Unrelated donors are commonly used for hematopoietic stem cell transplants, but graft-versus-host disease (GVHD) is a major problem. We investigated whether transplantation of purified mobilized peripheral-blood CD34+ stem cells from unrelated donors would prevent acute and chronic GVHD in pediatric patients with leukemia and avert the need for pharmacologic immunosuppression. Thirty-one pediatric patients with acute lymphoblastic leukemia (ALL, n = 16), acute myeloid (n = 7), chronic myeloid (n = 6), or juvenile myelomonocytic leukemia (n = 2) underwent transplantation. The median purity of CD34+ cells after positive magnet-activated cell sorting was 98.5%. Patients received a median of 8.0 × 106 CD34+ cells and 6 × 103 CD3+ T lymphocytes per kilogram, with no posttransplantation pharmacologic immunosuppression. Primary acute GVHD ≥ grade II was seen in only 10% of patients (n = 3) and occurred only after human herpesvirus 6 (HHV 6) infection. Two patients had limited chronic GVHD. Engraftment occurred in all patients (primary engraftment, n = 26; engraftment after reconditioning, n = 5). The 2-year survival estimate was 38% for all patients and 63% for patients with ALL in complete remission. Patients with myeloid malignancies had a poor outcome. In comparison to a historical control group who received unmanipulated bone marrow, our patients had a lower incidence of GVHD (P < .001). No difference was observed in the probability of relapse or survival. Study patients with ALL in remission showed a trend toward better survival (P = .07). Transplantation of purified peripheral-blood CD34+ cells from unrelated donors effectively minimizes GVHD and may be a good therapeutic option for patients with relapsed ALL.
Introduction
Childhood leukemias such as acute lymphoblastic leukemia (ALL) in second or subsequent remission,1,2high-risk or relapsed acute myeloid leukemia (AML),3,4 and chronic myeloid leukemia (CML)5,6 are increasingly treated by hematopoietic stem cell transplantation. Because only 30% of patients have a histocompatible sibling, alternative donors must be chosen for many patients.7-9 Although closely matched or completely matched unrelated donors are sought, graft-versus-host disease (GVHD) and early posttransplantation toxicity are major problems.10-12 Pharmacologic prophylaxis with cyclosporine and methotrexate can reduce the risk of severe GVHD from 50% to 24%,13 and ex vivo depletion of donor T cells can further decrease the risk of GVHD.14-19 It has not been conclusively established whether T-cell depletion can improve leukemia-free survival. Some investigators have observed higher rates of graft failure and leukemic relapse with T-cell–depleted grafts,20-25 but clinical results are influenced by the depletion methods used26 and thus must be interpreted cautiously. Techniques differ in the efficacy of depletion, the recovery of progenitor cells, the specificity of antibodies used (T-cell–specific antibodies versus antibodies that can also target other immune cells), and the mechanics of cell separation (centrifugation, agglutination, or immunomagnetic separation).26 Recent progress in immunomagnetic separation techniques allows the positive selection of CD34+ cells and the reproducible and profound depletion of T and B cells.27
Because transplantation of CD34+ progenitors from mismatched family donors has produced encouraging results,28 29 we evaluated whether transplantation of CD34+ stem cells from the peripheral blood of unrelated donors would prevent acute and chronic GVHD and eliminate the need for pharmacologic immunosuppression without increasing the risk of graft failure.
Patients and methods
Thirty pediatric patients and one young adult with high-risk leukemia who received consecutive transplants of purified CD34+ stem cells from unrelated donors at our institution between January 1997 and December 2000 were included in this study and compared to a historical control group who received transplants of unmanipulated bone marrow from matched unrelated donors (MUD; Table 1). Informed consent was obtained from the legal guardians or patients.
Age ranged from 1.5 to 24 years and body weight from 9.6 to 98 kg. Sixteen patients had ALL, 7 had AML, 6 had CML, and 2 had juvenile myelomonocytic leukemia (JMML). Of the 23 patients with relapsed ALL or AML, 16 were in second (n = 15) or third (n = 1) remission after treatment on the German ALL-BFM REZ95 and AML REZ protocols. Of the 23 patients, 4 were considered for transplantation during first remission (cases of ALL that responded poorly to prednisone or high-risk AML), 3 patients were not in remission at the time of transplantation, 5 of the 6 patients with CML were in chronic phase 1 or 2, and one had accelerated disease. Neither child with JMML was in remission.
