The human T-cell leukemia virus type 1 (HTLV) is the first isolated human retrovirus, but its receptor has yet to be identified, in part due to its ubiquitous expression. Here we report that quiescent CD4 and CD8 T lymphocytes do not express this receptor, as monitored with a soluble receptor-binding domain derived from the HTLV envelope. However, HTLV receptor is an early activation marker in neonatal and adult T lymphocytes, detected as early as 4 hours following T-cell–receptor (TCR) stimulation. This induced surface expression of the HTLV receptor requires de novo protein synthesis and results in a wide distribution on the surface of activated lymphocytes. Moreover, the distribution of the HTLV receptor is independent of TCR/CD3-capped membrane structures, as observed by confocal immunofluorescence microscopy. To determine whether HTLV receptor up-regulation specifically requires TCR-mediated signals or, alternatively, is dependent on more generalized cell cycle entry/proliferation signals, its expression was monitored in interleukin 7 (IL-7)–stimulated neonatal and adult T cells. Neonatal, but not adult, lymphocytes proliferate in response to IL-7 and HTLV receptor expression is restricted to the former population. Thus, HTLV receptor expression appears to be an early marker of cell cycle entry. Up-regulation of the HTLV receptor, via signals transmitted through the IL-7 cytokine receptor as well as the TCR, is likely to contribute to the mother-to-infant transmission and spreading of HTLV-1.
Introduction
The human T-cell leukemia virus type 1 (HTLV-1), the first characterized human retrovirus,1 is present in all areas of the world as either an endemic or a sporadic infectious agent.2 In endemic areas, HTLV-1 transmission seems to occur mostly from mother to infant through breast-feeding.3 The exceptionally broad tropism of HTLV-1 in vitro4,5 contrasts with the finding that in vivo, HTLV-1 is found primarily in CD4+ lymphocytes and less frequently in other mononuclear blood cells.6,7 Studies of this apparent discrepancy have been hindered by the high cytotoxicity of HTLV envelopes and their dependence on cell-to-cell contact for infection and spreading.4,8,9 Moreover, investigations have been limited because the HTLV envelope receptor remains unidentified, even though multiple cell surface components including adhesion molecules,10,11 matrix-associated proteins,12 lipids,13 and lipid rafts,14 have been implicated in HTLV envelope (Env)–mediated membrane fusion and virus transmission.
The HTLV-1 Env receptor-binding determinants are entirely contained within the extracellular surface component (SU).4,15 Recently, we demonstrated that the amino-terminal 215 amino acids of the SU harbors the receptor-binding domain (RBD) of HTLV Env.16 The binding specificity of a soluble tagged construct encompassing this region (HRBD) has been demonstrated by its efficient competition with HTLV Env-mediated cell fusion and infection (F.J.K., E. N. Garrido, N.M., M.S., J.-L.B., manuscript in preparation).
Using the RBD of HTLV Env, we have now tracked HTLV receptor expression on T lymphocytes, which have been reported to be a major HTLV-1 reservoir in vivo. Circulating T lymphocytes are almost entirely in the G0 phase of the cell cycle. Activation of these cells via their cognate antigen receptor is the predominant feature of an efficient immune response. On activation, expression of numerous surface markers is modulated,17,18 cytokines are secreted, and cells can undergo as many as 6 to 8 divisions. Here, we have assessed whether expression of the HTLV receptor on T lymphocytes is modulated by their activation state. Although it has previously not been possible to identify mammalian cell types that do not express the HTLV receptor,4 15 we now report that quiescent T lymphocytes do not express the HTLV receptor. Rather, receptor expression on T lymphocytes is induced by T-cell–receptor (TCR) engagement and requires de novo protein synthesis. Furthermore, interleukin 7 (IL-7)–stimulated neonatal and adult T lymphocytes demonstrate distinct cell surface HTLV receptor levels, with significantly higher expression in the immature neonatal T-cell population.
Materials and methods
Generation of HTLV and amphotropic MLV Env fusion proteins
The construction of a pCSI expression vector19 encoding the amino terminal 215 amino acids of the SU region of HTLV Env comprising the signal peptide and the SU RBD fused to a carboxy-terminal rabbit immunoglobulin Fc (rFc) tag, herein referred to as HRBD, is detailed elsewhere (F.J.K., E. N. Garrido, N.M., M.S., J.-L.B., manuscript in preparation). A similar construct, comprising the N-terminal 379 amino acids of the amphotropic murine leukemia virus (MLV) Env SU and fused to the carboxy-terminal rFc tag, was also generated and is herein referred to as ARBD. The RBD of HTLV-2 comprising the N-terminal 178 amino acids was fused to the enhanced green fluorescence protein (EGFP)–coding sequence (from pEGFP-N3; Clontech, Palo Alto, CA) lacking the ATG initiation codon by polymerase chain reaction (PCR) amplification in the pCSI expression vector and is herein referred to as HRBD-EGFP. Cloning details are available on request.
