To better understand the relationship between the embryonic hematopoietic and vascular systems, we investigated the establishment of circulation in mouse embryos by examining the redistribution of yolk sac–derived primitive erythroblasts and definitive hematopoietic progenitors. Our studies revealed that small numbers of erythroblasts first enter the embryo proper at 4 to 8 somite pairs (sp) (embryonic day 8.25 [E8.25]), concomitant with the proposed onset of cardiac function. Hours later (E8.5), most red cells remained in the yolk sac. Although the number of red cells expanded rapidly in the embryo proper, a steady state of approximately 40% red cells was not reached until 26 to 30 sp (E10). Additionally, erythroblasts were unevenly distributed within the embryo's vasculature before 35 sp. These data suggest that fully functional circulation is established after E10. This timing correlated with vascular remodeling, suggesting that vessel arborization, smooth muscle recruitment, or both are required. We also examined the distribution of committed hematopoietic progenitors during early embryogenesis. Before E8.0, all progenitors were found in the yolk sac. When normalized to circulating erythroblasts, there was a significant enrichment (20- to 5-fold) of progenitors in the yolk sac compared with the embryo proper from E9.5 to E10.5. These results indicated that the yolk sac vascular network remains a site of progenitor production and preferential adhesion even as the fetal liver becomes a hematopoietic organ. We conclude that a functional vascular system develops gradually and that specialized vascular–hematopoietic environments exist after circulation becomes fully established.
Introduction
A functional circulatory system is an early requirement for survival and growth of the mammalian embryo and is the first organ system to develop in the embryo.1 The circulatory system is composed of vascular, hematopoietic, and cardiac components, each formed from discrete regions of mesoderm. The first endothelial cells and blood cells are generated in yolk sac blood islands beginning at embryonic day 7 (E7.0) in the mouse. By E8.0, thousands of nucleated primitive red blood cells have formed within a vascular plexus in the yolk sac.2-4 Concurrently, the aorta and the peristaltic beating heart tube form in the embryo proper. In the next 36 hours, there is a remarkable increase in complexity of vascular and hematopoietic systems. The vascular plexus remodels and expands into an arborized network of specialized arteries and veins. Primitive erythroblasts from the yolk sac continue to divide and mature, and a second wave of hematopoiesis creating definitive (adultlike) progenitors originates in the yolk sac.5 6 Thus, the early vascular system is the hematopoietic environment for primitive and definitive lineages until the specialized stromal microenvironment of the fetal liver (beginning E10), and later the adult bone marrow, is available. However, the nature of any specific interactions between early embryonic hematopoietic cells and endothelial cells is unclear.
A peristaltic beating heart tube and the connection of the yolk sac and embryo proper vasculature have generally been considered sufficient evidence for functional circulation. This is thought to occur at E8.5 (8-10 somite pairs [sp]).7,8 More recently, unpublished results have been used to contend that hematopoietic progenitors “freely circulate between extra- and intra-embryonic compartments” as early as E8.25 or 5 sp.9 In contrast, we found that definitive hematopoietic progenitors remain almost entirely restricted to the yolk sac as late as E9.25.5This suggests that the circulation is initially very limited or that the yolk sac is a specialized hematopoietic environment despite circulation. Studies reported here present evidence supporting both these possibilities by taking advantage of the initial asymmetry of mammalian blood synthesis to more carefully determine the onset of circulation in staged mouse embryos.
Materials and methods
Embryo dissection and cell dissociation
ICR mice (Taconic, Germantown, NY) were mated overnight, and vaginal plugs were checked the following morning (E0.5). Maternal tissues and Reichert membrane were removed, and the yolk sac was rapidly detached from the embryo proper. For numerical analysis of erythroblasts in E9.0 and later embryos, dissections were performed on concepti in individual dishes while embryonic circulation was intact. Although there was no discernible flow from dissected yolk sacs, blood did continue to flow from the umbilical arteries of E9.5 and older embryos. Accordingly, all blood was collected from the dish and was included in the embryo proper values. Occasional contaminating maternal red blood cells were easily distinguished by size from the primitive erythroblasts and were excluded from cell counts. Tissues were dissociated at room temperature in a 1:30 dilution of 0.25% trypsin (Worthington Biochemical, Lakewood, NJ) in phosphate-buffered saline (PBS)/1 mM EDTA (ethylenediaminetetraacetic acid) with occasional trituration by pipette to aid in digestion. Once samples were dissociated into single cells, an equal volume of Iscove modified Dulbecco medium (IMDM; Gibco BRL, Grand Island, NY)/20% plasma-derived serum (PDS; Animal Technologies, Tyler, TX) was added, and cells were centrifuged and resuspended in IMDM/20% PDS. Total cell numbers and viability were determined by trypan blue staining (Sigma, St Louis, MO).