The histocompatibility of patient and donor was determined by serologic (HLA-A and -B) and high-resolution molecular (HLA-DRB1) typing methods. Of 31 patient-donor pairs, 15 were completely matched for HLA-A, -B, and -DRB1, and 16 were mismatched for 1 or 2 antigens (3 HLA-A mismatches, 4 HLA-B mismatches, 6 HLA-DRB1 mismatches, and 3 HLA-A or -B and HLA-DRB1 mismatches; Table 1). All mismatches were assessed as split-antigen or molecular mismatches.
Stem cell mobilization and selection of CD34+progenitors
Thirty patients' donors agreed to donate peripheral-blood stem cells (PBSCs). One patient received enriched CD34+ cells derived from bone marrow. Donor PBSCs were mobilized by administration of 10 μg/kg of granulocyte colony-stimulating factor (G-CSF) daily for 5 days and were harvested by 1 to 2 leukapheresis procedures. We sought to obtain 5 to 10 × 106 CD34+progenitors per kilogram of patient body weight. In 4 patients who experienced graft failure the relevant donors accepted a second mobilization. CD34+ stem cells were selected by using a modified semiautomated magnetic-activated cell sorting (MACS) technique (SuperMACS, Miltenyi Biotec, Bergisch Gladbach, Germany) as previously described27 or by using the automated CLINIMACS device (Miltenyi Biotec).30 Because T cells were efficiently depleted by the positive selection of CD34+ cells, no additional in vitro–depletion step was necessary. The enriched grafts were analyzed by fluorescence-activated cell sorting (FACS) on FACSscan or FACScalibur instruments (Becton Dickinson, Munich, Germany). Debris, dead cells, cell aggregates, and platelets were excluded by gating on forward and side light scatter and subsequently on CD45+, propidium iodide–negative cells. Cell viability was assayed by trypan blue dye exclusion. The cell suspensions (median volume, 50 mL) were infused within a 3-minute time period via a central venous catheter.
Treatment protocol
The myeloablative conditioning regimens were based on either total body irradiation (TBI, 6 fractions of 2 Gy each) or busulfan (16 mg/kg for age > 3 years and 20 mg/kg for age < 3 years), with specific modifications according to patients' pretreatment, diagnosis, and age. Fourteen patients with ALL received TBI, etoposide (40 mg/kg), rabbit antithymocyte globulin (ATG, 10 mg/kg daily for 3 days), and either thiothepa (10 mg/kg) or cyclophosphamide (120 mg/kg). Two children with ALL received busulfan combined with etoposide (40 mg/kg), cyclophosphamide (120 mg/kg), and ATG (30 mg/kg). Five patients with CML received TBI, thiotepa (10 mg/kg), cyclophosphamide (120 mg/kg), and ATG (30 mg/kg). One patient with CML received busulfan, thiotepa, cyclophosphamide, and ATG. Six patients with AML received busulfan, melphalan (140 mg/m2), cyclophosphamide, and ATG. One patient with chemotherapy-induced pretransplantation aplasia underwent a reduced conditioning regimen (thiotepa, cyclophosphamide, fludarabine, and ATG). The 2 patients with JMML received busulfan, cyclophosphamide, and ATG with either thiotepa (n = 1) or melphalan (n = 1).
In the case of graft failure, an 18-day immunologic reconditioning protocol consisting of murine monoclonal anti-CD3 antibody OKT 3 (0.1 mg/kg) and methylprednisolone (maximum dosage, 5 mg/kg, subsequently tapered) was administered as previously described.31Alternatively, a fludarabine-based regimen (fludarabine 200 mg/m2, cyclophosphamide 40 mg/kg, and rabbit ATG 30 mg/kg) was given. CD34+ cells from the same donor were then infused. With the exception of a short course of cyclosporine given to the first patient, no posttransplantation immunosuppressive therapy was administered.
Assessment of engraftment, chimerism, and immune reconstitution
The day of engraftment was defined as the first of 3 consecutive days on which the absolute neutrophil count (ANC) was > 0.5 × 109/L. Chimerism was assessed weekly in peripheral blood samples until day 100 and was then assessed every 3 months. Reconstitution of CD3+, CD4+, CD8+, CD19+, and CD56+ lymphocytes was monitored weekly by FACS analysis until T-cell recovery began and was subsequently assessed every 3 months.