HRBD, ARBD, and HRBD-EGFP proteins were produced by transfecting 293 T cells with the appropriate constructs or with the empty control vector using the calcium phosphate method. After transfection, cells were washed twice with phosphate-buffered saline (PBS) and fresh medium was added. Media containing the various soluble RBDs were harvested 1 day later and clarified by a 5-minute centrifugation at 13 000 rpm at 4°C.
Cell isolations and culture conditions
Adult peripheral blood (APB), obtained from healthy adult donors, as well as umbilical cord (UC) blood, obtained immediately after delivery of full-term infants, was collected in heparinized tubes. CD4+ T cells were purified by negative selection using tetrameric complexes in which one antibody recognizes a surface antigen on B cells, monocytes, natural killer (NK) cells or CD8+ cells and the other recognizes glycophorin A on the surface of red blood cells (RosetteSep, Stemsep Technologies, Vancouver, BC, Canada). Non-CD4+ T cells were then pelleted on Ficoll-Hypaque separation. The purity of each cell isolation was monitored on a FACScalibur (Becton Dickinson, San Jose, CA) following staining with fluorescein isothiocyanate (FITC)–conjugated αCD3 and phycoerythrin (PE)–conjugated αCD4 monoclonal antibodies (mAbs; Immunotech, Marseille, France). Lymphocytes were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), penicillin, and streptomycin. Cells were stimulated with immobilized αCD3 (UCHT1; a generous gift from D. Cantrell, Imperial Cancer Research Fund, London, United Kingdom) and αCD28 (9.3; kindly provided by C. June, University of Pennsylvania, Philadelphia) mAbs (1 μg/mL) and after 2 days, cultured in the presence of recombinant IL-2 (100 U/mL; Chiron, Emeryville, CA). Alternatively, cells were cultured in the presence of recombinant IL-7 (10 ng/mL; Peprotech, London, United Kingdom). When indicated, cycloheximide was added to cell cultures at a concentration of 5 μg/mL (Sigma, St Louis, MO).
The Jurkat T-cell clone 77-6.8, generously provided by Dr K. A. Smith (New York, NY), was maintained in RPMI 1640 medium supplemented with 10% FCS.
Flow cytometry for Env binding, surface markers, and cell cycle analysis
To evaluate binding of HRBD and ARBDexperiments, 5 × 105 CD4+ T cells were washed with PBA (PBS containing 1% bovine serum albumin [BSA] and 0.1% sodium azide), incubated with 0.3 mL control, HRBD, or ARBD supernatants for 30 minutes at room temperature, washed, and labeled for 20 minutes on ice with an FITC-conjugated sheep anti-rFc antibody (1:500 dilution; Sigma). To detect expression of CD4, CD8, CD25, CD69, CD45RA, and CD45RO, cells were incubated for 20 minutes on ice with the appropriate PE-conjugated mAbs or PE-conjugated isotype control mAbs (Immunotech). In all cases, cells were immediately analyzed on a FACSCalibur (Becton Dickinson) and data analysis was performed using CellQuest software (Becton Dickinson).
The percentage of cells in the S-G2/M phases of the cell cycle was determined by propidium iodide (PI) staining. At the indicated time point, cells were resuspended in PI (50 μg/mL) diluted in PBS with 5% glycerol and 0.1% Triton X-100 and incubated for 15 minutes prior to analysis. Cell cycle was analyzed on the FL2-A wavelength after gating out signals due to cell debris.
Confocal immunofluorescence microscopy
CD4+ T cells (5 × 105) were washed with PBS and incubated with HRBD-EGFP supernatants as well as either αCD4 or αCD3 mAbs (0.5 μg) for 30 minutes at room temperature. Cells were then fixed with 3.7% paraformaldehyde in PBS, washed with ice-cold PBS containing 0.1% BSA, and labeled with Cy3-conjugated sheep αmouse-IgG (1:500; Sigma) for 20 minutes on ice. Following PBS washes, cells were seeded on slides (Superfrost; Menzel-Glaser, Braunscheig, Germany) that were coated with poly-l-lysine (0.01%; Sigma) and mounted in Mowiol (Calbiochem, La Jolla, CA). Slides were analyzed on a Leica confocal microscope and acquisitions were performed using an Agfa CoolSpan camera and the MetaMorph software. For comparative analyses, all photographs were taken using the same exposure conditions.
