The Epstein-Barr virus (EBV)–encoded LMP1 protein is expressed in EBV-positive Hodgkin disease and is a potential target for cytotoxic T-lymphocyte (CTL) therapy. However, the LMP1-specific CTL frequency is low, and so far the generation of LMP1-specific CTLs has required T-cell cloning. The toxicity of LMP1 has prevented the use of dendritic cells (DCs) for CTL stimulation, and we reasoned that an inactive, nontoxic LMP1 mutant (ΔLMP1) could be expressed in DCs and would enable the activation and expansion of polyclonal LMP1-specific CTLs. Recombinant adenoviral vectors expressing LMP1 or ΔLMP1 were tested for their ability to transduce DCs. LMP1 expression was toxic within 48 hours whereas high levels of ΔLMP1 expression were achieved with minimal toxicity. ΔLMP1-expressing DCs were able to reactivate and expand LMP1-specific CTLs from 3 healthy EBV-seropositive donors. LMP1-specific T cells were detected by interferon-γ (IFN-γ) enzyme-linked immunospot assay (ELISPOT) assays using the HLA-A2–restricted LMP1 peptide, YLQQNWWTL (YLQ). YLQ-specific T cells were undetectable (less than 0.001%) in donor peripheral blood mononuclear cells (PBMCs); however, after stimulation the frequency increased to 0.5% to 3.8%. Lysis of autologous target cells by CTLs was dependent on the level of LMP1 expression. In contrast, the frequency of YLQ-specific CTLs in EBV-specific CTLs reactivated and expanded using lymphoblastoid cell lines was low and no LMP1-specific cytotoxic activity was observed. Thus, ΔLMP1 expression in DCs is nontoxic and enables the generation of LMP1-specific CTLs for future adoptive immunotherapy protocols for patients with LMP1-positive malignancies such as EBV-positive Hodgkin disease. Targeting LMP1 in these malignancies may improve the efficacy of current adoptive immunotherapy approaches.
Introduction
Immunotherapy with cytotoxic T cells (CTLs) is increasingly used to treat malignancies and viral infections.1,2 For example, polyclonal Epstein-Barr virus (EBV)–specific CTLs have been used for the prevention and treatment of posttransplantation EBV-associated lymphoma (PTLD).3EBV-specific CTLs persisted long-term, reconstituted immunity against EBV, and produced antiviral and antilymphoma effects. We have also used EBV-specific CTLs to treat 13 patients with EBV-positive Hodgkin disease and, while the results have been promising, no patient with bulky disease has been cured.1,4 One explanation for this failure is that current methods of EBV-specific CTL generation produce CTL lines that are dominated by clones reactive to EBV proteins not expressed in the malignant Reed-Sternberg cells (H-RS cells) of EBV-positive Hodgkin disease.5 Only a limited number of EBV-derived antigens (EBNA1, BARF0, LMP1, and LMP2) are present in H-RS cells,6,7 and the immunodominant response of CTL lines is against EBNAs 3A, B, and C, which are not expressed by the tumor cells. Of the EBV proteins expressed in H-RS cells, only LMP2 and LMP1 are potential targets for CD8+ T cells, because EBNA 1 is mainly presented on major histocompatibility complex (MHC) class II molecules8 and the level of BARF0 expression might be too low for CTL recognition.9 Our group and others have recently reported the generation of LMP2-specific CTLs10-12; however, for future clinical protocols it is desirable to generate CTLs against more than one tumor-associated antigen expressed in H-RS cells to reduce the risk of T-cell escape mutants13-15 and to ensure that good CTL epitopes are available regardless of the patient's HLA type. LMP1-specific CTLs are rarely detected in healthy EBV-seropositive individuals,5and reactivation of LMP1-specific CTL lines has been difficult, in part because LMP1 is toxic when expressed at high levels.16 It has been possible to generate LMP1-specific CTLs by cloning using LMP1-derived peptide epitopes17 or antigen-presenting cells infected with a recombinant vaccinia virus overexpressing LMP1.18 The generated T-cell clones were able to lyse targets expressing LMP1 and autologous lymphoblastoid cell lines (LCLs), providing the rational for the development of an adoptive immunotherapy for EBV-associated malignancies like Hodgkin disease or nasopharyngeal carcinoma. However, the adoptive immunotherapy with T-cell clones is not ideal, because the generation of T-cell clones is labor-intensive, limiting its application for adoptive immunotherapy protocols. Moreover, T-cell clones (1) do not persist in patients without the presence of specific CD4+ T helper cells19 and (2) carry the inherent risk that they may be evaded by epitope escape mutants.13-15
Thus, we have taken a different approach and generated polyclonal LMP1-specific CTL lines by stimulating peripheral blood mononuclear cells (PBMCs) with dendritic cells (DCs) expressing a functionally inactive, nontoxic LMP1 mutant. We used a 43–amino acid N-terminal deletion mutant, which renders the molecule inactive and nontoxic.20 About 90% of the molecule remains intact for antigen processing, and the 2 previously identified HLA-A2–restricted epitopes remain intact.17 Here we show that high levels of LMP1 expression can be achieved in human dendritic cells (DCs) with little toxicity using a recombinant ΔLMP1 adenovirus (Ad_ΔLMP1). Moreover, stimulation of PBMCs with DCs expressing ΔLMP1 led to an at least 500- to 3800-fold increase in the frequency of LMP1-specific CTLs, which were able to lyse wild-type LMP1-expressing target cells.
Materials and methods
Blood donors and cell lines
The study was performed on a Baylor College of Medicine institutional review board (IRB)–approved protocol, and informed consent was obtained from all donors. Autologous and mismatched LCLs were established as previously described.21 Primary autologous skin fibroblasts were isolated by punch biopsies taken from the volar surface of the forearm. LCLs and fibroblasts were maintained in RPMI 1640 (HyClone, Logan, UT) containing 2 mM GlutaMAX-I (Invitrogen, Carlsbad, CA) and 10% fetal calf serum (FCS) (HyClone).
HLA type of cell lines
The HLA class I type was for donor A HLA-A2, -B27, and -B51; donor B HLA-A2, -A3, -B35, and -B57; and donor C HLA-A2, -A3, -B7, and -B44. Mismatched LCLs for donor A were HLA-A1, -A31, -B7, and -B8; for donor B HLA-A24, -A33, -B7, and -B65; and for donor C HLA-A11, -A31, -B8, and -B38.