Supportive care
All patients received prophylactic acyclovir, fluconazole, co-trimoxazole (trimethoprim-sulfamethoxazole), and metronidazole. Immunoglobulin was given intravenously each week until day 100. G-CSF was administered to most patients. Patients who were positive for cytomegalovirus (CMV) or for whom a CMV-negative donor could not be found received high-dose acyclovir. Polymerase chain reaction (PCR) screening for CMV, herpes simplex virus, human herpesvirus 6 (HHV 6), and Epstein-Barr virus (EBV) was performed weekly. Adenovirus surveillance comprised antigen detection in stool and, if positive, PCR testing of blood. Additional antiviral treatment was started immediately in the case of positive PCR findings.
Statistical analysis
The collected data were transferred to the Institute for Medical Information Processing, University of Tuebingen, for further analysis. The probability of estimators for survival and relapse-free survival were evaluated by the method of Kaplan and Meier.32 A univariate analysis of prognostic factors was performed by using the log-rank test. Risk ratios and multivariate analysis of survival were evaluated by Cox regression after verification of the proportional hazard assumption by log-minus-log plots. Prognostic factors for the occurrence of GVHD and engraftment were analyzed by logistic regression33 or by Fisher exact test.
The following factors were included in the analysis: age (< 11 versus ≥ 11 years), donor mismatch (no mismatch versus 1- or 2-antigen mismatch), diagnosis (ALL vs AML vs CML or JMML), disease status at the time of transplantation (remission vs active disease), number of contaminating T cells (≤ 6000/kg body weight vs > 6000/kg body weight), number of infused CD34+ stem cells (< 10 × 106/kg body weight vs ≥ 10 × 106/kg body weight), and type of graft (unmanipulated bone marrow vs purified CD34+ stem cells). All of these factors were tested and found to have no colinearity or interaction. Differences between the test and control groups in age, disease status, diagnosis, and number of antigen mismatches were examined by the chi square test and Fisher exact test. All tests were performed by using SAS software (Version 8; SAS Institute, Cary, NC).
Results
Graft composition
The patients received a median of 8.0 × 106CD34+ progenitor cells per kilogram of body weight (range, 1 × 106 to 54 × 106 cells). The median purity of the CD34+ cells after separation was 98.5%, and the viability of the cells was consistently > 95%. The extent of T-cell depletion was approximately 5 logs, and the median number of residual T cells was 6000 cells/kg (range, 500 to 34 000 cells/kg) (Table 1).
Engraftment
Primary engraftment occurred in 26 patients (84%). The median time to an ANC > 0.5 × 109/L with G-CSF stimulation was 11 days (range, 9 to 20 days) (Table2).
Five patients (16%) had primary graft failure (rejection, n = 3; nonengraftment, n = 2). However, successful engraftment was achieved in all of these patients after reconditioning with steroids and OKT 3, or with fludarabine, cyclophosphamide, and ATG, and subsequent reinfusion of CD34+ progenitors from the original donors (n = 4) or from another unrelated donor (n = 1). Thus, all patients were successfully engrafted. A univariate analysis revealed no association between engraftment and age, diagnosis, or number of CD3+ and CD34+ cells in the graft. However, no graft failure occurred when 10 × 106 or more CD34+ cells per kilogram were infused. HLA mismatch significantly influenced engraftment: all patients with a matched donor experienced primary engraftment, and graft failure occurred only in the mismatched group (P = .04) with an incidence of 31% (5 of 16). These 5 patient-donor pairs showed one (4 pairs) or 2 (1 pair) split antigen or molecular mismatches (A31 vs A32; B49 vs B50; DRB1 1101 vs 1104; DRB1 1101 vs 1104; B54 vs B55, and DRB1 1401 vs 1405).
Graft-versus-host disease
Primary acute GVHD.
Of 30 evaluable patients, 23 (77%) showed no symptoms of primary acute GVHD. One patient who received donor T cells on day 0 could not be evaluated for primary GVHD; 4 patients (13%) experienced grade I GVHD; 3 patients who had acute HHV 6 infection had grade II GVHD (n = 1) or grades III or IV GVHD (n = 2) at the beginning of T-cell recovery, 90 to 120 days after transplantation. Because no other patients had severe GVHD, the HHV 6 infection was the apparent cause of GVHD in these patients. The incidence of acute GVHD ≥ grade II was 10% (3 of 30). A univariate analysis revealed no relationship between GVHD and HLA mismatch, number of residual T cells, age, or diagnosis.