Results
Expression of the HTLV receptor on human T lymphocytes is induced by TCR engagement
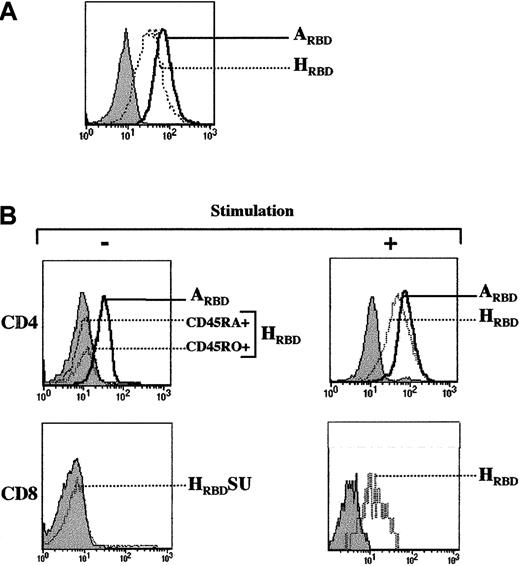
A 215-amino acid truncation of the SU of HTLV Env, herein referred to as HRBD, retained the capacity to bind the HTLV Env receptor as monitored by its ability to specifically interfere with HTLV Env-mediated cell binding, cell fusion, and infection (F.J.K., E. N. Garrido, N.M., M.S., J.-L.B., manuscript in preparation).16 This truncated fragment fused at its C-terminus to a rabbit Fc-tag was used to study HTLV receptor expression on human T-cell subsets. Binding experiments were first validated in the Jurkat T-cell leukemia cell line. As previously demonstrated using the entire HTLV SU,15 HRBDbound efficiently to these cells (Figure1A). PiT-2, the receptor for amphotropic MLV Env,20 is expressed on all T-cell subsets,21 and as such was used as a control throughout this study. As expected, binding of an Fc-tagged amphotropic MLV Env SU fragment (ARBD) was readily detectable in Jurkat cells (Figure 1A). Significant binding of the HRBD to Jurkat T cells was observed as early as 30 minutes after incubation at either 21°C or 37°C, but not at 4°C. In contrast, binding of the amphotropic RBD (ARBD) was observed at all 3 temperatures (data not shown). Whether these differences involve conformational changes of the receptors, the envelopes, or other membrane components remains to be determined.
HTLV receptor expression on CD4+ and CD8+ lymphocyte subsets requires T-cell–receptor stimulation.
(A) Expression of the HTLV Env receptor on Jurkat T cells was assessed using a truncated receptor-binding domain (RBD) of HTLV Env tagged with the Fc domain of rabbit IgG (HRBD). Cells were then incubated with an FITC-conjugated sheep αrabbit IgG antibody and binding was detected by fluorescence-activated cell sorting (FACS) analysis. Similarly, binding of the amphotropic MLV envelope to its receptor was monitored with an amphotropic SU fragment fused to the Fc domain of rabbit IgG (ARBD). Filled histograms depict binding in the presence of the secondary FITC-conjugated sheep antirabbit antibody alone. (B) CD4+ lymphocytes were purified by negative selection and expression of the HTLV Env and amphotropic MLV Env receptors was immediately assessed on the naive (CD45RA+) and memory (CD45RO+) populations as indicated. Expression of the HTLV Env and amphotropic MLV Env receptors was then monitored on CD4+ lymphocytes that had been prestimulated with immobilized αCD3 and αCD28 mAbs (+) for 4 days (upper panels). HTLV Env receptor expression on freshly isolated CD8+ lymphocytes and αCD3/αCD28-stimulated CD8+ lymphocytes (+) is shown (lower panels). Control binding with the secondary FITC-conjugated antibody is shown in all histograms (filled). Data are representative of results obtained in 5 independent experiments.
HTLV receptor expression on CD4+ and CD8+ lymphocyte subsets requires T-cell–receptor stimulation.
(A) Expression of the HTLV Env receptor on Jurkat T cells was assessed using a truncated receptor-binding domain (RBD) of HTLV Env tagged with the Fc domain of rabbit IgG (HRBD). Cells were then incubated with an FITC-conjugated sheep αrabbit IgG antibody and binding was detected by fluorescence-activated cell sorting (FACS) analysis. Similarly, binding of the amphotropic MLV envelope to its receptor was monitored with an amphotropic SU fragment fused to the Fc domain of rabbit IgG (ARBD). Filled histograms depict binding in the presence of the secondary FITC-conjugated sheep antirabbit antibody alone. (B) CD4+ lymphocytes were purified by negative selection and expression of the HTLV Env and amphotropic MLV Env receptors was immediately assessed on the naive (CD45RA+) and memory (CD45RO+) populations as indicated. Expression of the HTLV Env and amphotropic MLV Env receptors was then monitored on CD4+ lymphocytes that had been prestimulated with immobilized αCD3 and αCD28 mAbs (+) for 4 days (upper panels). HTLV Env receptor expression on freshly isolated CD8+ lymphocytes and αCD3/αCD28-stimulated CD8+ lymphocytes (+) is shown (lower panels). Control binding with the secondary FITC-conjugated antibody is shown in all histograms (filled). Data are representative of results obtained in 5 independent experiments.
