculture with GM-CSF, together with CpG, did not induce these cells to become efficient stimulators of T cells.
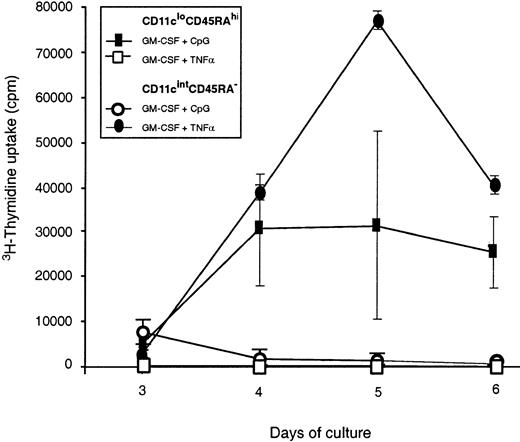
T-cell stimulatory capacity of in vitro–activated mouse pre-DCs.
Purified mouse blood pre-DC populations were tested for their ability to stimulate naive CD4+ T cells in an allostimulatory MLR. Freshly isolated pre-DC populations did not yield counts greater than the background obtained with T cells alone (fewer than 1000 counts per well), and only the results from in vitro–stimulated pre-DCs are shown. Pre-DCs were cultured overnight with their respective optimal stimuli: CD45RA−CD11cint pre-DCs in GM-CSF and TNF-α and CD45RAhiCD11clo/int pre-DCs in GM-CSF and CpG. In addition, the cells were cultured overnight with the optimal stimulus of the other population. After such preculture, the activated pre-DCs were washed, and live cells were counted and added to T cells for the MLR culture assay. The average cpm of triplicate values is shown for each time point, and the error bars represent the range of triplicate values. Data shown are from a single experiment that compared mouse pre-DC populations purified directly or after in vitro stimulation. Similar results were obtained in a second experiment. Activated CD45RAhiCD11cint pre-DCs from mouse spleen gave similar results, stimulating proliferation in allogeneic T cells though less effectively than conventional DCs.
T-cell stimulatory capacity of in vitro–activated mouse pre-DCs.
Purified mouse blood pre-DC populations were tested for their ability to stimulate naive CD4+ T cells in an allostimulatory MLR. Freshly isolated pre-DC populations did not yield counts greater than the background obtained with T cells alone (fewer than 1000 counts per well), and only the results from in vitro–stimulated pre-DCs are shown. Pre-DCs were cultured overnight with their respective optimal stimuli: CD45RA−CD11cint pre-DCs in GM-CSF and TNF-α and CD45RAhiCD11clo/int pre-DCs in GM-CSF and CpG. In addition, the cells were cultured overnight with the optimal stimulus of the other population. After such preculture, the activated pre-DCs were washed, and live cells were counted and added to T cells for the MLR culture assay. The average cpm of triplicate values is shown for each time point, and the error bars represent the range of triplicate values. Data shown are from a single experiment that compared mouse pre-DC populations purified directly or after in vitro stimulation. Similar results were obtained in a second experiment. Activated CD45RAhiCD11cint pre-DCs from mouse spleen gave similar results, stimulating proliferation in allogeneic T cells though less effectively than conventional DCs.
Maturation of CD11cloCD45RAhi blood pre-DCs in culture
When cultured in medium without exogenous cytokines, the CD11cloCD45RA+ population died rapidly without maturation. In contrast to the CD11cintCD45RA−cells, the CD11cloCD45RAhi population did not respond by maturation to the GM-CSF/TNF-α combination; instead it died rapidly. GM-CSF alone improved viability but did not induce maturation or proliferation. However, if CpG was used as a stimulus, together with GM-CSF, IL-3, or both, rapid differentiation to cells that resembled mature DCs was observed. The optimal time for mature DC production was 36 hours, when the cells had increased in size, developed dendrites, and displayed higher forward and side scatter. The overall recovery of viable cells at this time was 80%. CpG induced the up-regulation of MHC class 2 and CD11c (Figure 4B). In contrast to the reports for human CD11c− pre-DCs, mouse CD11cloCD45RA+ pre-DCs did not acquire DC phenotype or morphology in the presence of IL-3, or IL-3 plus GM-CSF, unless an additional microbial-derived stimulus was present. CpG also induced the expression of the high levels of CD8α on approximately 50% of the cells, but only marginal staining for CD4 was obtained.