GVHD after infusion of donor T cells.
One patient (ALL, active disease) experienced grade II GVHD after an infusion of donor T cells (50 000 cells/kg body weight), which was given on day 0; 2 patients who showed no sign of primary GVHD received donor T cells 1 to 3 months after transplantation; 1 patient received 5 × 25 000 cells/kg because of increasing mixed chimerism. The other received 3 infusions of 50 000, 1 × 106, and 1 × 107 donor T cells/kg because of leukemic relapse. Both patients subsequently experienced grade IV GVHD.
Chronic GVHD.
Patients were considered evaluable for chronic GVHD if they engrafted and survived for 100 days; 2 of 27 patients (7%) had transient and limited chronic GVHD.
Survival
As of February 2002, 11 of the 31 patients survived free of disease, with a median follow-up of 2.8 years (range, 1.3 to 5 years). One patient who had a relapse after transplantation entered a third complete remission after treatment with a mild chemotherapy regimen of prednisone, vincristine, and asparaginase and several infusions of donor T cells. Eighteen patients had died of relapse (n = 8) or infection (viral, n = 5; fungal, n = 2; bacterial, n = 1; unknown origin, n = 2). One patient died of HHV 6–associated GVHD. No other transplantation-related complications, such as EBV lymphoproliferative disease or veno-occlusive disease, were seen (Table 2).
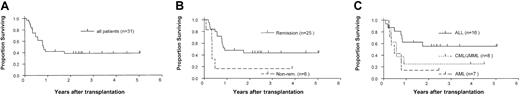
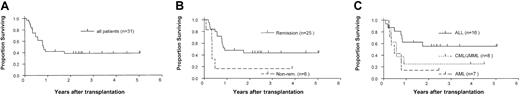
The overall 2-year survival estimate was 38% (Figure1A). Age, HLA mismatch, and number of CD34+ and CD3+ cells infused did not influence survival, whereas remission status (P = .03) and diagnosis (P = .06) were predictive. Of the 6 patients who had active disease at the time of transplantation, 5 died. Only one patient in this group (CML in accelerated phase) survived at the time of this report. Therefore, the probability of 2-year survival was lower in the group with active disease (17%) than in the group in remission (44%;P = .03) (Figure 1B). Patients with AML and CML/JMML also had poorer outcomes (2-year survival estimates of 14% and 25%, respectively) than patients with ALL (56%) (Figure 1C). The largest patient group (ALL in remission, n = 14) had a 63% probability of 2-year survival, whereas both patients with active ALL died.
Survival of patients in the study group.
(A) Kaplan-Meier estimate of the probability of survival for all patients who received positively selected peripheral-blood CD34+ cell grafts. (B) The probability of survival according to remission status at the time of transplantation. (C) The probability of survival according to type of leukemia.
Survival of patients in the study group.
(A) Kaplan-Meier estimate of the probability of survival for all patients who received positively selected peripheral-blood CD34+ cell grafts. (B) The probability of survival according to remission status at the time of transplantation. (C) The probability of survival according to type of leukemia.
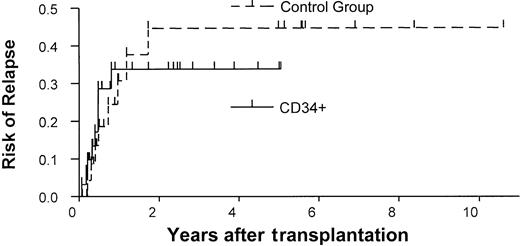
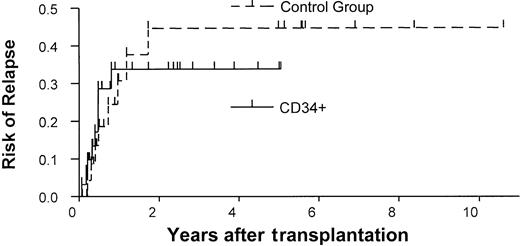
Relapse
The overall probability of relapse at 1 year was 34% in the study group (Figure 2). In a univariate analysis, remission status was predictive in the study group. Patients who were not in remission at the time of transplantation had a risk of relapse 6 times that of patients who were in remission at the time of transplantation (P = .003, log-rank test). Age also influenced the probability of relapse. Patients younger than 11 had a higher risk of relapse than did older patients (risk ratio = 7.7,P = .02). In a multivariate analysis, P values for remission status (P = .05) and age (P = .11) were not confirmed. However, high risk ratios of 3.8 and 5.6, respectively, were found again.