The in vivo profile of the HTLV receptor is not known and, specifically, its expression on T lymphocyte populations has not yet been elucidated. Using the HRBD construct, we were unable to detect binding to freshly isolated naive (CD45RA) or memory (CD45RO) CD4+ or CD8+ lymphocyte subsets, although in some donors low-level expression could be detected on a maximum of 5% of lymphocytes (Figure 1B and data not shown). The lack of binding was specific to HRBD because ARBD binding was readily detectable on all lymphocyte subsets (Figure 1B and data not shown). Significant differences between Jurkat cells and primary T lymphocytes include cell cycle progression and their general “activation” state. Thus, unlike Jurkat cells, primary T cells are almost entirely in the G0 phase of the cell cycle and do not express activation markers. To induce cell cycle progression and an “activated” profile, T lymphocytes were activated through their cognate antigen receptor (TCR) using αCD3 and αCD28 mAbs. In marked contrast with quiescent T lymphocytes, there was a significant level of HRBD binding to TCR-stimulated CD4+ as well as CD8+ lymphocytes.
To directly visualize the distribution of the HTLV receptor on CD4+ T lymphocytes, we used an HRBD fused directly to EGFP, referred to herein as HRBD-EGFP. In agreement with the FACS data, HRBD-EGFP binding was not detected on unstimulated lymphocytes but was widely distributed on the surface of activated lymphocytes (Figure2A). The lack of HRBD-EGFP binding was not due to an intrinsic difficulty in detecting staining in small quiescent T cells because equivalent binding of an αCD4 mAb was observed on unstimulated and TCR-stimulated CD4+lymphocytes (Figure 2A). Using an αCD3 mAb, a redistribution of TCR/CD3 complexes to capped membrane structures was induced.22 In contrast, this process did not result in a redistribution of the HTLV receptor (Figure 2B). These data were observed whether or not cells were fixed immediately following addition of the primary αCD3 mAb. It should nevertheless be noted that some variation of HRBD-EGFP staining on the surface of cells was observed both in the absence and presence of the αCD3 mAb, most likely due to the convoluted state of the image. Altogether, these data demonstrate a high expression of the HTLV receptor in stimulated T lymphocytes and strongly suggest that the receptor does not partition with CD3/ TCR clusters.
Distinct localizations of the TCR and HTLV Env receptor in activated CD4+ lymphocytes.
(A) Unstimulated or αCD3/αCD28-stimulated CD4+lymphocytes were incubated with an αCD4 mAb, detected with a goat antimouse IgG-Cy3, and the HTLV Env SU RBD fused to EGFP (HRBD-EGFP). Cells were examined by confocal immunofluorescence microscopy. (B) Capping was induced in prestimulated CD4+ lymphocytes using an αCD3 mAb. After a 30-minute induction at room temperature, cells were fixed and incubated with a goat αmouse IgG-Cy3 and HRBD-EGFP to visualize the localization of CD3 and the HTLV receptor, respectively. Original magnification, × 100.
Distinct localizations of the TCR and HTLV Env receptor in activated CD4+ lymphocytes.
(A) Unstimulated or αCD3/αCD28-stimulated CD4+lymphocytes were incubated with an αCD4 mAb, detected with a goat antimouse IgG-Cy3, and the HTLV Env SU RBD fused to EGFP (HRBD-EGFP). Cells were examined by confocal immunofluorescence microscopy. (B) Capping was induced in prestimulated CD4+ lymphocytes using an αCD3 mAb. After a 30-minute induction at room temperature, cells were fixed and incubated with a goat αmouse IgG-Cy3 and HRBD-EGFP to visualize the localization of CD3 and the HTLV receptor, respectively. Original magnification, × 100.