Functional testing in the MLR system revealed that freshly isolated CD11cloCD45RA+ did not induce proliferation by naive allogeneic T cells. However, preincubation overnight with CpG and GM-CSF to produce a DC morphology and phenotype produced cells able to stimulate T cells (Figure 5). Stimulator capacity was less than that of the DCs produced by the CD11cintCD45RA−population and less than that of the freshly isolated splenic DCs. Optimal activation stimuli of the CD11cintCD45RA− population, GM-CSF, and TNF-α did not induce T-cell stimulator capacity in the CD11cintCD45RA− population (Figure 5).
Cytokine production by mouse blood pre-DCs
Analysis of mouse blood pre-DCs revealed similarities between the murine CD11cintCD45RA− population and human blood pre-DC1 monocyte precursors of human “myeloid” DC1 and between the murine CD11cloCD45RA+ population and human blood plasmacytoid pre-DC2. To further check these similarities, we examined their capacity to produce 2 key cytokines, type 1 IFN and IL-12 p70. Production of type 1 IFN is a hallmark of the plasmacytoid human pre-DC2.16,18 Human pre-DC1 have been reported to produce bioactive IL-12 p70 and to induce TH1 responses29; however, in apparent contradiction to this, it is the murine CD8+ DCs that are the major IL-12 p70 producers among murine splenic DCs.26 30
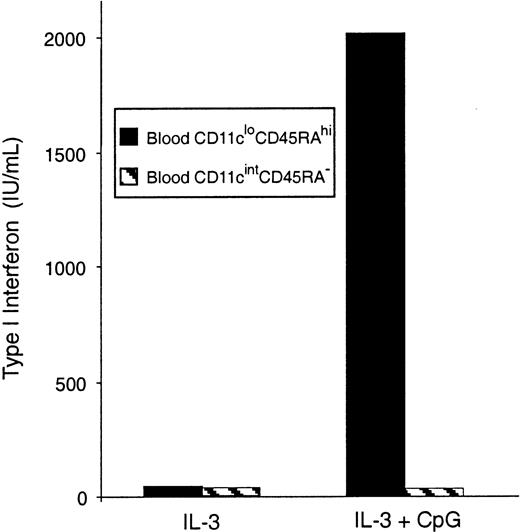
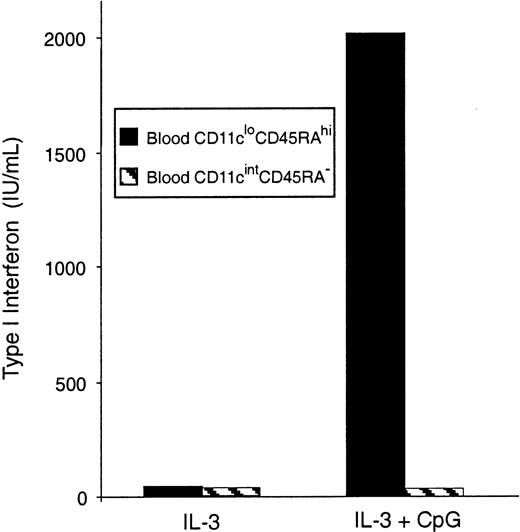
Freshly isolated mouse blood pre-DCs were separated, sorted into the 2 subtypes, and stimulated in culture with CpG, an effective inducer of type 1 IFN. Type 1 IFNs were detected in the supernatants of CD11cloCD45RAhi pre-DCs, but not in the supernatants of CD11cintCD45RA− pre-DCs, by a type 1 IFN bioassay (Figure 6) and an IFN-α–specific ELISA (data not shown). This supports the correspondence between the CD11cloCD45RAhipre-DCs and the human plasmacytoid pre-DC2.
IFN-α production by mouse blood pre-DCs.
Sorted populations of mouse blood pre-DCs were stimulated in culture with CpG and IL-3 or IL-3 alone. Supernatants were harvested after 36 hours, then analyzed by bioassay for type 1 IFN production. Data are from one experiment; replicate samples produced identical results. Similar results were obtained in 3 experiments. Results were also confirmed by ELISA for IFN-α.
IFN-α production by mouse blood pre-DCs.
Sorted populations of mouse blood pre-DCs were stimulated in culture with CpG and IL-3 or IL-3 alone. Supernatants were harvested after 36 hours, then analyzed by bioassay for type 1 IFN production. Data are from one experiment; replicate samples produced identical results. Similar results were obtained in 3 experiments. Results were also confirmed by ELISA for IFN-α.