Rates of relapse in the study and control groups.
Kaplan-Meier estimates of the probability of relapse in the CD34+ transplant group versus the control group (unmanipulated bone marrow transplants).
Rates of relapse in the study and control groups.
Kaplan-Meier estimates of the probability of relapse in the CD34+ transplant group versus the control group (unmanipulated bone marrow transplants).
Comparison with a historical control group
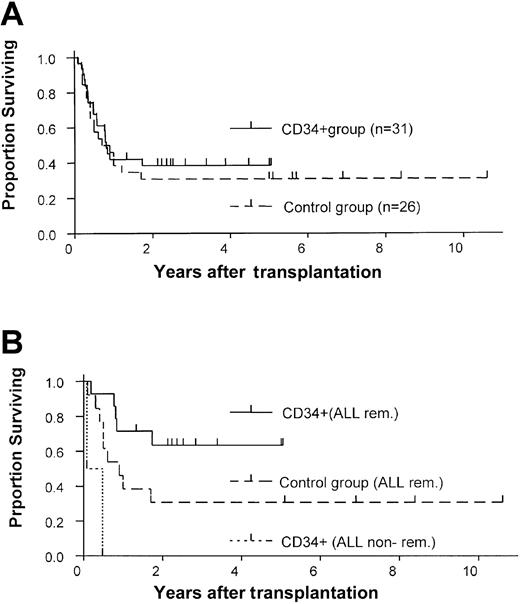
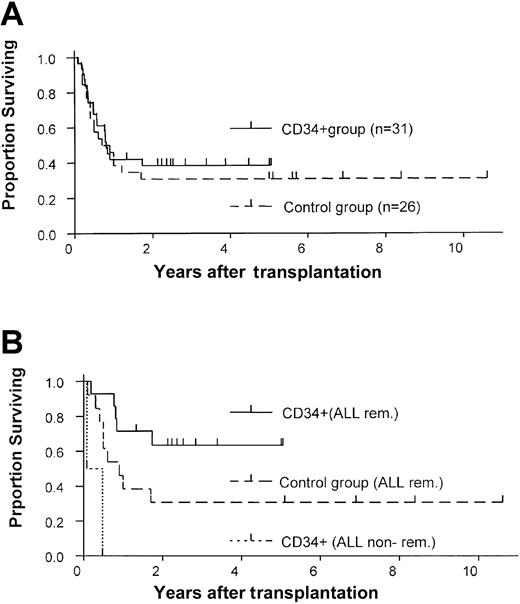
The results of our study group were compared with those of a historical control group treated at our institution (Table 2). The control group comprised 26 patients who consecutively underwent transplantation between 1990 and December 2000 with unmanipulated bone marrow from matched unrelated donors and who received GVHD prophylaxis consisting of cyclosporine and methotrexate. No patients were excluded. No differences were found between the study and control groups in age, diagnosis, and remission state. The CD34+ group included more patients with HLA-mismatched donors (n = 16) than did the control group (n = 1; P < .001). However, the likelihood of 1-year survival was similar in the 2 groups (38% vs 31%), and the CD34+ group had a slight survival advantage (Figure 3A). Similarly, no difference was observed between the 2 groups in the 1-year probability of relapse (34% vs 31%; Figure 2). Primary engraftment was observed in 84% of patients in the CD34 group compared with 100% in the control group (P = .06).
Comparison of survival estimates in the study and control groups.
Kaplan-Meier estimates of survival for (A) the entire study and control groups and (B) only for ALL patients who received CD34+transplants or unmanipulated bone marrow transplants while in remission.
Comparison of survival estimates in the study and control groups.
Kaplan-Meier estimates of survival for (A) the entire study and control groups and (B) only for ALL patients who received CD34+transplants or unmanipulated bone marrow transplants while in remission.
A lower probability of death from GVHD was observed in the CD34+ group than in the control group (risk ratio, 7.2;P = .03). The incidence of acute GVHD ≥ grade II was lower in the CD34+ group (10% vs 77%,P < .001), as was the incidence of chronic GVHD (7% vs 41%). Patients with ALL in remission who received CD34+-enriched grafts showed a trend toward a survival advantage over similar patients in the control group (63% versus 31% 2-year survival estimate; P = .07) (Figure 3B). The 2 patients with ALL not in remission at time of transplantation died.