TCR-induced HTLV receptor expression on CD4+ T lymphocytes precedes proliferation and requires de novo protein synthesis
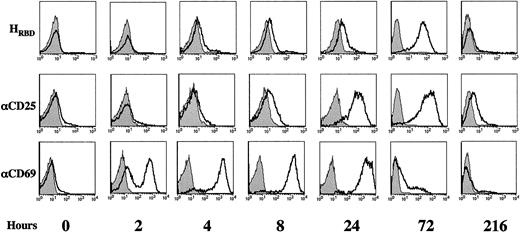
To determine the kinetics of HTLV receptor expression at the surface of activated CD4+ T lymphocytes, cells were stimulated with immobilized αCD3/αCD28 mAbs and binding of HRBD was assessed at 2, 4, 8, 24, 72, and 216 hours following stimulation (Figure 3). Cell surface expression of the HTLV receptor was compared with expression of 2 early activation markers, CD25 (IL-2Rα chain) and CD69 (Figure 3). Cell surface CD69 and CD25 are detected at approximately 4 and 16 hours after stimulation,17 respectively, whereas T lymphocytes enter S phase at approximately 36 hours following TCR engagement23 (J. Garcia-Sanz, personal oral communication, April 2002). As expected, CD69 expression was detected on the vast majority of cells within 8 hours after activation and reached maximal levels at approximately 24 hours. The profile of CD25 induction was slower; levels increased gradually between 4 and 24 hours and plateau levels were maintained for 72 hours. The kinetics of HTLV receptor expression was fairly comparable with that observed for CD25, with a steady increase in receptor levels occurring between 4 and 72 hours after stimulation: the HTLV receptor was expressed on 30% to 40% of cells at 24 hours, whereas high receptor levels were detected on the vast majority of cells at 72 hours (Figure 3).
Kinetics of HTLV Env receptor expression following TCR engagement.
Freshly isolated CD4+ lymphocytes were activated with immobilized αCD3 and αCD28 mAbs and expression of the HTLV Env receptor was followed after 2, 4, 8, 24, 72, and 216 hours of culture using HRBD. Cells were simultaneously evaluated for expression of the CD25 (IL-2Rα) and CD69 activation markers using PE-conjugated mAbs. Staining profiles are shown and filled histograms depict staining with an FITC-conjugated secondary mAb alone or with isotype-matched PE-conjugated control mAbs. Results are representative of data obtained in 4 independent experiments.
Kinetics of HTLV Env receptor expression following TCR engagement.
Freshly isolated CD4+ lymphocytes were activated with immobilized αCD3 and αCD28 mAbs and expression of the HTLV Env receptor was followed after 2, 4, 8, 24, 72, and 216 hours of culture using HRBD. Cells were simultaneously evaluated for expression of the CD25 (IL-2Rα) and CD69 activation markers using PE-conjugated mAbs. Staining profiles are shown and filled histograms depict staining with an FITC-conjugated secondary mAb alone or with isotype-matched PE-conjugated control mAbs. Results are representative of data obtained in 4 independent experiments.
HTLV receptor expression did not remain stable following TCR activation but diminished significantly as T lymphocytes returned to their resting state. In experiments performed with CD4+ T lymphocytes isolated from 4 individual donors, HTLV receptor expression decreased to basal levels between 8 and 14 days after stimulation. Expression of the CD69 and CD25 activation markers also decreased, albeit with different kinetics; CD69 levels were largely decreased by 72 hours, whereas CD25 levels returned to baseline with a longer lag time than the HTLV receptor (12-24 days).
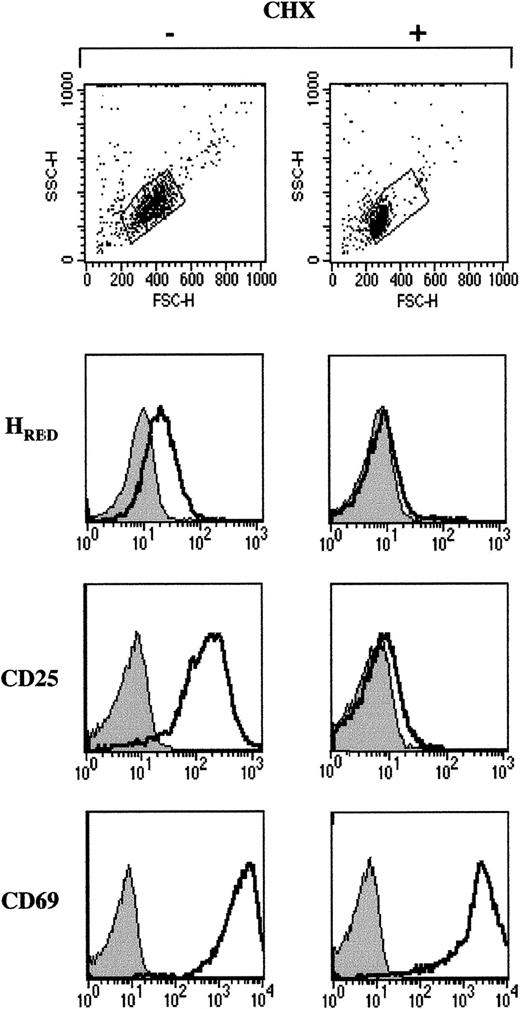
The TCR-induced proliferation of T lymphocytes is preceded by a 7-to 10-fold increase in protein synthesis.24 Indeed, translation is crucial for the propagation of TCR-induced signals because in its absence, activation is inhibited.25 To assess whether surface expression of the HTLV receptor required de novo protein synthesis, freshly isolated quiescent CD4+lymphocytes were stimulated with αCD3/αCD28 mAbs in the presence of the protein synthesis inhibitor, cycloheximide (CHX). Although a 24-hour culture in the presence of 5 μg/mL CHX did not reduce cell viability (not shown), it inhibited TCR-activated blast formation as demonstrated by significantly lower forward and side angle light scatters (Figure 4). Up-regulation of the HTLV receptor as well as CD25 expression were completely abrogated by CHX treatment (Figure 4). In contrast, CD69 was induced to levels observed in control T cells, suggesting that the rapid kinetics of CD69 up-regulation is due mainly to translocation of intracellular stores of CD69 to the cell membrane. Expression of the amphotropic envelope receptor PiT-2 does not require TCR activation and in agreement with this observation, PiT-2 was present on CHX-treated lymphocytes (not shown). Altogether, these data demonstrate that distinct metabolic pathways regulate cell surface expression of the HTLV receptor and CD25, on the one hand, and CD69 on the other hand.