Construction of recombinant adenoviruses
The plasmid pSG5-LMP1 was provided by Bill Sugden, University of Wisconsin, Madison. LMP1 was subcloned into pEGFP-C1 (BD Biosciences, Palo Alto, CA) using BamHI and BglII sites. TheSmaI/XbaI LMP1 fragment of pEGFP-C1-LMP1 was inserted into the XbaI and NotI (Klenow filled in) sites of pShuttleX (BD Biosciences). ΔLMP1 was generated by digesting pShuttleX-LMP1 with NheI and BsrGI, Klenow fill in, and subsequent self-ligation. From pShuttleX-LMP1/-ΔLMP1 the expression cassette containing the CMV promoter, LMP1 or ΔLMP1 and the BGH polyA, was cloned into the E1/E3-deleted adenoviral backbone vector pAd5F35 using pI-SceI and I-CeuI sites.22The resultant plasmids were sequenced to confirm the sequence of LMP1 and ΔLMP1 (SEQwright, Houston, TX). Recombinant adenoviruses were generated as described in the literature.23 Plaques positive for LMP1 and ΔLMP1 expression by Western blot were expanded, purified, and titered by standard procedures.22 23
Vaccinia viruses
The vaccinia recombinants24 expressing EBV latency antigens EBNA-3A, -3B, and -3C were a gift from Elliot Kieff, Boston, MA, and the vaccinia-EGFP virus was constructed according to published procedures24 using the enhanced green fluorescent protein (EGFP) fragment of pEGFP-C1 and the shuttle plasmid pSC11 (gift from Bernard Moss, Bethesda, MD).
Peptides for ELISPOT and cytotoxicity assays
The 2 identified LMP1 HLA-A2–restricted peptide epitopes17 YLQQNWWTL (YLQ) and YLLEMLWRL (YLL) were prepared by Martin Campbell, Synthetic Antigen Laboratory, The University of Texas MD Anderson Cancer Center, Houston, TX. The YLQ peptide is conserved between EBV strains.25 The YLL epitope varies in between EBV strains, and the YLL epitope is not present in the LMP1 sequence used in this study (YLLEILWRL; group B virus).25 Therefore, the peptide served as a negative control, and no activity of LMP1-specific CTLs against YLL peptide was observed.
DC generation
DCs were generated by the “adherence method.”26,27 Briefly, peripheral blood mononuclear cells (PBMCs) were purified by Ficoll (Lymphoprep, Nycomed, Oslo, Norway) gradient separation. A total of 4 × 107 to 5 × 107 mononuclear cells were plated in Cell Genix media (Technologie Transfer, Freiburg, Germany) containing 2 mM GlutaMAX-I in T-75 flasks for 2 hours. The nonadherent cells were removed by washing with phosphate-buffered saline (PBS), and the adherent cells were cultured in Cell Genix/GlutaMAX-I media with 800 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (Sargramostim Leukine, Immunex, Seattle, WA) and 1000 U/mL interleukin-4 (IL-4) (R&D Systems, Minneapolis, MN) for 5 days. IL-4 and GM-CSF were replenished on day 2 and 4. On day 5, cells were harvested and transduced with recombinant adenovirus. DCs were cultured for 2 more days in Cell Genix/ GlutaMAX-I media containing 800 U/mL GM-CSF, 1000 U/mL IL-4 and, for maturation, 10 ng/mL IL-1β, 100 ng/mL IL-6, 10 ng/mL tumor necrosis factor-α (TNF-α) (all R&D Systems), and 1 μg/mL (prostaglandin E2) (PGE2) (Sigma, St Louis, MO).12 28
CTL generation
A total of 2 × 106 PBMCs per well of a 6-well plate were cocultured with 1 × 105 per well autologous, irradiated, Ad_ΔLMP1–transduced DCs (DC_ΔLMP1) in 2 mL complete medium (45% RPMI 1640, 45% Click [Eagle Ham amino acids; Irvine Scientific, Santa Ana, CA], 2 mM GlutaMAX-1, 10% FCS). Cultures were restimulated on day 10 and, after that, weekly with DC_ΔLMP1 at a responder-stimulator ratio of 10:1. IL-2 (Proleukin, Chiron, Emeryville, CA), 40 U/mL, was first added with the third stimulation and then twice weekly.
Flow cytometry
The expression of LMP1 and ΔLMP1 was detected by fluorescence-activated cell sorter (FACS) analysis. Briefly, transduced DCs were fixed for 10 minutes in 4% paraformaldehyde (PFA) and then permeablized with 1% saponin. The monoclonal LMP1 antibody cocktail,29 CS1-4 (Research Diagnostics, Flanders, NJ), recognizing the common C-terminus of LMP1 and ΔLMP1, was used as primary antibody and goat antimouse–fluorescein isothiocyanate (FITC) (BD Biosciences) as secondary antibody. Samples were acquired on a FACScan flow cytometer (BD Biosciences), and the data were analyzed using CellQuest software (BD Biosciences). Nontransduced DCs served as negative control. Expression of the surface molecules was measured on nonfixed, nonpermeabilized DCs using phycoerythrin (PE)–conjugated monoclonal antibodies: anti-CD3, -CD14, -CD16, -CD19, -CD56, -CD80, -CD83, -CD86, and anti-DR peridinin chlorophyll protein (PerCP). CTL lines were analyzed with anti-CD8 FITC, anti-CD16 FITC, anti–T-cell receptor (anti-TCR) γ/δ FITC, anti-CD4 PE, anti-CD56 PE, anti-CD16 PE, anti-TCR α/β PE, and anti-CD3 PerCP. All monoclonal antibodies were obtained from BD Biosciences except anti-CD16 PE, anti-CD56 PE, and anti-CD83 PE (Immunotech, Marseille, France).
ELISPOT assays
The enzyme-linked immunospot (ELISPOT) assay was performed with modifications as described in the literature.30 A multiscreen 96-well plate (Millipore, Bedford, MA) was precoated with “capture” antibody against interferon-γ (IFN-γ) (Mabtech, Nacka, Sweden) overnight at 4°C. The wells were washed 3 times with phosphate-buffered saline (PBS) and then blocked with RPMI 1640/2 mM GlutaMAX-I containing 10% FCS for at least 1 hour. PBMCs and EBV- and LMP1-specific CTLs were washed once and resuspended in RPMI 1640/2 mM GlutaMAX-I containing 5% human serum (HS) (C-6 Diagnostic, Germantown, WI). Cells were set up in triplicates in a separate 96-well plate to avoid membrane damage of the multiscreen plate and serial diluted; for PBMCs the undiluted well contained 1 × 105 cells and for CTLs 1 × 104 cells. The blocking medium was removed from the multiscreen plate, the wells were washed twice with PBS, and 100 μL of the serial-diluted cells was transferred into each well; 100 μL of autologous, irradiated LCLs (1 × 105 cells) or peptide (10−5 M) were resuspended in RPMI 1640/2mM GlutaMAX-I containing 5% HS and 200 U/mL IL-2. In each assay, negative controls included PBMCs, CTLs, LCLs, or peptide alone and, as positive controls, PBMCs or CTLs stimulated with 2.5 μg/mL phorbol myristate acetate (PMA) (Sigma) and 1 μg/mL ionomycin (Sigma). After 16 to 20 hours, the plates were washed 6 times with PBS/0.05% Tween 20 and incubated for 2 hours with biotinylated “detection” antibody against IFN-γ (Mabtech). After an additional 6 washes with PBS/0.05% Tween 20, 100 μL of avidin-peroxidase complex (AEC; prepared according to manufacturer instructions; Vector Laboratories, Burlingame, CA) was added per well for 1 hour at room temperature. The plates were washed 3 times with PBS/0.05% Tween 20, followed by 3 washes with plain PBS. AEC substrate (Sigma) was prepared by dissolving one AEC tablet in 2.5 mL dimethylformamide and adding 47.5 mL sodium acetate buffer and 25 μL of 30% hydrogen peroxide. Prior to use the AEC substrate was filtered through a 0.45-μM filter, and 100 μL was added per well. After 4 minutes the reaction was stopped by washing with deionized water, and the plates were dried overnight prior to membrane removal. The spot number was determined in an independent blinded fashion (ZellNet Consulting, New York, NY) using a high-resolution automated ELISPOT Reader System (Carl Zeiss, Thornwood, NY) using KS ELISPOT 4.3 software. In all assays the background was fewer than 5 spot-forming cells (SFCs) per 100 000 cells. Linear regression analysis and statistical analysis (Wilcoxon signed rank test) was performed using GB-STAT (Dynamic Microsystems, Silver Spring, MD).
Cytotoxicity assays
The CTLs were tested for specific cytotoxicity against autologous fibroblasts either uninfected or infected with recombinant adenovirus or vaccinia virus constructs. Fibroblasts were exposed to 100 U/mL IFN-γ (R&D Systems) 24 to 48 hours prior to the cytotoxicity assay and infected on the day of the cytotoxicity assay with vaccinia virus recombinants containing EBNA-3A, -3B, -3C, and EGFP or 24 hours before with adenovirus recombinants containing an empty expression cassette, LMP1 ΔLMP1. Recombinant vaccinia virus expressing LMP1 could not be used due to the additive toxicity of LMP1 and vaccinia virus infection. Autologous LCLs, HLA class I–mismatched LCLs, and autologous phytohemagglutinin (PHA) blasts were also tested. Where indicated, PHA blasts were loaded with peptide for 1 hour after51chromium (51Cr) labeling. A total of 1 × 106 target cells were labeled with 0.1 mCi (3.7 MBq) 51Cr and mixed with various numbers of effector cells to give effector-target ratios of 40:1, 20:1, 10:1, and 5:1. Target cells incubated in complete medium alone or in 1% Triton X-100 were used to determine spontaneous and maximum 51Cr release, respectively. After 4 hours (LCLs, PHA blasts) or 5 hours (fibroblasts), supernatants were collected and radioactivity was measured on a gamma counter. The mean percentage of specific lysis of triplicate wells was calculated as 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release).
Western blot
Lysates of LCLs and fibroblasts were prepared by washing cells once with ice-cold PBS and boiling samples for 5 minutes in Laemmli buffer (Pierce Biotechnology, Rockford, IL). Fibroblasts were transduced with Ad_LMP1 or Ad_ΔLMP1 24 hours prior to harvest as for cytotoxicity assays. Nontransduced fibroblasts served as negative control. Samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Pall Life Science, Ann Arbor, MI). Membranes were blocked in Tris (tris(hydroxymethyl)aminomethane)–buffered saline containing 0.1% Tween 20 (TBS-T) and 5% milk powder (MP). Membranes were washed twice with TBS-T and incubated for 1 hour with anti-LMP1 antibody CS1-4 in TBS-T containing 2% MP. Membranes were washed 4 times with TBS-T and incubated with a horseradish peroxidase (HRP)–conjugated secondary antibody (Amersham Biosciences, Piscataway, NJ) for 1½ hours in TBS-T containing 1% MP. The membrane was washed 4 times with TBS-T, and bound HRP was detected by enhanced chemiluminescence (ECL Plus; Amersham Biosciences).
Results
LMP1 expression is toxic in dendritic cells
Different adenoviral vectors have been tested for their efficiency in transducing human DCs.31 The most efficient vector identified has a serotype 5 capsid containing the short-shafted fiber protein of serotype 35 (Ad5F35). Recombinant Ad5F35 vectors containing LMP1 and ΔLMP1 were generated according to Yotnda et al,22 and the resultant constructs are shown in Figure1. To evaluate the effect of LMP1 and ΔLMP1 expression, DCs were transduced with increasing multiplicities of infection (MOIs) of Ad_LMP1 and Ad_ΔLMP1. Forty-eight hours after transduction, the number of viable and LMP1-positive cells was determined by FACS analysis. A total of 32% of DCs were positive for LMP1 expression after transduction with Ad_LMP1 at an MOI of 3. Higher MOIs led to no significant increase in transduction efficiency but resulted in more than 60% cell death (Figure2). In contrast, more than 80% of DCs were positive for ΔLMP1 at all MOIs tested. There was an adenoviral dose-dependent decrease in DC viability, but 90% of DC were viable at an MOI of 3, indicating that high levels of ΔLMP1 expression could be achieved with minimal toxicity (Figure 2). The marked cytopathic effects of LMP1 expression in DCs prevented the further use of Ad_LMP1-transduced DCs for CTL generation.
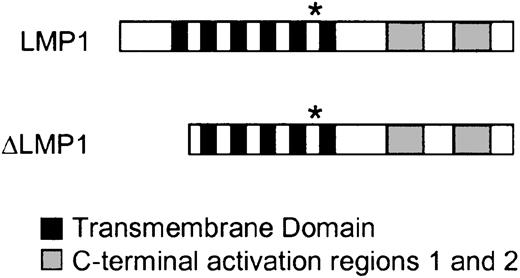
Structure of LMP1 and ΔLMP1.
LMP1 consists of a short N-terminus, 6 transmembrane domains, and 2 C-terminal activation regions. The first transmembrane domain is homologous to the retroviral transmembrane domain p15a. * indicates the position of the HLA-A2–restricted YLQQNWWTL peptide used in this study. ΔLMP1 lacks the N-terminus and the first transmembrane domain.
Structure of LMP1 and ΔLMP1.
LMP1 consists of a short N-terminus, 6 transmembrane domains, and 2 C-terminal activation regions. The first transmembrane domain is homologous to the retroviral transmembrane domain p15a. * indicates the position of the HLA-A2–restricted YLQQNWWTL peptide used in this study. ΔLMP1 lacks the N-terminus and the first transmembrane domain.
LMP1 expression is toxic in dendritic cells.
Day 5 immature DCs were transduced with increasing MOIs of Ad_LMP1 and Ad_ΔLMP1. After transduction, the DCs were matured with a cytokine cocktail for 2 days. (A) LMP1 expression and (B) the percentage of viable large cells were determined by FACS analysis. The transduction efficiency of Ad_LMP1 was lower than Ad_ΔLMP1, and LMP1 expression was toxic.
LMP1 expression is toxic in dendritic cells.
Day 5 immature DCs were transduced with increasing MOIs of Ad_LMP1 and Ad_ΔLMP1. After transduction, the DCs were matured with a cytokine cocktail for 2 days. (A) LMP1 expression and (B) the percentage of viable large cells were determined by FACS analysis. The transduction efficiency of Ad_LMP1 was lower than Ad_ΔLMP1, and LMP1 expression was toxic.
ΔLMP1 is functionally inactive in dendritic cells
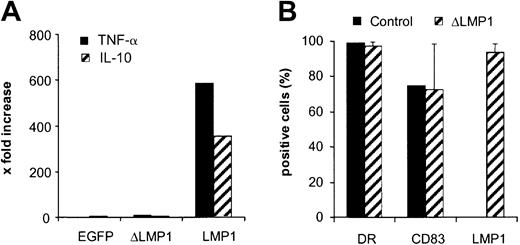
To confirm that ΔLMP1 was functionally inactive in DCs, we measured their production of TNF-α and IL-10, 2 cytokines that are known to be induced by LMP1.32 Immature DCs were transduced with Ad_EGFP, Ad_LMP1, and Ad_ΔLMP1, and 48 hours after transduction the concentration of the 2 cytokines was determined by FACS using the Cytokine Cytometric Bead Array kit. DC-expressing LMP1 produced high amounts of TNF-α and IL-10, while ΔLMP1- and EGFP-expressing DCs did not, indicating that ΔLMP1 is functionally inactive (Figure 3A).
ΔLMP1 is functionally inactive in dendritic cells.
(A) Day 5 immature DCs were transduced with Ad_EGFP, Ad_ΔLMP1, and Ad_LMP1 at an MOI of 3. After 48 hours, cell-free media were collected and the concentration of TNF-α and IL-10 was determined by FACS using the Cytokine Cytometric Bead Array kit (BD Biosciences). Only LMP1 induced high levels of TNF-α and IL-10. (B) Day 5 immature DCs were transduced at an MOI of 6 and matured with a cytokine cocktail for 2 days prior to FACS analysis for DR, CD83, and ΔLMP1 expression (donors A, B, and C; n = 15). ΔLMP1 expression did not affect the expression of DR and CD83 in comparison with nontransduced DCs (control).
ΔLMP1 is functionally inactive in dendritic cells.
(A) Day 5 immature DCs were transduced with Ad_EGFP, Ad_ΔLMP1, and Ad_LMP1 at an MOI of 3. After 48 hours, cell-free media were collected and the concentration of TNF-α and IL-10 was determined by FACS using the Cytokine Cytometric Bead Array kit (BD Biosciences). Only LMP1 induced high levels of TNF-α and IL-10. (B) Day 5 immature DCs were transduced at an MOI of 6 and matured with a cytokine cocktail for 2 days prior to FACS analysis for DR, CD83, and ΔLMP1 expression (donors A, B, and C; n = 15). ΔLMP1 expression did not affect the expression of DR and CD83 in comparison with nontransduced DCs (control).
The ΔLMP1-transduced DCs were further characterized to demonstrate that ΔLMP1 expression had no effect on DC maturation. Immature DCs were transduced on day 5 with Ad_ΔLMP1 at an MOI of 6 and cultured for 2 more days in the presence of a cytokine cocktail containing IL-4 and GM-CSF and, for maturation, TNF-α, IL-1β, IL-6, and PGE2. DCs were analyzed by FACS for DR (bright), CD83, and ΔLMP1 expression. A total of 97% of DCs were positive for ΔLMP1, and expression of DR and CD83 was unchanged compared with controls (Figure 3B), indicating that Ad_ΔLMP1 leads to high levels of ΔLMP1 expression without changing the DC phenotype.
CTLs generated with ΔLMP1-expressing DCs recognize an HLA-A2–restricted LMP1 peptide epitope
LMP1-specific CTLs were generated from 3 healthy, HLA-A2, EBV-seropositive donors by stimulating PBMCs with autologous DC-expressing ΔLMP1 (DC_ΔLMP1). The phenotype of DC_ΔLMP1-induced CTLs was similar for all 3 donors, with more than 95% being CD3+. More than 80% of the CD3+ T cells were positive for CD8, 11% to 20% for CD4, and more than 95% were TCR-αβ+. Less than 5% of cells were positive for natural killer cell markers (CD3− and CD56+).
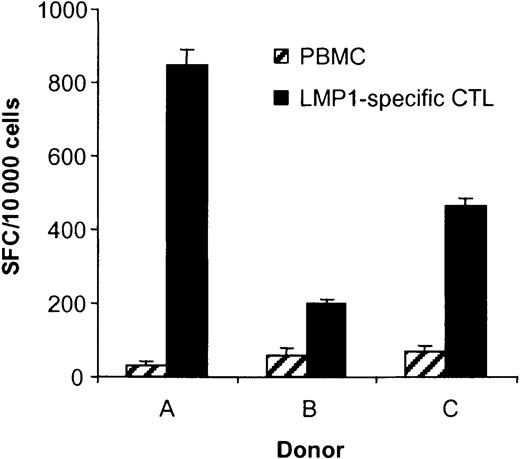
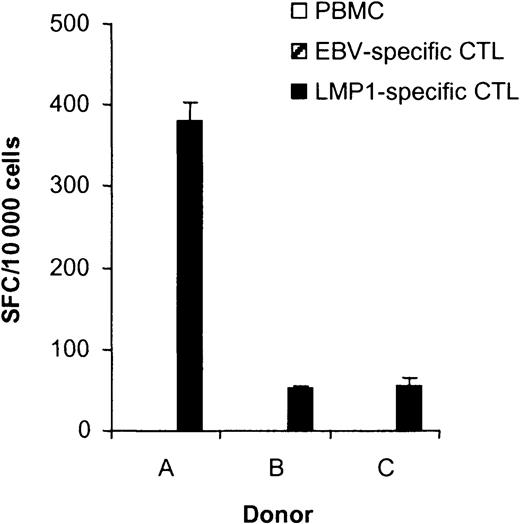
To determine if stimulation of PBMCs with DC_ΔLMP1 induced the expansion of LMP1-specific CTLs, IFN-γ ELISPOT assays were performed using the HLA-A2–restricted LMP1 peptide epitope YLQQNWWTL (YLQ). This epitope is located between the fifth and sixth transmembrane domain of LMP1 (Figure 1) and is conserved in between the laboratory EBV strain B95-8, European and Chinese EBV isolates.25Depending on the donor, 0.5% to 3.8% of the CTLs secreted IFN-γ in response to YLQ peptide, demonstrating that the generated CTLs had specificity for this particular HLA-A2–restricted LMP1 epitope (Figure4). No YLQ-specific CTLs were observed when PBMCs of donor B were stimulated with untransduced dendritic cells (data not shown).
LMP1-specific CTL lines recognize the HLA-A2–restricted LMP1 epitope YLQQNWWTL (YLQ).
The ability of T cells in PBMCs, EBV-specific CTLs, and LMP1-specific CTLs to secrete IFN-γ in response to the LMP1 peptide YLQ was determined in ELISPOT assays. Spot-forming cells (SFCs) were quantified by Zellnet Consulting. No reactive cells were observed in PBMCs and EBV-specific CTLs for all 3 donors. The frequency of YLQ peptide–reactive LMP1-specific CTLs was 3.82% for donor A, 0.52% for donor B, and 0.55% for donor C. YLQ peptide–reactive CTLs were undetectable in PBMCs and EBV-specific CTLs.
LMP1-specific CTL lines recognize the HLA-A2–restricted LMP1 epitope YLQQNWWTL (YLQ).
The ability of T cells in PBMCs, EBV-specific CTLs, and LMP1-specific CTLs to secrete IFN-γ in response to the LMP1 peptide YLQ was determined in ELISPOT assays. Spot-forming cells (SFCs) were quantified by Zellnet Consulting. No reactive cells were observed in PBMCs and EBV-specific CTLs for all 3 donors. The frequency of YLQ peptide–reactive LMP1-specific CTLs was 3.82% for donor A, 0.52% for donor B, and 0.55% for donor C. YLQ peptide–reactive CTLs were undetectable in PBMCs and EBV-specific CTLs.
The frequency of YLQ-specific CTLs was less than 0.01% (undetectable) in EBV-specific CTLs generated using LCLs as antigen-presenting cell lines (APCs), indicating that only DC_ΔLMP1 directs the stimulation of LMP1-specific CTLs (Figure 4). To estimate the expansion of YLQ-specific CTLs after DC_ΔLMP1 stimulation, their frequency was determined in PBMCs. The frequency in all 3 donors was below the detection limit of the ELISPOT assay (0.001%), indicating that the frequency of YLQ-specific CTLs had increased at least 500- to 3800- fold after 4 to 6 stimulations (30 to 50 days) (Figure 4).
LMP1-specific CTLs recognize LCLs
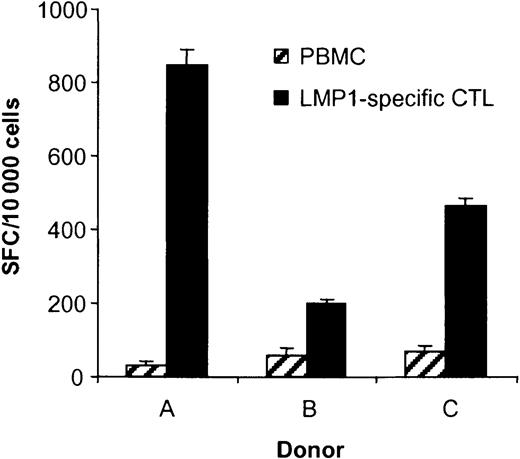
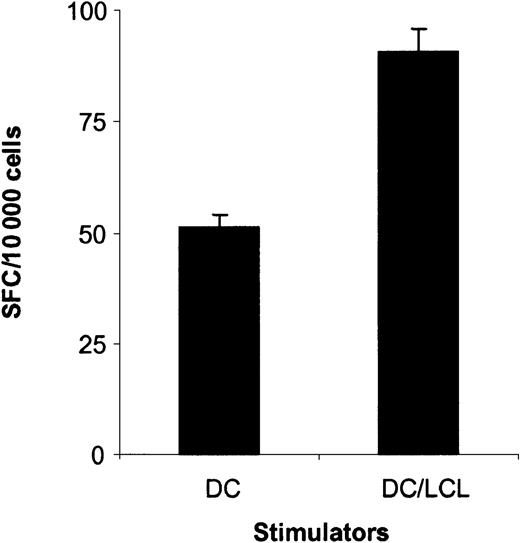
Having established that the DC_ΔLMP1-activated CTLs recognize the LMP1-derived YLQ peptide epitope, it was important to determine if the generated CTLs recognize autologous LCLs, in which LMP1 is naturally expressed by EBV. Depending on the donor, 2% to 8.5% of CTLs recognized LCLs as judged by IFN-γ secretion in ELISPOT assays (Figure 5), in contrast to less than 0.7% in PBMCs prior to stimulation. The frequency of CTLs recognizing LCLs in EBV-specific CTL lines for all 3 donors was 38% to 49% (data not shown).
LMP1-specific CTL lines recognize autologous LCLs.
The ability of T cells in PBMCs, EBV-specific CTLs, and LMP1-specific CTLs to secrete IFN-γ in response to autologous LCLs was determined in ELISPOT assays. Spot-forming cells (SFCs) were quantified by Zellnet Consulting. The frequency of LCL-reactive CTLs in LMP1-specific CTLs was 8.5% for donor A, 2.0% for donor B, and 4.6% for donor C and, in PBMCs, 0.34%, 0.57%, and 0.68%, respectively.
LMP1-specific CTL lines recognize autologous LCLs.
The ability of T cells in PBMCs, EBV-specific CTLs, and LMP1-specific CTLs to secrete IFN-γ in response to autologous LCLs was determined in ELISPOT assays. Spot-forming cells (SFCs) were quantified by Zellnet Consulting. The frequency of LCL-reactive CTLs in LMP1-specific CTLs was 8.5% for donor A, 2.0% for donor B, and 4.6% for donor C and, in PBMCs, 0.34%, 0.57%, and 0.68%, respectively.
Cytotoxic activity of LMP1-specific CTLs
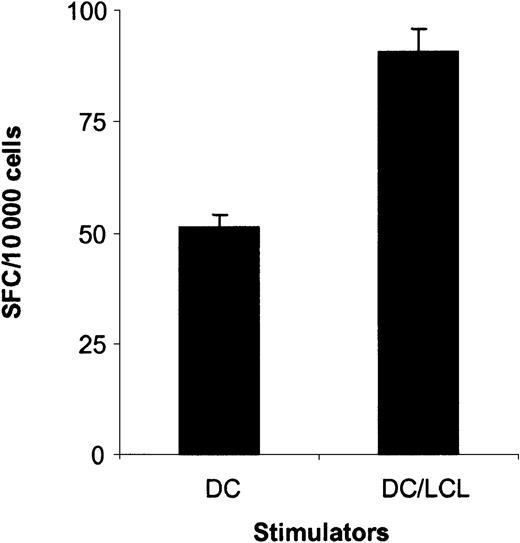
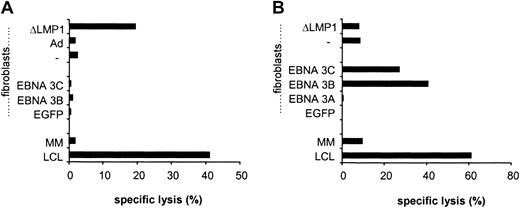
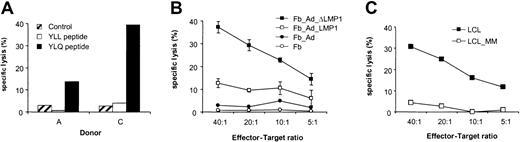
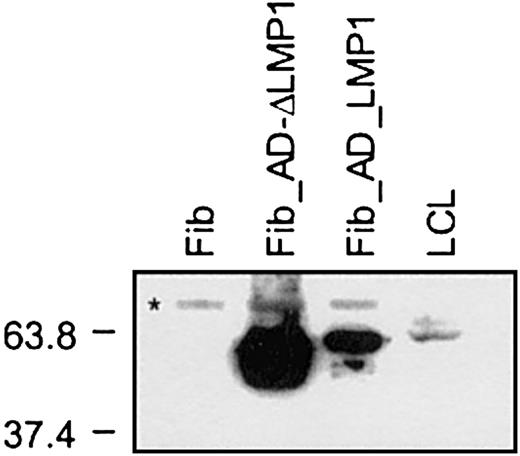
To determine if the generated LMP1-specific CTLs not only secreted IFN-γ after YLQ peptide or LCL stimulation but also had cytotoxic activity, CTLs were tested against a panel of targets in cytotoxicity assays. LMP1-specific CTLs lysed autologous PHA blasts loaded with YLQ peptide, whereas PHA blasts alone or with an unrelated HLA-A2–restricted peptide were resistant to killing (Figure6A). In addition, LMP1-specific CTLs lysed autologous fibroblasts expressing LMP1 and ΔLMP1 from the adenoviral vector (Figure 6B). No lysis of targets transduced with an adenovirus without a transgene was observed, indicating that the stimulation protocol did not lead to the expansion of adenovirus-specific CTLs. LMP1-specific CTLs of all 3 donors were tested for their ability to lyse autologous LCLs in which LMP1 is solely expressed by EBV. Only CTL from donor A, who had the highest frequency of reactive CTLs against YLQ peptide and LCLs, lysed autologous LCLs (Figure 6C). Decreased susceptibility to CTL lysis might be explained either by sequence variation of LMP1, differences in the level of LMP1 expression in fibroblasts and LCLs, or by the low frequency and/or affinity of LMP1-specific CTLs.30,33 34The LMP1 sequence of B95-8 EBV used to generate LCLs is almost identical (99.1% homology) to the LMP1 used for CTL generation, excluding epitope variation that would result in LCL resistance to CTL lysis. The level of LMP1 and ΔLMP1 expression in fibroblasts after adenoviral transduction was much higher than in LCLs (Figure7), indicating that the lysis of target cells depends on the level of antigen expression. Thus, the frequency and/or affinity of LMP1-specific CTLs in donor B and C only allows for killing of targets that express LMP1 at higher levels than LCLs. In an attempt to increase the frequency and/or affinity of LMP1-specific CTLs for donor B, we combined initial DC_ΔLMP1 stimulations with subsequent autologous LCL stimulations. The resultant LMP1-specific CTL line had a 2-fold higher frequency of YLQ-specific CTLs in comparison to stimulations with DC_ΔLMP1 alone (Figure8). In addition, killing of autologous fibroblasts expressing ΔLMP1 as well as autologous LCLs was observed (Figure 9A). To exclude that stimulation with LCLs after initial DC_ΔLMP1 stimulations led to an expansion of CTLs recognizing immunodominant EBV proteins, cytoxicity assays were performed using autologous fibroblasts expressing EBNA-3A, -3B, and -3C. No lysis of targets was observed, in contrast to LCL-activated EBV-specific CTL, which killed EBNA-3B– and -3C–expressing targets (Figure 9). Moreover, EBV-specific CTLs did not lyse LMP1-expressing targets, confirming the ELISPOT assay results that only DC_ΔLMP1 and not LCLs direct the expansion of LMP1-specific CTLs (Figure 9B).
Cytotoxic activity of LMP1-specific CTL lines.
LMP1-specific CTL lines were tested for their ability to lyse autologous targets. (A) Autologous PHA blasts of donor A and C loaded with YLQ peptide were killed in cytotoxicity assays by LMP1-specific CTLs at an effector-target ratio of 40:1. PHA blasts alone or loaded with a control peptide (YLL) were not lysed. (B) Autologous fibroblasts of donor B expressing LMP1 and ΔLMP1 were killed by LMP1-specific CTLs. Nontransduced fibroblasts and fibroblasts transduced with an adenoviral vector without transgene were not lysed. (C) LMP1-specific CTLs of donor A killed autologous LCLs. No cytotoxic activity was observed against mismatched targets.
Cytotoxic activity of LMP1-specific CTL lines.
LMP1-specific CTL lines were tested for their ability to lyse autologous targets. (A) Autologous PHA blasts of donor A and C loaded with YLQ peptide were killed in cytotoxicity assays by LMP1-specific CTLs at an effector-target ratio of 40:1. PHA blasts alone or loaded with a control peptide (YLL) were not lysed. (B) Autologous fibroblasts of donor B expressing LMP1 and ΔLMP1 were killed by LMP1-specific CTLs. Nontransduced fibroblasts and fibroblasts transduced with an adenoviral vector without transgene were not lysed. (C) LMP1-specific CTLs of donor A killed autologous LCLs. No cytotoxic activity was observed against mismatched targets.
LMP1 expression in fibroblasts and LCLs.
Fibroblasts were harvested 24 hours after transduction with Ad_LMP1 and Ad_ΔLMP1; 2 × 103 cells per lane of fibroblasts and 2 × 105 cells per lane of LCLs were loaded. LMP1 expression was detected by Western blot using the monoclonal antibody cocktail CS1-4 and HRP-conjugated secondary antibodies. LMP1 expression was detected in transduced fibroblasts as well as LCLs, and the level of LMP1 expression was higher in fibroblasts than in LCLs. Protein standards are indicated at the left in kilodaltons (*cross-reactive band only present in fibroblasts).
LMP1 expression in fibroblasts and LCLs.
Fibroblasts were harvested 24 hours after transduction with Ad_LMP1 and Ad_ΔLMP1; 2 × 103 cells per lane of fibroblasts and 2 × 105 cells per lane of LCLs were loaded. LMP1 expression was detected by Western blot using the monoclonal antibody cocktail CS1-4 and HRP-conjugated secondary antibodies. LMP1 expression was detected in transduced fibroblasts as well as LCLs, and the level of LMP1 expression was higher in fibroblasts than in LCLs. Protein standards are indicated at the left in kilodaltons (*cross-reactive band only present in fibroblasts).
LMP1-specific CTLs generated by initial DC_ΔLMP1 stimulations followed by autologous LCL stimulations recognize the HLA-A2–restricted LMP1 epitope YLQQNWWTL (YLQ).
The ability of LMP1-specific CTLs generated by DC_ΔLMP1 stimulations followed by autologous LCL stimulations to secrete IFN-γ in response to the LMP1 peptide YLQ was determined in ELISPOT assays. Spot-forming cells (SFCs) were quantified by Zellnet Consulting. The frequency of YLQ-reactive CTLs was 0.9% in contrast to 0.52% for LMP1-specific CTLs, which were only stimulated with DC_ΔLMP1 (P < .001; Wilcoxon signed rank test)
LMP1-specific CTLs generated by initial DC_ΔLMP1 stimulations followed by autologous LCL stimulations recognize the HLA-A2–restricted LMP1 epitope YLQQNWWTL (YLQ).
The ability of LMP1-specific CTLs generated by DC_ΔLMP1 stimulations followed by autologous LCL stimulations to secrete IFN-γ in response to the LMP1 peptide YLQ was determined in ELISPOT assays. Spot-forming cells (SFCs) were quantified by Zellnet Consulting. The frequency of YLQ-reactive CTLs was 0.9% in contrast to 0.52% for LMP1-specific CTLs, which were only stimulated with DC_ΔLMP1 (P < .001; Wilcoxon signed rank test)
Cytotoxic activity profile of LMP1- and EBV-specific CTLs.
(A) LMP1-specific CTLs were generated by DC_ΔLMP1 stimulations followed by autologous LCL stimulations and (B) EBV-specific CTLs by LCL stimulations alone. Both were tested at an effector-target ratio of 40:1 against a panel of autologous targets, including autologous LCLs (LCL), mismatched LCLs (MM), fibroblasts alone (−), fibroblasts infected with recombinant vaccinia virus expressing EGFP, EBNA-3A, -3B, or -3C, or fibroblasts infected with recombinant adenovirus without transgene (Ad) or ΔLMP1. Both CTL lines killed autologous LCLs; however, only LMP1-specific CTLs killed LMP1-expressing targets. No cytotoxic activity of LMP1-specific CTLs was observed against EBNA-3B– and -3C–expressing targets in contrast to EBV-specific CTLs.
Cytotoxic activity profile of LMP1- and EBV-specific CTLs.
(A) LMP1-specific CTLs were generated by DC_ΔLMP1 stimulations followed by autologous LCL stimulations and (B) EBV-specific CTLs by LCL stimulations alone. Both were tested at an effector-target ratio of 40:1 against a panel of autologous targets, including autologous LCLs (LCL), mismatched LCLs (MM), fibroblasts alone (−), fibroblasts infected with recombinant vaccinia virus expressing EGFP, EBNA-3A, -3B, or -3C, or fibroblasts infected with recombinant adenovirus without transgene (Ad) or ΔLMP1. Both CTL lines killed autologous LCLs; however, only LMP1-specific CTLs killed LMP1-expressing targets. No cytotoxic activity of LMP1-specific CTLs was observed against EBNA-3B– and -3C–expressing targets in contrast to EBV-specific CTLs.
Discussion
We demonstrate that human DCs, expressing functionally inactive LMP1, efficiently activate and expand LMP1-specific CTLs from PBMCs of healthy, HLA-A2, EBV-seropositive donors. Polyclonal LMP1-specific CTL lines recognized an HLA-A2–restricted LMP1 peptide, YLQ, and lysed target cells expressing wild-type LMP1 in cytotoxicity assays. In contrast, expression of wild-type LMP1 was toxic in DCs, preventing CTL generation, and no LMP1-specific CTLs were detected in LCL-activated EBV-specific CTLs, indicating that only DC_ΔLMP1 can direct the activation and expansion of LMP1-specific CTLs.
Different methods have been developed to deliver antigens to DCs for CTL activation and expansion, such as (1) loading with peptides, proteins, or tumor cell lysate, (2) fusion of DCs with tumor cells, and (3) gene transfer with DNA, RNA, or recombinant viral vectors.35,36 Genetic modification of DCs offers several advantages including persistent expression of full-length antigen, allowing for the presentation of multiple/undefined epitopes and the ability to genetically modify antigens to make them more potent or—as for LMP1—less toxic. For clinical applications recombinant adenoviruses are attractive vectors for the genetic modification of DCs, because high transduction rates are achieved without interfering with DC function in comparison to other viral vectors, like vaccinia virus or herpes simplex virus.37,38 Adenoviral-transduced DCs have been used successfully in vitro to generate specific CTLs against a variety of tumor-associated antigens.10,39,40 In addition, these vectors can be produced in high titers under current good manufacturing conditions, and in phase 1 clinical trials their safety record has been encouraging. One concern of overexpression of antigens in APCs for CTL activation and expansion is the generation of low-affinity, low-avidity T cells41; this has not been observed with adenoviral vectors but may occur with other viral vectors, like vaccinia virus, which are capable of expressing antigens at much higher levels.
To separate the functional properties of LMP1 from its potential epitopes for antigen presentation, we constructed recombinant adenoviral vectors expressing LMP1 or ΔLMP1, a functionally inactive LMP1. ΔLMP1 is not only inactive and nontoxic but also lacks its first transmembrane domain, which shares homology with the retroviral immunosuppressive domain p15a.42,43 Peptides derived from this domain inhibit T-cell proliferation, and because LMP1 fragments can be detected in the supernatant of LMP1-expressing cells, LMP1 might have a direct immunosuppressive effect on T cells. High levels of ΔLMP1 expression with little toxicity were achieved in DCs, and ΔLMP1 did not induce cytokines like TNF-α or IL-10. In contrast, LMP1 expression was toxic in human DCs, preventing our planned investigation of how LMP1 expression in DCs affects their ability to activate and stimulate T cells. However, the observed induction of IL-10 is an indication that expression of LMP1 may impair DC function, because IL-10 down-regulates HLA-class II expression and inhibits their activation of TH1 immune responses.44 Further studies are needed to establish how LMP1 modulates the cellular immune response, because other investigators have observed potential immunosuppressive as well as stimulatory effects.42,45 46
The DC_ΔLMP1-induced CTLs contained CD4+ as well as CD8+ T cells, which is considered advantageous for adoptive immunotherapy protocols, because infused CD8 T cells do not persist long-term without the presence of CD4 cells.19 The specificity of the generated CD4 T cells has not been determined, and the identification of MHC class II–restricted epitopes is in progress. Of the expanded CTLs, 0.5% to 3.8% recognized the HLA-A2–restricted LMP1 epitope YLQ. In polyclonal EBV-specific CTL lines, frequencies between 6% and 8% have been reported for single EBNA-3A and -3C epitopes47 using tetramer analysis, and in LMP2-specific CTL lines up to 20% of cells may bind to a tetramer of a single LMP2 epitope.10 Most likely, these differences are a function of the CTL precursor frequency in PBMCs for a specific antigen, and the frequency for LMP1 epitopes is lower than for EBNA-3A, -3C, and LMP2 epitopes.5 Indeed, we were not able to detect YLQ-specific T cells in PBMCs of any of the 3 donors. In addition, IFN-γ ELISPOT assays are likely to underestimate the frequency of epitope-specific T cells in comparison to tetramer analysis, because only 30% to 80% of tetramer-positive T cells secrete IFN-γ after specific stimulation.48,49 The remainder of the LMP1-specific CTLs were either specific for other undefined LMP1 epitopes or were produced as a result of nonspecific CTL stimulation. The only other HLA-A2–restricted LMP1 epitope, YLLEMLWRL (YLM), currently identified17 was not present in the LMP1 sequence used in these experiments, and studies are currently in progress to identify novel LMP1-specific CTL epitopes.
If LMP1-specific CTLs are to be of therapeutic benefit, they must recognize LMP1 expressed at physiologic levels from its natural promoter. Therefore, we determined the frequency of CTLs recognizing LCLs in LMP1-specific CTL lines. The frequency of LCL-reactive CTLs in IFN-γ ELISPOT assays was 2% to 8.5%, establishing that LMP1-specific CTLs recognize LCLs. In EBV-specific CTLs generated by LCL stimulation from all 3 donors, the frequency of LCL-reactive CTLs was 38% to 49% in IFN-γ ELISPOT assays, indicating that LMP1-specific CTL lines contain a higher percentage of CTLs that were of low affinity, had activities other than IFN-γ secretion, or were nonspecific.
LMP1-specific CTL lines killed PHA blasts loaded with YLQ peptide and autologous fibroblasts expressing wild-type LMP1 or ΔLMP1. The killing of fibroblasts expressing LMP1 was lower than that of fibroblasts expressing ΔLMP1, because LMP1 expression in fibroblasts as in DCs was toxic and lower levels of gene expression were achieved in comparison to ΔLMP1. Only CTLs from donor A, who had the highest frequency of reactive CTLs against the LMP1-derived YLQ peptide and LCLs in ELISPOT assays, lysed autologous LCLs, which expressed LMP1 at a lower level than fibroblasts transduced with Ad_LMP1 or Ad_ΔLMP1. This finding is consistent with previous published results that lysis of target cells is dependent on the level of antigen expression and the frequency and/or affinity of antigen-specific CTLs.30,33,34 Thus, the frequency and/or affinity of LMP1-specific CTLs in donors B and C only allows killing of targets that express LMP1 at higher levels than LCL. However, the level of LMP1 expression in LCLs is lower than in malignant H-RS cells of EBV-positive Hodgkin disease.50,51 Therefore, it would be of great interest to test the ability of LMP1-specific CTLs to lyse EBV-positive H-RS cell lines, but no cell line is readily available for such studies. In an effort to increase the frequency and/or affinity of LMP1-specific CTLs in the generated T-cell lines, we used LCLs to restimulate DC_ΔLMP1-reactivated CTL lines. The resultant LMP1-specific CTL line lysed ΔLMP1-expressing fibroblast and autologous LCLs; no killing of targets was observed expressing the immunodominant EBV proteins recognized by LCL-activated EBV-specific CTLs from the same donor. Other investigators have used the sequential combinations of APCs to generate antigen-specific T cells17,18,52; most likely, nonspecific T-cell proliferation is reduced and/or the affinity of the generated antigen-specific T cells is increased because the level of antigen expression varies between different APCs. Moreover, for the ex vivo generation of LMP1-specific CTLs for adoptive immunotherapy protocols, the use of LCLs to expand DC_ΔLMP1-activated CTLs is advantageous because it decreases the patient's blood volume required for DC generation. The efficacy of LMP1-specific T-cell therapy for the adoptive immunotherapy of EBV-positive Hodgkin disease may be determined only in a clinical trial. So far we have only generated LMP1-specific CTLs from healthy donors. In the past we have shown that EBV-specific CTLs can be generated from patients with EBV-positive Hodgkin disease; the expansion of EBV-specific CTLs was found to be about 10-fold lower than in healthy donors, and the generation of sufficient numbers of CTLs for infusion required the use of a mitogenic cocktail.1 4 These results indicate that the generation of LMP1-specific CTLs from patients with Hodgkin disease should be feasible.
No adenovirus-specific killing was observed in the present study. This finding is in accordance with results of other investigators using similar DC-based CTL stimulation protocols.10,12,39,40 The absence of adenovirus-specific CTLs may be protocol-specific because in most stimulation protocols adenoviral-transduced DCs are cultured for 2 days to allow for maturation and transgene expression. This most likely results in loss of adenoviral-derived epitopes, because adenoviral-specific CTLs could be reactivated when DCs were cultured with PBMCs immediately after adenoviral transduction.53
In summary, we described the generation of LMP1-specific CTLs for future adoptive immunotherapy protocols for patients with LMP1-positive malignancies, like EBV-positive Hodgkin disease or nasopharyngeal carcinoma. The generation of LMP1-specific CTLs was possible only using an inactive, nontoxic LMP1 mutant. The strategy of rendering a protein inactive to allow antigen presentation may be applicable to other tumor-associated antigens that are cytotoxic, immunosuppressive, or oncogenic in their native form.
We thank Malcolm K. Brenner for helpful discussion and advice.
Prepublished online as Blood First Edition Paper, October 31, 2002; DOI 10.1182/blood-2002-05-1514.
Supported by National Institutes of Health grants RO1 CA61384 and RO1 CA74126; peptide synthesis was supported by Core grant CA-16672. S.G. is the recipient of a Doris Duke clinical scientist development award. H.E.H. is the recipient of a Doris Duke distinguished clinical scientist award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen Gottschalk, Center for Cell and Gene Therapy, Baylor College of Medicine, 6621 Fannin St MC 3-3320, Houston, TX 77030; e-mail: smg@bcm.tmc.edu.