Immune reconstitution
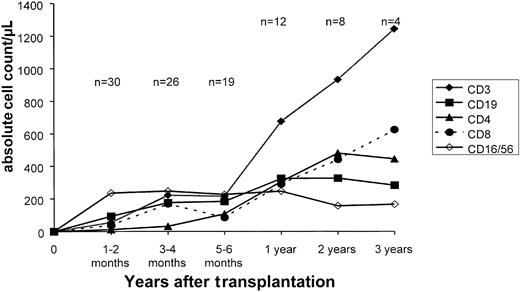
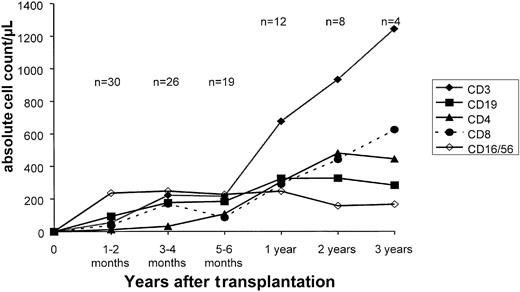
Figure 4 shows the recovery of subsets of immune cells in the study group. A median of 154 days was required to reach a CD3+ cell count > 100/μL, and a median of 185 days was required to reach a CD3+/CD4+ T helper cell count > 100/μL. Patients' T-cell counts were completely normal after one year. However, one patient developed a severe T-cell deficiency because of a chronic varicella zoster infection and died after 1.5 years. In contrast, the recovery of CD56+ natural killer (NK) cells was rapid, and a clear NK cell peak was observed only 1 to 2 months after transplantation. B-cell recovery was not delayed in most patients, although a sustained deficiency of CD19+ cells occurred in one patient. This patient also developed a pure red cell aplasia 2 years after transplantation.
Immune reconstitution.
Reconstitution of CD3+, CD4+, CD8+, CD19+, CD16+/CD56+ T, B, and NK cells after transplantation of CD34+ cells. Points represent the mean values at each time point.
Immune reconstitution.
Reconstitution of CD3+, CD4+, CD8+, CD19+, CD16+/CD56+ T, B, and NK cells after transplantation of CD34+ cells. Points represent the mean values at each time point.
Discussion
Transplantation of purified peripheral-blood CD34+cells from unrelated donors effectively minimized acute and chronic GVHD in our study group. Moderation of this side effect and its direct and indirect sequelae is essential to improvement of the outcome of transplantations using matched unrelated donors. Removal of T lymphocytes from the graft is a reliable method for preventing GVHD, and a number of negative selection techniques for T-cell depletion have been described and evaluated in clinical trials.17,18,34-43 However, most of these techniques cannot deplete T cells sufficiently to eliminate the need for posttransplantation pharmacologic immunosuppression. In addition, transplantation with T-cell–depleted grafts is associated with a higher incidence of posttransplantation EBV-associated lymphoproliferative syndrome44-47 and a high incidence of nonengraftment or rejection.16 17
Our method, unlike others, depletes T and B cells indirectly by positive selection of peripheral-blood CD34+ cells. We have shown previously that CD34+ stem cells can be purified to a high degree (98%) by this method, with a CD34+ cell recovery of approximately 70%27 30 and a very low proportion of residual T and B lymphocytes. In the present study, a median of 8.0 × 106 (range, 1 to 54 × 106) CD34+ stem cells per kilogram were transplanted, and a median of only 6 × 103 (range, 0.5 to 34 × 103) residual T cells per kilogram were co-infused. In addition, the use of antithymocyte globulin for rejection prophylaxis in our conditioning regimens also may result in a further in vivo T-cell depletion. Contaminating B lymphocytes comprised < 0.5% of cells in the grafts (data not shown).