Expression of the HTLV receptor on TCR-stimulated lymphocytes requires de novo protein synthesis.
Freshly isolated CD4+ lymphocytes were activated with immobilized αCD3 and αCD28 mAbs in the absence or presence of the protein synthesis inhibitor cycloheximide (CHX; 5 μg/mL) for 24 hours. The size and density of viable cells were monitored by forward angle light scatter (FSC) and side angle scatter (SSC) on a flow cytometer. HTLV receptor expression as well as CD25 and CD69 levels were assessed using HRBD and the appropriate conjugated mAbs, in the absence and presence of CHX. Filled histograms depict control binding. Data are representative of results obtained in 2 independent experiments.
Expression of the HTLV receptor on TCR-stimulated lymphocytes requires de novo protein synthesis.
Freshly isolated CD4+ lymphocytes were activated with immobilized αCD3 and αCD28 mAbs in the absence or presence of the protein synthesis inhibitor cycloheximide (CHX; 5 μg/mL) for 24 hours. The size and density of viable cells were monitored by forward angle light scatter (FSC) and side angle scatter (SSC) on a flow cytometer. HTLV receptor expression as well as CD25 and CD69 levels were assessed using HRBD and the appropriate conjugated mAbs, in the absence and presence of CHX. Filled histograms depict control binding. Data are representative of results obtained in 2 independent experiments.
Induction of HTLV receptor expression in IL-7–stimulated neonatal T cells
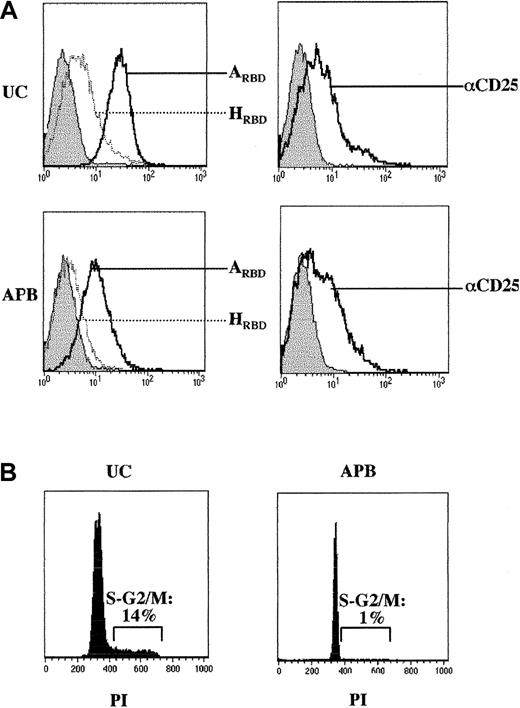
TCR engagement in primary T lymphocytes leads to the induction of an “activated” profile with high expression of CD25 and CD69 at the cell surface as well as an acquisition of a memory phenotype (Figure 3 and data not shown). Because the IL-7 cytokine serves as a T-cell survival factor without inducing an extensive activation profile,26-28 we assessed its effects on HTLV receptor expression. In T cells from APB, relatively low levels of CD25 were induced by IL-7 and cell surface expression of the HTLV receptor was marginal as monitored by binding of HRBD (Figure5A). In contrast, surface expression of the HTLV receptor was detected in IL-7–treated neonatal CD4+ lymphocytes isolated from UC blood, albeit at significantly lower levels than those observed in TCR-activated lymphocytes (Figure 5A). UC-derived lymphocytes are particular in that they are naive T cells that have recently emigrated from the thymus and intriguingly IL-7 induces their proliferation29 30 but not the proliferation of APB CD4+ T cells (Figure 5B).