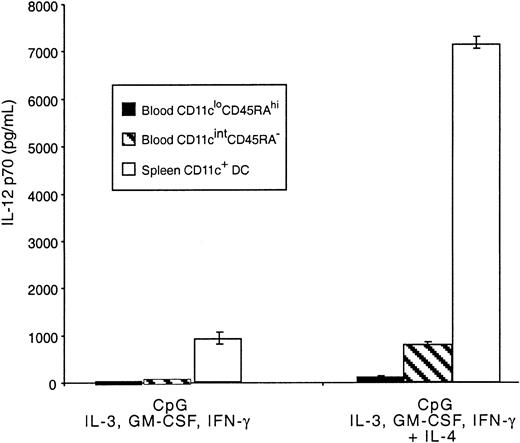
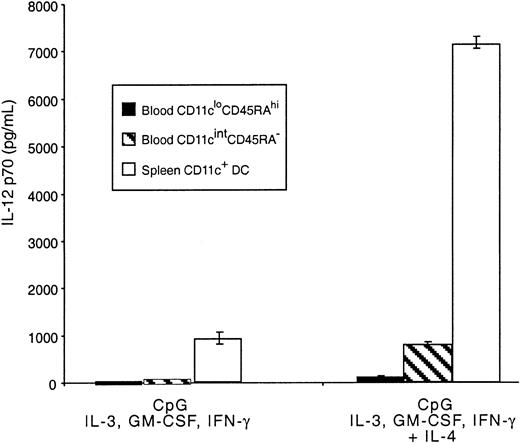
Mouse blood pre-DCs were also tested for the production of IL-12 in response to CpG and the mixture of cytokines previously shown to lead to optimal production of the bioactive form of this cytokine.27 The response was compared with that of freshly isolated splenic CD11c+ DCs. The CD11cintCD45RA− population produced IL-12 p70, though at levels substantially lower than total mature splenic CD11c+ DCs (Figure 7) and, therefore, at very much lower production than the 20% CD8+splenic DCs that account for most splenic DC IL-12 p70.27Levels of IL-12 p70 produced by these cells were more comparable to the levels produced by DCs from the thymus.26 Very low, but still detectable, levels of IL-12 p70 were also produced by the CD11cloCD45RAhi population. As with mature DCs, IL-12 p70 production induced by CpG in both pre-DC populations was much higher when IL-4 was included in the cytokine mix.27,31Although IL-4 alone reduced the viability of the CD11cloCD45RAhi cells, no loss of viability was evident in the presence of CpG, GM-CSF, or IL-3. These results support the correspondence between mouse CD11cintCD45RA− and human blood–derived pre-DC1, which have been shown to produce similar levels of IL-12 p70,27,31 32 indicating a marked quantitative difference in IL-12 p70 production compared with mouse spleen CD8+DCs.
IL-12 production by mouse blood pre-DCs.
Sorted populations of mouse blood pre-DCs or purified mouse spleen DCs (0.5 × 106/mL) were stimulated in culture with CpG, IFN-γ, IL-3, GM-CSF, with or without IL-4. Supernatants were harvested after 36 hours then analyzed by ELISA for IL-12 p70 production. Data are from one experiment; error bars represent the range of replicate samples. Similar results were obtained in 3 experiments.
IL-12 production by mouse blood pre-DCs.
Sorted populations of mouse blood pre-DCs or purified mouse spleen DCs (0.5 × 106/mL) were stimulated in culture with CpG, IFN-γ, IL-3, GM-CSF, with or without IL-4. Supernatants were harvested after 36 hours then analyzed by ELISA for IL-12 p70 production. Data are from one experiment; error bars represent the range of replicate samples. Similar results were obtained in 3 experiments.
Discussion
We have identified 2 major populations of pre-DCs in mouse blood, together with a minor group of immature DCs. This allows, for the first time, a direct comparison of the DC-lineage cells in human and mouse blood. It is now clear that similar populations of pre-DCs are found circulating in both species and that they have similar biologic functions.
The CD11cintCD45RA−CD11b+ mouse blood pre-DC subset identified in this study displays similarities to the human CD11c+ pre-DC1 monocytes. These murine cells, like their human counterparts, express myeloid markers, differentiate into mature DCs in culture in response to GM-CSF and TNF-α, and do not produce IFN-α, but they are able to produce moderate amounts of IL-12 p70 in response to microbial stimulus. Mouse blood, like human blood, contains more developed DCs that may be downstream forms of these pre-DCs. However, unlike the analogous human population, the CD11cintCD45RA−CD11b+ pre-DCs do not express CD4, nor do they express CD8α, found on subsets of murine lymphoid tissue but not found on any human DCs or pre-DCs.
The CD11cloCD45RA+ pre-DC subset of mouse blood is clearly analogous to the human plasmacytoid pre-DC2 subset. The lack of myeloid markers, the morphology, the differentiation into mature DCs under the influence of IL-3 and CpG rather than GM-CSF and TNF-α, and, above all, the production of high levels of type 1 IFN in response to CpG identifies this murine cell population as the equivalent of the human plasmacytoid cell.
There are some differences between mouse blood plasmacytoid pre-DCs and human blood plasmacytoid pre-DCs, but the apparent species differences diminish if mouse lymphoid tissue plasmacytoid pre-DCs are included in the comparison. We and others33-36 have analyzed the plasmacytoid pre-DCs from mouse spleen, thymus, and lymph nodes, and their basic properties and surface phenotype are similar to those in mouse blood. Both are CD45RAhiCD11clow/int, and both produce type 1 IFN and transform into CD8+ DCs when stimulated by CpG. However, many of the mouse lymphoid tissue forms of this cell, in contrast to those in blood, express CD4 and low levels of IL-3R. Many of the mouse lymphoid tissue forms are, therefore, closer to human plasmacytoid pre-DCs, which express CD4 and IL-3R. However, apart from CD4, many lymphoid tissue plasmacytoid pre-DCs also express CD8, a molecule not found on the surfaces of human or mouse plasmacytoid pre-DCs.
It is of note that the human blood pre-DC populations appear to express comparatively higher levels of MHC class 2 than the mouse blood pre-DCs we have isolated. In addition, human blood pre-DC2 cells do not require an added microbial stimulus, such as CpG, for DC differentiation in the presence of IL-3; they also express higher levels of IL-3R. Furthermore, there are several reports of a low-level T-cell stimulation by human blood pre-DCs, particularly pre-DC1,37,38 whereas the mouse pre-DC subsets we have isolated require maturation in culture before detectable T cell stimulation is observed in MLR. We have also found that lymphoid tissue plasmacytoid pre-DCs require activation by bacterial products before they can induce the stimulation of allogeneic T cells in MLR.36 All this suggests that human blood pre-DCs are slightly more mature or activated than are mouse pre-DC subsets. This may be a species difference, but it might also indicate a difference between the pathogen-free environment of our laboratory mice compared with a level of exposure to microbial stimuli for the human blood donors.
The relationship between the pre-DC populations we have found in mouse blood and the mature DC subtypes in mouse lymphoid tissue must now be considered. The CD11cintCD45RA−CD11b+ pre-DCs produce CD4−CD8−CD11b+ mature DCs in culture, and the simplest interpretation would be that these are pre-DCs that are the direct precursors of myeloid-type CD4−CD8−CD11b+ found in mouse spleen and lymph nodes. The CD11cloCD45RA+CD11b− pre-DCs produce CD4−CD8+CD11b− DCs in culture, and the simplest interpretation would be that these are the direct precursors of the CD4−CD8+CD11b− DCs previously termed the lymphoid DCs, found in mouse thymus, spleen, and lymph nodes. However, such a direct precursor-product relationship cannot be assumed. Randolph et al39 have suggested that the normal progeny of the monocyte-derived DCs may be infrequent in normal mice—a phagocytic stimulus within tissues might be required to induce progeny. In a similar vein, our culture studies suggest a microbial stimulus such as CpG may be required to produce DC progeny from the plasmacytoid CD11cloCD45RA+ precursors.
The large number of mice required makes extensive precursor-product studies on mouse blood pre-DCs an impractical enterprise. Our expensive and tedious studies on mouse blood have, however, served the purpose of demonstrating the close relationship of the DC systems of the 2 species, provided the same source material and similar direct isolation procedures are used in the investigation. Treating mice with DC poietins, such as Flt3-L, may prove to be a useful technique to further study the pre-DCs of mouse blood using fewer mice. Apart from increasing DC numbers, treatments with cytokines such as Flt3-L do alter some of the biologic properties of DCs28 and so it is necessary that the basic properties of pre-DCs in the normal mouse be understood first. We and others33,35 36 have found a more plentiful source of mouse plasmacytoid pre-DCs in bone marrow, thymus, spleen, and lymph nodes. We are currently using these sources in more direct studies of DC precursors to DC product relationships in vivo.
Prepublished online as Blood First Edition Paper, October 10, 2002; DOI 10.1182/blood-2002-03-0974.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Meredith O'Keeffe, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, Victoria 3050, Australia; e-mail:okeeffe@wehi.edu.au.