The effective reduction of T cells in the grafts allowed us to omit all posttransplantation pharmacologic immunosuppression and maintain a very low incidence of GVHD. Of 30 evaluable patients, 23 (77%) remained free of GVHD and 4 had grade I GVHD (13%). Late-onset GVHD in 3 patients (grades II-IV) was probably triggered by acute HHV 6 infection; GVHD began between days 90 and 120 after transplantation, at the time of T-cell reconstitution. Other investigators have reported that HHV 6 can play a role in the initiation and exacerbation of GVHD also after transplantation of unmanipulated bone marrow.48,49 The incidence of primary grades II-IV GVHD (10%, 3 of 30) was significantly lower than that in our historical control group. Importantly, only 2 patients experienced chronic GVHD, and these cases were limited and transient. In a study comparing peripheral stem cells to bone marrow using unrelated donors for transplantation in adult patients, the incidence of acute GVHD grades II-IV and chronic GVHD in patients receiving transplants of unmanipulated bone marrow was 32% and 76%, respectively.50 In this study, the incidence of acute GVHD was lower and chronic GVHD was higher than in our historical control group. Indeed, chronic GVHD and its long-term sequelae, especially in pediatric patients, is a major problem in matched unrelated transplantations, which can be addressed by using positively selected CD34+ stem cells without compromising the overall outcome.
Two children experienced severe GVHD after receiving donor T-cell infusions. One patient with JMML received consecutive infusions of 50 000, 1 × 106, and 1 × 107 T cells/kg because of a leukemic relapse. Although remission was induced, the patient died of grade IV GVHD. The second patient received 3 infusions of only 25 000 cells/kg because of increasing mixed chimerism. She developed severe GVHD, probably because of reactivation of CMV infection, despite these extremely low T-cell doses. Therefore, the alloreactive potential of even small T-cell infusions was not predictable, and we have not identified a safe T-cell dose to date. For that reason, we did not routinely administer donor T-cell infusions to induce a graft versus leukemia effect.
None of our patients developed EBV-associated lymphoproliferative disease, despite the extensive T-cell depletion of the graft and the use of antithymocyte globulin, both of which are considered to be risk factors.44 In contrast, between 14% and 29% of recipients with unrelated or mismatched related donors have been reported to experience this serious side effect when T cells are directly depleted without removal of B cells by methods such as E-rosetting or the use of anti-CD6 antibodies.45-47 This disparity is likely to reflect the indirect depletion of B lymphocytes by our one-step positive selection of CD34+ cells, which considerably reduces the number of co-infused B cells that may harbor EBV.
Primary engraftment was achieved in 26 patients (84%), with a median of 11 days required to reach an ANC > 0.5 × 109/L. Three patients experienced graft rejection, and 2 experienced nonengraftment. There was an increased risk of primary graft failure as compared to that of our control group, a finding also observed in other T-cell–depletion studies.16,17 In a univariate analysis, only HLA mismatch was negatively associated with engraftment. However, graft failure occurred only in patients who received a stem cell dose < 10 × 106/kg. Therefore, as observed also in the 3-loci haploidentical setting, major or minor HLA barriers can be overcome by the tolerance-inducing effect of an increased number (megadose) of transplanted CD34+ stem cells.28Although additional verification is needed, our experience suggests that the optimal dose of matched unrelated-donor CD34+ stem cells is > 10 × 106/kg. For most pediatric patients, this number can be obtained by harvesting peripheral mobilized stem cells rather than bone marrow.
It is important to note that all patients who experienced graft failure or rejection were successfully engrafted after additional immunosuppression with either cyclophosphamide and fludarabine or a course of anti–CD3 antibody OKT-3 and steroids31 followed by reinfusion of purified CD34+ stem cells from the same donor. This high rate of successful secondary engraftment is likely to reflect the lower rate of early transplantation-related toxicity in these patients, who received no immunosuppression, than in patients who receive unmanipulated bone marrow and are given cyclosporine and methotrexate for GVHD prophylaxis. The overall rate of engraftment in our 31 patients was 100%. A primary engraftment of only 69% was achieved in our patients grafted with mismatched stem cells. In order to further overcome the HLA barrier in these patients, an increased CD34+ dose with > 10 × 106 cells/kg and/or an intensified conditioning regimen with the inclusion of fludarabine or adding a short course of an anti-CD3 therapy with OKT 3 and steroids after transplantation, as described for the haploidentical setting,28 may be helpful to avoid reconditioning procedures in the future. Decreasing the nonengraftment/rejection rate in this mismatched setting would increase the availability of the donor pool for a significant number of patients.
Although there was no difference in overall survival between our study and control groups, study patients with ALL who received transplants while in remission had a higher probability of survival than did comparable control patients. While relapse was the most common cause of death in both groups, viral and fungal infections were the second most frequent cause of death in the CD34+ group, whereas organ toxicity and acute and chronic GVHD caused most transplantation-related mortalities in the unmanipulated group. The absence of organ toxicity in the CD34+ group is likely to be associated with the absence of posttransplantation GVHD prophylaxis, as cyclosporine is believed to play a role in its pathogenesis.51
Delayed reconstitution of T lymphocytes caused the higher incidence of viral and fungal infections in the CD34+ group. The absolute numbers of T-cell subsets were low during the first 6 months after transplantation (Figure 4), when essentially all of the infectious episodes occurred. Six months after transplantation, with increasing numbers of circulating T lymphocytes and their subsets, viral and fungal infections became rare and were not life-threatening. The actuarial risk of lethal viral and fungal infections in the CD34+ group was 37%. Patients with AML and CML appeared to have more infections (3 of 7 = 43% and 5 of 8 = 62%) than patients with ALL (2 of 14 = 14%). However, this observation must be interpreted with caution because of the small number of patients. The transplantation of higher doses of purified CD34+ stem cells may hasten immune reconstitution. We have shown that the transplantation of large numbers of CD34+ cells (> 20 × 106/kg) is associated with faster reconstitution of T lymphocytes in recipients of haploidentical grafts than in recipients of grafts from matched unrelated donors.52 It should be mentioned that survivors in our study group had excellent quality of life, being spared the severe late effects of chronic GVHD. Chronic GVHD and its treatment can have a disastrous impact on growth and development, especially in young children. We suggest that prevention of chronic GVHD may be more important in this set of patients than in adults and that the prevention of GVHD in children may counterbalance an increased risk of viral infection.
We found no difference between the study and control groups in the risk of relapse (Figure 2). This finding may be explained by the rapid recovery of CD16+/CD56+ NK cells (Figure 4), which may exert an antileukemic effect in vivo.53 This premise is consistent with the good results we obtained in patients with ALL in remission (63% 2-year survival) and with those of other investigators who observed that T-cell depletion appeared to exert no negative impact in ALL. We also have showed that patients with high posttransplantation NK cell activity have a lower rate of relapse and a better outcome than patients with low posttransplantation NK cell activity.54 Our results were less promising in patients with CML/JMML and AML. In CML, a T-cell–dependent graft versus leukemia effect clearly exists,20,55 and extensive T-cell depletion may not be the optimal strategy for these patients. In AML, it is unclear whether there is an antileukemic effect mediated by NK cells or T cells. An impressive antileukemic effect exerted by alloreactive NK cells against myeloblasts has been described in 3-loci haploidentical transplantation with megadoses of purified CD34+ stem cells.56 Because all of our donors were matched or had minor mismatches, this alloreactive NK cell effect may have been absent in patients with AML. Such patients may benefit from 3-loci haploidentical transplantation, if a donor with alloreactive NK cells can be identified.
An additional advantage of our method is that it provides well-characterized, highly pure grafts that contain precisely known numbers of CD34+ cells with profound depletion of T cells. Such a graft provides an excellent basis for further graft engineering. Selected cell subsets may be added to a basic CD34+ cell graft, such as clearly enumerated T cells, specific T-cell subsets, nonalloreactive T cells, or NK cells.
In summary, the transplantation of purified peripheral CD34+ stem cells from unrelated donors effectively minimizes acute and chronic GVHD, with a high overall rate of engraftment. Despite T-cell depletion, we observed no increase in the frequency of relapse. Children with ALL in remission appeared to derive the greatest benefit from this approach. Relapse and viral or fungal infection were the main causes of death. Although it is not clear how the relapse rate can be decreased, infections may be reduced by intensive screening for viruses and fungi and preemptive antiviral and antifungal therapies. This method may be of clinical importance in transplantation for pediatric patients with ALL, and larger studies in this patient population are warranted.
We thank Olga Bartuli, Christiane Braun, Gabi Hochwelker, and Ulrike Krauter for excellent technical assistance. We thank all the volunteers who donated mobilized peripheral stem cells, especially those who were willing to donate a second time after graft rejection.
Prepublished online as Blood First Edition Paper, October 10, 2002; DOI 10.1182/blood-2002-04-1203.
Supported by a grant from the Deutsche Forschungsgemeinschaft and the German José Carreras Foundation (DJCLS-R3).
P.L. and R.H. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rupert Handgretinger, St Jude Children's Research Hospital, 332 N Lauderdale St, Memphis, TN 38105-2794; e-mail: rupert.handgretinger@stjude.org.