Induction of HTLV receptor expression on IL-7–stimulated neonatal CD4+ lymphocytes.
(A) Adult and neonatal CD4+ T cells were isolated from adult peripheral blood (APB) and umbilical cord blood (UC), respectively, and cultured for 7 days in the presence of recombinant IL-7 (10 ng/mL). Expression of the HTLV and amphotropic MLV receptors as well as CD25 levels were monitored by FACS using HRBD, ARBD, and a PE-conjugated αCD25 mAb. Filled histograms depict control binding. (B) Cell cycle entry of IL-7–stimulated APB and UC CD4+ lymphocytes was monitored at day 7 by assessing the DNA content of PI-stained cells on FACS. Results are representative of data obtained in 3 independent experiments.
Induction of HTLV receptor expression on IL-7–stimulated neonatal CD4+ lymphocytes.
(A) Adult and neonatal CD4+ T cells were isolated from adult peripheral blood (APB) and umbilical cord blood (UC), respectively, and cultured for 7 days in the presence of recombinant IL-7 (10 ng/mL). Expression of the HTLV and amphotropic MLV receptors as well as CD25 levels were monitored by FACS using HRBD, ARBD, and a PE-conjugated αCD25 mAb. Filled histograms depict control binding. (B) Cell cycle entry of IL-7–stimulated APB and UC CD4+ lymphocytes was monitored at day 7 by assessing the DNA content of PI-stained cells on FACS. Results are representative of data obtained in 3 independent experiments.
Expression of the HTLV receptor in IL-7–stimulated UC lymphocytes was not directly associated with expression of the CD25 activation marker as demonstrated by the findings that CD25 was induced to similar levels in IL-7–treated neonatal and adult CD4+ lymphocytes, whereas only the former bound HRBD at significant levels, and HTLV receptor expression in UC lymphocytes was not restricted to the CD25-expressing population (not shown). Expression of the HTLV receptor was not a constitutive characteristic of UC lymphocytes but was directly dependent on IL-7 treatment; HRBD binding was not observed in freshly isolated quiescent UC T lymphocytes. Moreover, on TCR engagement, HRBD binding to UC lymphocytes was induced to equivalent levels and with comparable kinetics as that observed in adult CD4+ lymphocytes (not shown). Thus, the HTLV receptor, which demonstrates a differential expression profile in IL-7–stimulated lymphocyte populations, represents a distinct activation marker in neonatal and adult CD4+ lymphocytes.
Discussion
Previous to this study, vertebrate cell lines that do not express the HTLV receptor had not been identified. Paradoxically, although T cells appear to constitute a major HTLV reservoir in vivo, we report here that quiescent T lymphocytes lack cell surface expression of the HTLV receptor. However, we demonstrate that HTLV receptor expression is induced under conditions where lymphocytes are stimulated to enter into cell cycle, either via TCR engagement or in the case of neonatal T cells, following IL-7 stimulation. Notably though, expression of the HTLV receptor demonstrates a profile that is distinct from that of other activation markers. The kinetics of HTLV receptor expression was less rapid than that of the CD69 activation marker in TCR-stimulated cells. Moreover, the induction and down-regulation of the IL-2Rα subunit (CD25) did not correlate with HTLV receptor expression in either IL-7–stimulated neonatal or adult T-cell subsets. Whereas low levels of CD25 induction were observed in both IL-7–induced T-cell populations, HTLV receptor expression was detected only in the proliferating neonatal T cells. Thus, HTLV receptor expression appears to be a novel cell cycle entry/proliferation marker on human T cells.
In the context of TCR engagement, expression of the HTLV receptor was not limited to any particular T-cell subset; rather, it was detected on naive and memory lymphocytes as well as on both CD4+and CD8+ populations. Additionally, it should be noted that induction of HTLV receptor expression on naive T lymphocytes largely preceded the acquisition of a memory phenotype as acquisition of the memory CD45RO marker31 generally requires between 4 and 6 days under the conditions of TCR stimulation used here.32 The timing of HTLV receptor expression also preceded DNA synthesis, because the former was detected at 4 to 8 hours after TCR stimulation, whereas the latter generally begins at approximately 36 hours.23 Indeed, we found that HTLV receptor expression was independent of DNA synthesis. Treatment of TCR-stimulated T cells with an agent that blocks cell cycle progression at the end of the G1 phase (aphidicolin) did not abolish up-regulation of the HTLV receptor (S.K. and N.M., unpublished observations, September 2002).