Hematopoietic progenitor assays
Dissociated cells were plated in methylcellulose, as previously described,5 but IMDM and 0.8% methylcellulose were used with the inclusion of the following cytokines: interleukin-3 (IL-3; 20 ng/mL), IL-6 (20 ng/mL), stem cell factor (SCF; 60 ng/mL), granulocyte macrophage (GM; 2.5 ng/mL) (all from R&D Systems, Minneapolis, MN), and erythropoietin (EPO; 2 U/mL; Amgen, Thousand Oaks, CA). Cultures were incubated at 37°C, 5% CO2. Erythroid–colony-forming units (CFU-Es) were counted after 3 days of culture, and erythroid blast-forming unit (BFU-E) and macrophage–colony-forming unit (CFU-mac) colonies were counted after 7 days of culture. Culturing of high proliferative potential–colony-forming cells (HPP-CFCs) was carried out on agar plates, as previously described.10
In situ hybridization
Embryos were fixed in 4% paraformaldehyde in PBS overnight at 4°C, rinsed with PBS, dehydrated in methanol, bleached with 5% hydrogen peroxide in methanol for 5 hours, and then rinsed and stored in methanol. Digoxigenin-labeled antisense RNA probes corresponding to nucleotides 74 to 421 of βH1-globin mRNA (GenBank accession no.J00417) were synthesized using the DIG RNA Labeling Kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. Whole-mount in situ hybridization was carried out as described by Wilkinson11 with the following modifications: no proteinase K or RNase A treatments were performed, embryos were permeabilized with 3 washes, 10 minutes each, of RIPA buffer (150 mM NaCl, 50 mM Tris, pH 8, 1 mM EDTA, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS])12; hybridization buffer was at pH 4.5 with 0.1% Tween instead of SDS, and probe was added to a final concentration of 0.5 μg/mL. Additionally, posthybridization washes were simplified to 3 washes, 30 minutes each, at 70°C each of solution 1 and solution 3, followed by 3 washes, 5 minutes each, with TBST (0.14 M NaCl, 2.7 mM KCl, 25 mM Tris-HCl, pH 7.5, 0.1% Tween) at room temperature. After nitroblue tetrazolium/x-phosphate 5-bromo-4-chloro-3-indolyl-phosphate (NBT/BCIP) staining, embryos were dehydrated in methanol and rehydrated in TBST to darken the stain. Stained embryos were cleared in 50% glycerol in TBST, followed by 80% glycerol and 0.02% sodium azide in TBST.
Immunohistochemistry
Embryos were fixed and bleached as for in situ hybridization. After rehydration into PBT (PBS, 0.1% Tween 20), embryos were blocked for 2 hours in PBSMT (PBS, 3% instant milk, 0.1% Triton X-100) then incubated overnight at 4°C with 1:200 dilution of rat antimouse platelet endothelial cell adhesion molecule-1 (PECAM-1; BD Biosciences PharMingen, San Diego, CA) in PBSMT. Embryos were washed 3 times for 5 minutes and then 5 times for 1 hour in PBSMT at 4°C. Embryos were then incubated with secondary antibody and washed as for primary antibody. The secondary antibody was either 1:100 dilution of horseradish peroxidase–conjugated goat antirat immunoglobulin G (IgG) or 1:200 dilution of alkaline phosphatase-conjugated goat antirat IgG (both from Jackson ImmunoResearch, West Grove, PA). For horseradish peroxidase detection, embryos were incubated for 20 minutes in 0.3 mg/mL p-dimethylaminoazobenzene (DAB), 0.05% NiCl2 (Vector Labs, Burlingame, CA) in PBT, then developed with the addition of H2O2 to a concentration of 0.03%. The staining reaction was stopped by a PBT rinse. For alkaline phosphatase staining, the embryos were developed and cleared as for in situ hybridization.
Benzidine and o-dianisidine staining of hemoglobin-containing cells
By 5 sp, erythroblast hemoglobin content was sufficient to allow detection by benzidine staining of dissociated cells, as previously described.13 Hemoglobin-containing cells in intact embryos were visualized using o-dianisidine (Sigma) staining performed as described by O'Brien,14 with the following modifications: dissections were performed in PB-1 with 50 mM KCl added to stop the heartbeat.15 Embryos were then rinsed in PB-1, stained in o-dianisidine solution with hydrogen peroxide reduced to 0.15%, postfixed in 4% paraformaldehyde in PBS for 20 minutes, rinsed in PBS, and cleared in glycerol as for in situ hybridization. This method directly detects hemoglobin-containing cells and avoids difficulties associated with the asynchronous decrease in βH1-globin message that occurs in red blood cells beginning at E9 (data not shown).
Results
Red blood cell movement begins in 4 to 6 sp embryos but is extremely limited
Although it is generally assumed that 5 sp (E8.25) embryos demonstrate functional circulation, the onset and extent of blood flow have not been specifically examined. One of the difficulties in determining the timing of developmental events in the early mouse embryo is the variability of developmental stage within a particular embryonic day.16,17 This is caused by 2 factors. First, variability in developmental stages between and even within litters can be extensive. Second, embryos develop rapidly, and profound changes occur within a few hours. Accordingly, we used the number of somite pairs to more precisely and accurately stage individual embryos. Somite pairs are formed in the murine embryo between E8 and E10 at an approximate rate of 1 every 1.8 hours.17 To determine the kinetics of circulation, we took advantage of the fact that definitive hematopoietic progenitors do not produce circulating red blood cells until E12, so early embryo red blood cells are derived from primitive erythroid progenitors confined to the yolk sac.5,6Therefore, circulation could be detected as the redistribution of primitive nucleated red blood cells from yolk sac into the embryo proper. Yolk sac–derived primitive erythroblasts are also distinguished from the fetal liver–derived definitive erythrocytes by expression of embryonic hemoglobin.6 We used in situ hybridization to embryonic-specific βH1 globin mRNA to detect erythroblasts before they accumulated enough hemoglobin to be detected by direct staining by benzidine. Vascular development during embryogenesis was assessed by PECAM immunohistochemistry.18 We chose not to use the earlier marker of endothelial potential, vascular endothelial growth factor receptor-2 (VEGFR-2) (flk-1), because it is also expressed in early progenitors of blood and smooth muscle lineages and is down-regulated in many mature vascular beds.4 19
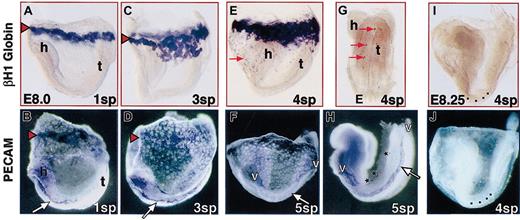
From 0 to 3 sp, erythroblasts are not only restricted to the yolk sac but are present in a narrow band of blood islands forming at the most proximal region (farthest from the embryo proper) of the yolk sac walls (arrowhead, Figure1A,C). We sought to determine whether vascular development in the yolk sac was similarly limited to blood islands. At presomite stages, the first PECAM-expressing cells are in the blood islands (data not shown).4 18 As somatogenesis begins, vascular development remains asymmetric in the yolk sac and is centered at the blood islands (Figure 1B). Simultaneously, angioblasts within the embryo proper begin to differentiate and coalesce into the aortae (Figure 1B, arrow). By 3 sp, vascular development has spread throughout the yolk sac, and extended aortic tubes are visible (Figure 1D); however, red blood cells remain restricted to the blood islands of the proximal yolk sac (Figure 1C). These findings indicate that there are hemangiogenic and angiogenic regions within the yolk sac.
Asymmetric blood formation and first blood flow in early somite pair embryos.
In rodents, the embryo proper develops within the amnionic sac, which itself is within the yolk sac. In this figure, the yolk sac was photographed with the embryo proper inside, except for panels D and I, where the embryo proper was dissected free of amnion and yolk sac. (Top row) Blood flow was monitored by the redistribution of yolk sac–derived primitive erythroblasts detected by in situ hybridization to the embryonic-specific βH1 globin mRNA. (Bottom row) Endothelial development was delineated by PECAM immunohistochemistry. No staining was seen in stage-matched negative controls of in situ hybridization or immunohistochemistry (see panels I and J for examples). (A-B) At 1 sp, red blood cells were restricted to blood islands (red arrowheads) in the proximal region (farthest from the embryo proper), where vascular development was most prominent. The yolk sac vascular plexus centered at the blood islands and the embryo proper aortae (arrow) had begun to form (h indicates head; t, tail). (C-D) By 3 sp, blood was still restricted to the proximal yolk sac, whereas vascular development had spread throughout the yolk sac, and extended aortic tubes were visible (arrow). (E-G) At 4 sp, the first few dispersed red blood cells were observed (red arrow) in the distal yolk sac (E) and in the embryo proper (G). At this stage, a well-developed vascular plexus extended throughout the distal yolk sac (F), forming the vitelline connections (v) with the vasculature of the embryo proper (H). Stars in panel H indicate sections of the forming cardinal vein. Somite pairs in panels I and J are marked with small squares. Original magnification A-J, × 75.
Asymmetric blood formation and first blood flow in early somite pair embryos.
In rodents, the embryo proper develops within the amnionic sac, which itself is within the yolk sac. In this figure, the yolk sac was photographed with the embryo proper inside, except for panels D and I, where the embryo proper was dissected free of amnion and yolk sac. (Top row) Blood flow was monitored by the redistribution of yolk sac–derived primitive erythroblasts detected by in situ hybridization to the embryonic-specific βH1 globin mRNA. (Bottom row) Endothelial development was delineated by PECAM immunohistochemistry. No staining was seen in stage-matched negative controls of in situ hybridization or immunohistochemistry (see panels I and J for examples). (A-B) At 1 sp, red blood cells were restricted to blood islands (red arrowheads) in the proximal region (farthest from the embryo proper), where vascular development was most prominent. The yolk sac vascular plexus centered at the blood islands and the embryo proper aortae (arrow) had begun to form (h indicates head; t, tail). (C-D) By 3 sp, blood was still restricted to the proximal yolk sac, whereas vascular development had spread throughout the yolk sac, and extended aortic tubes were visible (arrow). (E-G) At 4 sp, the first few dispersed red blood cells were observed (red arrow) in the distal yolk sac (E) and in the embryo proper (G). At this stage, a well-developed vascular plexus extended throughout the distal yolk sac (F), forming the vitelline connections (v) with the vasculature of the embryo proper (H). Stars in panel H indicate sections of the forming cardinal vein. Somite pairs in panels I and J are marked with small squares. Original magnification A-J, × 75.
Beginning at 4 sp and later, we observed some embryos with erythroblasts in the distal yolk sac, though they were not in large clusters as seen in proximal blood islands. Instead, dispersed single and small groups of erythroblasts were present within the vascular plexus throughout the distal yolk sac (Figure 1E-F). These observations suggest that primitive erythroblasts form in blood islands and then move into more distal regions of the yolk sac. Coincident with the first appearance of red blood cells in the distal yolk sac, we found a few red blood cells in the embryo proper of some 4 sp embryos (Figure1G). These cells were generally seen in the head and tail regions, where embryonic and yolk sac vasculatures meet anterior to the atrium and at the posterior extreme of the aortae (Figure 1F,H). Occasionally, a few erythroblasts were also observed in the central region of the aortae. Although this represented the beginning of blood flow into the embryo proper, it was an extremely small fraction of the total red blood cells (fewer than 20 of 10 000 red blood cells). Indeed, circulation throughout the embryo proper is not possible because the cardinal vein has not yet formed (Figure 1H, stars).
Blood flow increases only slightly from E8.5 to E8.75
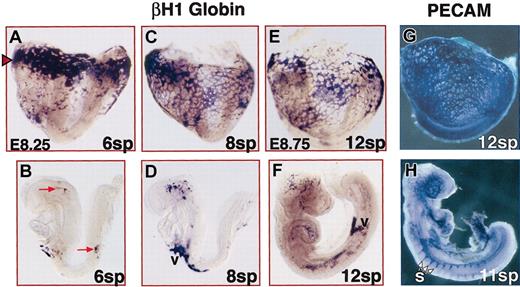
Beginning at 8 sp (E8.5), we observed an increase in numbers of erythroblasts in the head vessels and anterior aortae (compare Figure2B,D). Concurrently, red blood cells were more extensively localized in the distal yolk sac (compare Figure 2A,C). Despite the increase in numbers of red blood cells in the embryo proper from 8 to 12 sp, most red blood cells remained in the yolk sac (Figure 2E-F). Additionally, the distribution of red blood cells within the embryo was limited to the sites that most directly receive blood flow from the heart—head vessels and aortae. Interestingly, PECAM staining demonstrated that vascular development was more widespread than the distribution of red blood cells during this stage. In particular, PECAM staining of intersomitic arteries was present hours before red blood cells were detected (Figure 2H). These data indicate that though red blood cells do move into the embryo proper at early somite stages (4 to 6 sp), for the next 12 hours the early cardiovascular system moves only a small fraction of the total red blood cells to a subset of the developing endothelial network of the embryo.
Limited blood flow in E8.25 to E8.75 embryos.
In the top row, embryos proper and yolk sacs remain attached. In the bottom row, embryos proper were dissected free of extraembryonic tissues. Before 8 sp, an embryo proper is initially curved with its back arched. From 8 to 12 sp, the embryo proper then turns into the more traditional fetal position. Distribution of βH1 globin mRNA-containing erythroblasts was detected by in situ hybridization (A-F), and distribution of PECAM-expressing endothelial cells was detected by immunohistochemistry (G-H). (A-B) At 6 sp, most of the red blood cells were in the yolk sac and particularly concentrated at the proximal edge (red arrowhead). The few erythroblasts (red arrows) in the embryo proper were seen in the head and aorta. (C-D) At 8 sp, more red blood cells were seen throughout the yolk sac and within the aorta and head regions. (E-H) By 12 sp, while most blood was still in the yolk sac, increased numbers of red blood cells were seen in the embryo proper and were found throughout the length of the aortae. However, blood was not seen throughout the entire embryonic endothelial network (compare panels F and H). For example, blood was rarely seen in the intersomitic arteries (s) that were observed at this time. v indicates vitelline connections of yolk sac and embryo proper vasculature. Original magnification A-D, × 75; E-H, × 50.
Limited blood flow in E8.25 to E8.75 embryos.
In the top row, embryos proper and yolk sacs remain attached. In the bottom row, embryos proper were dissected free of extraembryonic tissues. Before 8 sp, an embryo proper is initially curved with its back arched. From 8 to 12 sp, the embryo proper then turns into the more traditional fetal position. Distribution of βH1 globin mRNA-containing erythroblasts was detected by in situ hybridization (A-F), and distribution of PECAM-expressing endothelial cells was detected by immunohistochemistry (G-H). (A-B) At 6 sp, most of the red blood cells were in the yolk sac and particularly concentrated at the proximal edge (red arrowhead). The few erythroblasts (red arrows) in the embryo proper were seen in the head and aorta. (C-D) At 8 sp, more red blood cells were seen throughout the yolk sac and within the aorta and head regions. (E-H) By 12 sp, while most blood was still in the yolk sac, increased numbers of red blood cells were seen in the embryo proper and were found throughout the length of the aortae. However, blood was not seen throughout the entire embryonic endothelial network (compare panels F and H). For example, blood was rarely seen in the intersomitic arteries (s) that were observed at this time. v indicates vitelline connections of yolk sac and embryo proper vasculature. Original magnification A-D, × 75; E-H, × 50.
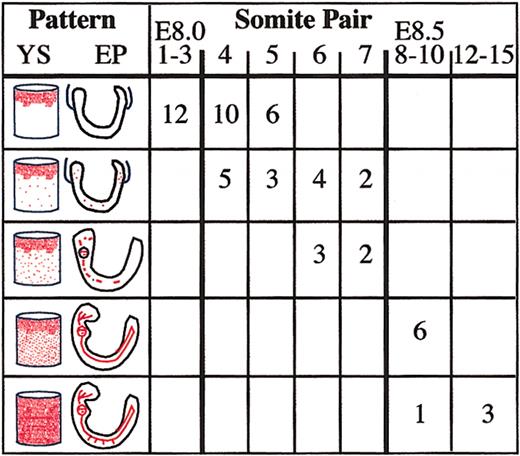
Changes in distribution of red blood cells are precisely temporally regulated
To determine how tightly the timing of the onset of blood flow is regulated in E8 embryos, we compared the distribution of red blood cells in 57 embryos. Embryos were categorized by their spatial patterns of erythroblasts (Figure3, schematics). In the yolk sac, 3 patterns were seen: red blood cells confined to blood islands, occasional dispersed distal cells, and diffuse distribution. Embryos proper were assigned 1 of 5 patterns: no red blood cells, small numbers of red blood cells, hundreds of red blood cells, thousands of red blood cells, and red blood cells within intersomitic arteries. As shown in Figure 3, there was a remarkable correlation of each pattern within a narrow range of somite pair numbers, indicating that the onset of circulation is precisely timed. Beginning with 4 sp embryos and including all embryos at 6 sp (or within 3 hours), small numbers of red blood cells were found in the distal yolk sac and the embryo proper. Because primitive erythroblasts originate in the yolk sac, their presence in the embryo proper indicates that some type of blood flow has begun. Additionally, examination of 25 4 to 6 sp embryos revealed a strict correlation between the presence of erythroblasts in the distal yolk sac and the presence of erythroblasts in the embryo proper. This suggests that the appearance of red blood cells in the distal yolk sac is caused by the same factors that cause the first movement of red blood cells into the embryo proper. All embryos with 8 or more somite pairs had a quantitative increase in numbers of red blood cells in the embryo proper; by 15 sp, red blood cells could be found in the intersomitic arteries closest to the heart. Changes in red blood cell distribution in the embryo proper also correlated with a marked increase in numbers of red blood cells observed in the distal yolk sac. The precision of these transitions suggests an incremental acquisition of circulatory function. Although the entire cardiovascular system continues to mature during E8, the changes in red blood cell distribution correlate well with dramatic changes in cardiac function that occur at 4 to 8 sp. Fusion of the heart primordia, development of myofibrillar bundles, and initiation of contractions occurs at 4 sp. The transition to a looped peristaltic beating heart occurs by 8 sp.7,20 21
Highly regulated timing of initial stages of blood flow in murine embryos.
Individual embryos were divided into 5 groups based on their yolk sac (YS) and embryo proper (EP) red blood cell patterns (indicated in red). The somite pair range of embryos that had each pattern is indicated.
Highly regulated timing of initial stages of blood flow in murine embryos.
Individual embryos were divided into 5 groups based on their yolk sac (YS) and embryo proper (EP) red blood cell patterns (indicated in red). The somite pair range of embryos that had each pattern is indicated.
Increasing circulation within the embryo proper does not achieve a steady-state density of red blood cells until E10
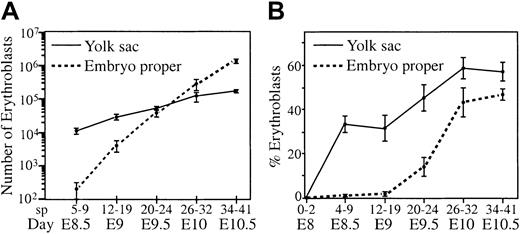
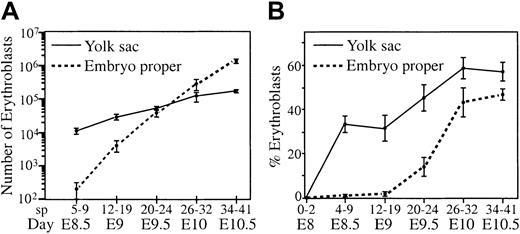
Our in situ analysis indicated that most primitive erythroblasts were retained in the yolk sac even 12 hours after the first movement of red blood cells into the embryo proper. We wanted to determine when a significant proportion of red blood cells moved into the embryo proper. Therefore, total cells and hemoglobin-containing cells were counted from individual concepti separated into embryo proper and yolk sac fractions. Over a 48-hour period, erythroblasts continued to divide and mature within the vascular system, causing an exponential increase in red blood cell numbers in the embryo proper and the yolk sac (Figure4A). There was also a redistribution of red blood cells caused by circulation. At E8.5 (5-9 sp), there were 100-fold more red blood cells in the yolk sac than in the embryo proper. Twenty-four hours later, at E9.5 (20-24 sp), half the red blood cells were in the embryo proper, and by E10.5 (35-40 sp), 10-fold more red blood cells were found in the embryo proper than in the yolk sac (Figure 4A).
Changes in red blood cell distribution and density in the embryo proper and yolk sac.
Embryos were dissected into embryo proper and yolk sac and dissociated into single cells, and total cells and erythroblasts (benzidine positive) were enumerated. (A) Absolute numbers of erythroblasts per tissue were plotted, and (B) percentages of total cells that were erythroblasts were plotted. Error bars indicate SEM (n = 5-13).
Changes in red blood cell distribution and density in the embryo proper and yolk sac.
Embryos were dissected into embryo proper and yolk sac and dissociated into single cells, and total cells and erythroblasts (benzidine positive) were enumerated. (A) Absolute numbers of erythroblasts per tissue were plotted, and (B) percentages of total cells that were erythroblasts were plotted. Error bars indicate SEM (n = 5-13).
We reasoned that a functional end point for the establishment of robust circulation is the provision of a sufficient density of red blood cells to adequately oxygenate tissue. Therefore, we determined the density of red blood cells as the percentage of total cells that were erythroblasts in the yolk sac and embryo proper between E8.0 and E10.5. The density of red blood cells in the yolk sac increased and remained high through E10.5 (Figure 4B), consistent with its highly vascular nature and previous reports of blood cell density in the yolk sac.22 From E8.0 to E9.0, the density of red blood cells in the embryo proper increased only slightly. However, after E9.0, there was a sharp increase in density of erythroblasts from 3% to 40%, reaching equilibrium by E10.0.
Distribution of red blood cells throughout the vascular system of the embryo proper is also delayed until E10.5
In addition to sufficient red blood cell density, functional circulation requires that blood flow in a circular loop throughout the entire vascular system. Throughout E8, erythroblasts were localized only in head vessels and the aortae (Figure 2). We wanted to determine whether the sharp increase in embryo proper red blood cell density, beginning at E9.0, was correlated with erythroblasts now distributed throughout the entire endothelial network. Alternatively, the rapid increase in total red blood cell density from E9 to E10 could be accompanied by the filling of progressively more vascular beds with red blood cells. The latter possibility has intriguing implications for inequities in flow, shear stress, and oxygenation within the vascular system at the time of extensive angiogenesis. We compared the distribution of red blood cells with the vascular pattern in stage-matched embryos to distinguish between these possibilities.
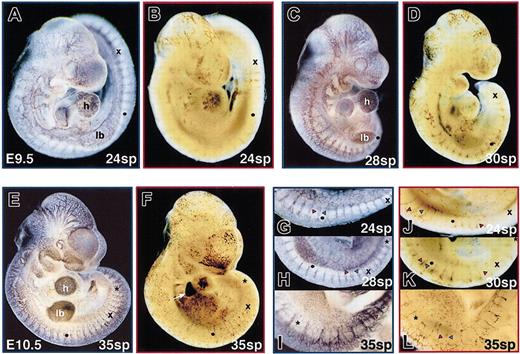
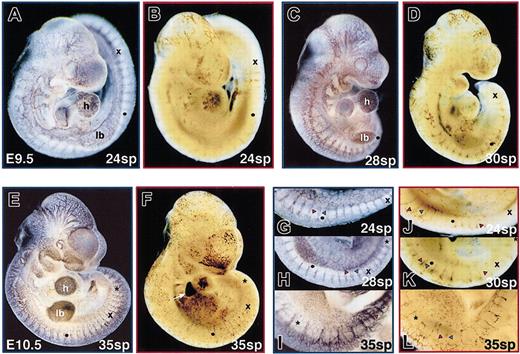
By 24 sp (E9.5), the basic structure of the endothelial system has changed from vascular plexuses connected to large aortae and cardinal veins into an arborized network (Figure5A). The arborized network continues to be elaborated, increasing in complexity through E10.5 (Figure 5C,E). From 25 to 35 sp, the red blood cell pattern also increases in complexity (Figure 5B,D,F). Comparisons of developmentally matched embryos indicated that at 24 sp, the red blood cells were still concentrated in a subset of the available vascular system. However, by 35 sp, the complexities of vascular and blood patterns were comparable. This change was particularly evident along the dorsal regions of the embryo proper. At 12 sp (E8.75), the initiation of angiogenesis was evident as intersomitic arteries branched off the aortae between each somite pair (Figure 2H, arrows). By E9.5 (24 sp), the venous component of intersomitic vessels became evident as more superficial intersomitic veins branched off the cardinal vein and connected to the vertebral arteries at the dorsal edge of the neural tube23 (Figure 5G, red and blue arrowheads). As each somite pair was formed, the new vascular loop was extended such that intersomitic vessels were present between even the most posterior somite pairs. In contrast, red blood cells were not found between the more posterior somite pairs (for example, blood was seen only through 16 sp in a 24 sp embryo; Figure 5J). In more anterior positions, stripes of red blood cells were observed in deep intersomitic arteries and in superficial intersomitic veins (Figure 5J, K, red and blue arrowheads, respectively). However, in the more posterior positions, a single stripe of red blood cells was observed in the deeper intersomitic arteries. By E10.5 (35 sp), erythroblasts were found between all somite pairs, including in the superficial intersomitic veins (Figure 5L). Therefore, blood was not fully dispersed in the endothelial network of the embryo proper until after E10.0. Additionally, there was a restriction of red blood cells to intersomitic vessels closer to the heart at E9.5. This restriction was no longer observed by E10.5. This closely followed the achievement of a plateau in the density of the red blood cells within the embryo proper (Figure 4B). These data suggest that from E9 to E10, there was a maturation of the cardiovascular system required for blood to flow in a complete circuit of the available vascular beds.
Distribution of red blood cells throughout the embryo proper vasculature occurs by 35 sp (E10.5).
The pattern of the embryo proper endothelial and red blood cell distribution was visualized in stage-matched embryos by PECAM immunohistochemistry (A, C, E, G-I) and o-dianisidine staining of hemoglobin (B, D, F, J-L), respectively. To facilitate comparison, the 12th sp is indicated by a dot, the 20th sp by the letter x, and the 25th sp by a star (h indicates heart ventricle; lb, limb bud). (A, C, E) The extensive arborized vascular network evident in the head and ventricle and between the somite pairs at E9.5 (24 sp) became progressively more complex by E9.75 (28 sp) and E10.5 (35 sp). (B, D, F) Red blood cells initially more concentrated in a subset of vessels at 24 sp became more evenly dispersed by 35 sp. Additionally, high levels of hemoglobin-containing cells were seen in the fetal liver (arrow) as it became the primary site of hematopoiesis by E10.5. (G-L) Along the back, paired intersomitic arteries and veins meet at capillary networks of increasing complexity between somite pairs during this time period. Initially, red blood cells were observed in paired intersomitic vessels in the anterior back, at mid positions in deeper arteries only, and not at all between posterior somite pairs. By 35 sp, red blood cells had penetrated intersomitic vasculature throughout the back. Red and blue arrowheads indicate intersomitic arteries and veins, respectively. Original magnifications A-F, × 20; G-L, × 50.
Distribution of red blood cells throughout the embryo proper vasculature occurs by 35 sp (E10.5).
The pattern of the embryo proper endothelial and red blood cell distribution was visualized in stage-matched embryos by PECAM immunohistochemistry (A, C, E, G-I) and o-dianisidine staining of hemoglobin (B, D, F, J-L), respectively. To facilitate comparison, the 12th sp is indicated by a dot, the 20th sp by the letter x, and the 25th sp by a star (h indicates heart ventricle; lb, limb bud). (A, C, E) The extensive arborized vascular network evident in the head and ventricle and between the somite pairs at E9.5 (24 sp) became progressively more complex by E9.75 (28 sp) and E10.5 (35 sp). (B, D, F) Red blood cells initially more concentrated in a subset of vessels at 24 sp became more evenly dispersed by 35 sp. Additionally, high levels of hemoglobin-containing cells were seen in the fetal liver (arrow) as it became the primary site of hematopoiesis by E10.5. (G-L) Along the back, paired intersomitic arteries and veins meet at capillary networks of increasing complexity between somite pairs during this time period. Initially, red blood cells were observed in paired intersomitic vessels in the anterior back, at mid positions in deeper arteries only, and not at all between posterior somite pairs. By 35 sp, red blood cells had penetrated intersomitic vasculature throughout the back. Red and blue arrowheads indicate intersomitic arteries and veins, respectively. Original magnifications A-F, × 20; G-L, × 50.
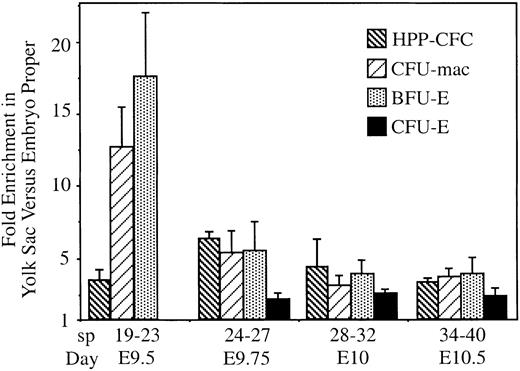
Hematopoietic progenitors do not circulate freely, even after the onset of circulation
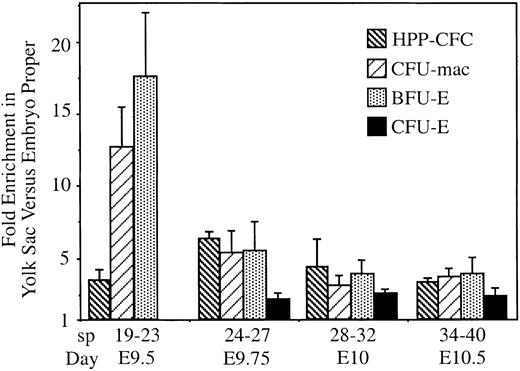
Along with primitive erythroblasts, the embryonic circulation also contains the first progenitor cells for definitive hematopoiesis arising in the yolk sac.5 Differentiation of definitive hematopoietic progenitors during fetal and adult periods is dependent on interactions within the stromal microenvironments of the liver and the bone marrow, respectively. In contrast, embryonic hematopoiesis is thought to consist of free-floating cells in the vascular system.2 If yolk sac–derived definitive hematopoietic progenitor cells were free floating, they should have entered the circulatory system of the embryo proper at the same rate as primitive erythroblasts. We tested this hypothesis by measuring the numbers of hematopoietic progenitors in the embryo proper when a significant proportion of erythroblasts entered the embryo proper (starting at 20 sp; Figure 4A). Erythroblast numbers were used to quantify the extent of circulation in individual embryos. We examined 3 unipotential definitive hematopoietic progenitors (erythroid-producing BFU-E and CFU-E and macrophage producing CFU-mac) and a multilineage HPP-CFC found exclusively in the yolk sac before 15 sp.5 10Enrichment of progenitors in the yolk sac versus the embryo proper was calculated by dividing the proportion of progenitors in the yolk sac by the proportion of erythroblasts there. If progenitors moved within the vascular system as freely as erythroblasts, this fold enrichment of progenitors would be 1. Because our question focused on progenitors within the yolk sac and embryo proper vascular systems, the developing liver buds were removed from embryos with 35 sp or more and were cultured separately. As expected, at 35 to 40 sp, we observed a high level of enrichment of BFU-Es and CFU-macs in the liver versus the embryo proper (70- to 30-fold, respectively; data not shown). However, because of their small size, the early liver buds still contained only a minority of the total progenitors in the conceptus at these stages.
We found a significant enrichment of progenitors in the yolk sac compared with the embryo proper at all stages examined (Figure6). For BFU-Es and CFU-macs, enrichment was highest at 19 to 23 sp, but it persisted even as the liver became the predominant site of hematopoiesis (35 sp). As previously reported, CFU-Es were not observed before 25 sp5 but, once detected, were also enriched in the yolk sac. Interestingly, the enrichment of CFU-Es in the yolk sac was consistently lower than that of its own progenitors, BFU-Es. This suggests that BFU-Es continue their preferential association with the yolk sac as they mature into CFU-Es but that this specific interaction decreases. However, decreasing yolk sac association with maturation cannot be a universal characteristic because the multilineage progenitor, HPP-CFC, actually circulates more extensively than the unilineage progenitors at 19 to 23 sp. Thus, though all progenitors examined were enriched in the yolk sac, there are indications of quantitative differences in the association with the yolk sac. Enrichment in the yolk sac could be attributed to the continued production of progenitors from fixed cells associated with the vascular system or to the preferential adherence of circulating progenitors to yolk sac endothelium. In either case, these data are not consistent with all definitive hematopoietic progenitors existing as free-flowing cells within the yolk sac. Rather, our findings suggest that hematopoietic progenitors specifically interact with a subset of the vascular bed, even after extensive circulation and liver hematopoiesis have been established.
Hematopoietic progenitors are retained in the yolk sac despite circulation.
Numbers of BFU-E, CFU-E, CFU-mac, and HPP-CFC hematopoietic progenitors and erythroblasts in the yolk sac versus the embryo proper were determined. Fold enrichment was calculated as the ratio of progenitors in yolk sac to embryo proper divided by the ratio of erythroblasts in the yolk sac to embryo proper. If progenitors circulated as freely as erythroblasts, the expected enrichment would have been 1. However, significantly more of all 3 types of progenitors were found in the yolk sac than would be expected, even as the fetal liver was becoming hematopoietic (E10.5). Three to 4 embryos were pooled for each experiment, and 3 to 6 independent experiments were performed for each time point. Error bars indicate SEM.
Hematopoietic progenitors are retained in the yolk sac despite circulation.
Numbers of BFU-E, CFU-E, CFU-mac, and HPP-CFC hematopoietic progenitors and erythroblasts in the yolk sac versus the embryo proper were determined. Fold enrichment was calculated as the ratio of progenitors in yolk sac to embryo proper divided by the ratio of erythroblasts in the yolk sac to embryo proper. If progenitors circulated as freely as erythroblasts, the expected enrichment would have been 1. However, significantly more of all 3 types of progenitors were found in the yolk sac than would be expected, even as the fetal liver was becoming hematopoietic (E10.5). Three to 4 embryos were pooled for each experiment, and 3 to 6 independent experiments were performed for each time point. Error bars indicate SEM.
Discussion
Onset of circulation
Ultimately the determination of when embryonic circulation begins is dependent on the definition of circulation. If circulation is defined simply as red blood cell movement within vessels, then the onset of circulation occurs in the mouse embryo within a narrow window of time between 4 and 6 sp, during E8.25. However, this definition fails to incorporate connotations of circulation critical for function, including the movement of sufficient blood in a circuit to oxygenate all tissues. Results reported here demonstrate a stepwise acquisition of characteristics of circulation over 36 hours of embryogenesis in the mouse. From E8.25 to E8.5 (4-7 sp), circulation is characterized by the movement of a few yolk sac–derived erythroblasts into the aortae of the embryo proper. The first movements of erythroblasts correlate temporally with the formation of a beating heart tube and linkage of embryonic and yolk sac vascular systems.3,7,20,21,57 These data are consistent with reports of the first erythroblasts entering the allantoic vasculature (contiguous with the distal end of the aortae) at 6 to 10 sp.22 At E8.5 to E9.0 (8-15 sp), we found evidence of increased flow as red blood cells penetrated further into the embryo proper arterial system. Nonetheless, only one tenth of the yolk sac–generated red blood cells entered the embryo proper by E9.0, though the embryo proper had 10 times more total cells than the yolk sac. These data suggest that the early cardiovascular system is insufficiently developed to establish vigorous circulation throughout the E8 conceptus.
Between E9 and E10, we observed a rapid increase in the extent of circulation so that by E10.5 (35 sp), erythroblasts were dispersed throughout the entire vascular system, and a steady state density of red blood cells within the embryo proper was achieved. The start of increased blood dispersal seen at E9.25 temporally correlated with a fundamental change in the structure of the vascular network. Between 15 and 25 sp, the netlike vascular plexuses and large aortae and cardinal veins were transformed by angiogenesis into an arborized vascular system (Figure 2H, 4A).8,24 Two additional major changes in the cardiovascular system, formation of a chambered heart and specialization of vessel walls with recruitment of vascular smooth muscle cells, occur later in embryogenesis. The heart tube begins to remodel into chambers only at E11, and recent data suggest that cardiac function increases steadily, without major transitions, from E8.25 to E10.5.25,57 Likewise, vascular smooth muscle cells only begin to differentiate along a limited area of the aorta at 30 sp,26,27 and the expression of transcripts for certain critical smooth muscle proteins does not begin until E10.28 In the context of these observations, our data suggest that a major factor in the achievement of robust circulation at E10 is the maturation and remodeling of the vascular network.
Our results imply, but do not prove, that functional circulation is not achieved until E10, concomitant with angiogenesis. This hypothesis is supported by the fact that embryos mutant in genes necessary for initial establishment of the endothelial networks (VEGFR-2), red blood cell synthesis (GATA1, LMO2, and Tal1), and regular heartbeat (NCX1) do not die before E9.5.29-33 Furthermore, these mutant embryos do not die earlier than embryos with defects blocking later angiogenesis or heart maturation (Tie2, EphrinB2, and MEF2C).24,34,35 Further delineation of how vascular remodeling, smooth muscle recruitment, and cardiac development specifically affect circulation awaits mutations altering only one of these processes.24 33-37
Vascular remodeling may require early circulation given that an embryo that does not establish a normal heartbeat because of a mutation of cardiac sodium–calcium exchange is defective in angiogenesis.33 An interesting implication of our data is that angiogenic remodeling that creates an arborized vascular structure between E9 and E10 is initiated under conditions of uneven circulation. Shear stress and oxygenation are known to be potent regulators of angiogenesis in the adult.37 The data presented suggest that inequities in these factors exist during the period of angiogenic remodeling and that the arterial vessels closest to the heart experience blood flow before distal arterial vessels or the venous system do. Whether this discrepancy is recognized and used during development of the vascular system remains to be explored.
Asymmetry of the yolk sac
The yolk sac is a deceptively simple bilayer structure of mesoderm and visceral endoderm. Visceral endoderm provides the embryo with liver and gut functions, including synthesis of serum proteins.2 Recently, the visceral endoderm has been shown to be a potent developmental regulator of specific anterior embryonic structures,38 and it may also be critical in directing yolk sac mesoderm fates.39,40 It has been generally held that during the initial formation of the vascular system, the yolk sac mesoderm is hemangiogenic, whereas the embryo proper mesoderm is angiogenic.8,41,42 Our work identifies hemangiogenic and angiogenic regions within the yolk sac. Large pools of red blood cells and sheets of endothelial cells—that is, blood islands—develop by E8.0 in the proximal region of the yolk sac. In contrast, vasculogenesis occurs without visible hematopoiesis within the distal yolk sac and embryo proper. Indeed, red blood cells are not found in the distal yolk sac unless red blood cell movement has been initiated. Drake and Fleming4 found a similar asymmetry of distal versus proximal yolk sac using immunohistochemical analysis of early vascular markers. The yolk sac later displays additional asymmetries in vascular remodeling, including arterial versus venous specification.43 Thus, the yolk sac has apparently compartmentalized processes central to the development of hematopoietic and endothelial fates.
Interconnections of endothelial and hematopoietic development
Increasing evidence supports the intertwining of endothelial and hematopoietic fates. Marker studies have found extensive overlap in gene expression in the earliest progenitors of both lineages, including LMO2, Tal1, VEGFR-2, Tie2, and CD34.4,44-47 Mutational analyses in mouse embryos have confirmed that the normal development of hematopoietic and vascular systems require functional LMO2, Tal1, and VEGFR-2 genes.44,47-49 There are also spatial and temporal correlations between the emergence of the first blood cells and endothelial cells in blood islands of the yolk sac. The most direct evidence of a common hemangioblast progenitor is the isolation of bipotential cells during the development of embryoid bodies from embryonic stem cells.50 The association of vascular and hematopoietic fates continues in later development as the first hematopoietic stem cells capable of engrafting an adult recipient are associated with the aorta and omphalomesenteric arteries at E10.5 in the mouse.51-53 This has led to the hypothesis that the adult hematopoietic system arises from a hemogenic endothelium with the embryo proper.42 In the adult, bone marrow is a source of hematopoietic stem cells and recently identified circulating endothelial stem cells.5455 Although the precise relationship between endothelial and hematopoietic potential in the bone marrow remains undefined, significant plasticity of cells fates can be uncovered experimentally.41,42 56 The results presented here support an additional relationship between hematopoietic and endothelial cells that occurs normally in the embryo. Before bone marrow or fetal liver stromas are available as hematopoietic microenvironments, observations of blood island development have led to the impression that embryonic blood cells form and float freely within the vascular network. No specific microenvironmental role has been proposed for endothelial cells. In contrast, we have observed that hematopoietic progenitors remain associated with a specific vascular bed (ie, yolk sac) despite maturation of the vascular system, onset of circulation, and colonization of the fetal liver. The identification of specific temporal–spatial associations of endothelial and hematopoietic cells in the embryo should facilitate future studies regarding the molecular underpinnings of the interactions between these systems.
We thank Dr Joe Miano, Dr Laurie Milner, and Dr Colin Phoon for critically reading the manuscript.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-08-2531.
Supported by National Institutes of Health grant R01 HL59484 and American Heart Association grant 0130503T.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kathleen E. McGrath, Center for Human Genetics and Molecular Pediatric Disease, University of Rochester, Box 703, 601 Elmwood Ave, Rochester, NY 14642; e-mail:kathleene_mcgrath@urmc.rochester.edu.