Expression of the HTLV receptor was strictly dependent on de novo protein synthesis. It has been shown that prior to T-lymphocyte proliferation, there is a 7- to 10-fold increase in protein synthesis and a 30- to 40-fold augmentation in mRNA synthesis.33This “pre-proliferation” characteristic appears to be a particularity of T lymphocytes and is not observed in other cell types. The required pre-proliferation burst in mRNA/protein synthesis likely results from the extremely low metabolic rate of resting G0T lymphocytes, which make up the vast majority of the circulating T-lymphocyte pool. Thus, it is intriguing to speculate that it is this low metabolic rate that accounts for the fact that T lymphocytes are the first and only cell type, identified to date, which do not constitutively express the HTLV receptor. Notably, Wodarz and Bangham have recently used a mathematical model to suggest that the rate of evolution of HTLV-1 is limited by the restricted availability of activated uninfected T cells, irrespective of the viral load.34 Distinctions between CD4+ and CD8+ T cells in serving as a reservoir for HTLV in vivo are probably not due to expression of the receptor itself, because we found that binding of HRBD depended on similar activation requirements and kinetics in these 2 cell types. Therefore, the distinct in vivo characteristics of these 2 cell types with regard to HTLV might be due to differences in the postentry steps of the infection. The precise associations between HTLV receptor expression, T-cell cycle progression, and infection remain to be elucidated, but our data suggest that quiescent and activated CD4+ and CD8+ T lymphocytes will provide important models in which to evaluate these questions.
Because of the previous lack of adequate tools and the cytotoxicity of the HTLV envelope, few of the molecular mechanisms regulating HTLV binding at the cell surface have been elucidated. On the other hand, a great deal of work has been performed with the HIV retroviral envelope and it is generally accepted that binding of the SUgp120 HIV-1 envelope glycoprotein to its receptors results in receptor cocapping.35 Notably, HTLV Env was widely distributed on target cells, as assessed by confocal microscopy (Figure 2), and no capped structures were observed. Moreover, under conditions where capping was induced with an antibody directed against the CD3ε epitope of the TCR, neither capping nor a coaggregation of the HTLV receptor was detected. Because TCR engagement results in the clustering of rafts,36 domains of lateral lipid clusters that are enriched in sphingolipids, cholesterols, and glycosylphosphatidylinositol (GPI)–anchored proteins as well as signal transduction assemblies,37 this suggests that HTLV envelope-receptor complexes do not partition in CD3-associated raft structures. The finding that HTLV-1–mediated syncytium formation is dependent on raft integrity,14 is likely due to the fact that the mechanisms regulating HTLV Env binding and syncytia formation are not identical.8 38
In conclusion, the absence of a functional HTLV receptor on quiescent CD4+ lymphocytes and its expression for a short period of time following TCR stimulation are likely to be crucial for the constitution of these cells as HTLV-1 reservoirs in vivo. In the case of neonatal T cells, our results suggest that a narrow window of susceptibility to HTLV-1 infection would result from either TCR stimulation or on contact with the IL-7 cytokine. Neonatal lymphocytes differ from their adult counterparts in that the former represent, almost solely, recent thymic emigrants. Thus, our finding that IL-7–stimulated neonatal T cells express the HTLV receptor suggests a possible mechanism via which HTLV can be transmitted from an infected mother to “nonactivated” recent thymic emigrants in the infant. The data presented here lay the groundwork for further in vitro and in vivo studies directed toward elucidating the pathophysiology of HTLV-1 infection and the role of the HTLV receptor in the T-cell activation process.
We are indebted to C. Boyer and the maternity staff at Clinique St Roch (Montpellier) without whose assistance this study would not have been possible. We thank P. Travo for expert advice on confocal microscopy, C. Mongellaz and L. Swainson for their support, and all the members of our laboratories for insightful discussions.
Prepublished online as Blood First Edition Paper, October 17, 2002; DOI 10.1182/blood-2002-09-2681.
N.M. and S.K. are funded by the Ecole Normale Supérieure (ENS) de Lyon and the European Community (HPMF-CT-2000-01035), respectively. F.J.K. has been funded by an award from the Philippe Foundation and successive fellowships from the Agence Nationale de Recherche sur le SIDA (ANRS), Association pour la Recherche sur le Cancer (ARC), and the Fondation de France. J.-L.B., N.T., and M.S. are supported by INSERM. This work was supported by grants from ARC and the Association Française contre les Myopathies (AFM) (M.S. and N.T), the ANRS and the March of Dimes (N.T.), and Fondation de France (M.S.).
N.M. and S.K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Naomi Taylor or Marc Sitbon, Institut de Génétique Moléculaire de Montpellier, 1919 Route de Mende, 34293 Montpellier, Cedex 5, France; e-mail:taylor@igm.cnrs-mop.fr orsitbon@igm.cnrs-mop.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal