Anaplastic Large CellLymphomas (ALCLs) carry translocations in which the anaplastic lymphoma kinase (ALK) gene is juxtaposed to various genes, the most common of which is the NPM/B23gene. ALK fusion proteins result in the constitutive activation of ALK tyrosine kinase, thereby enhancing proliferation and increasing cell survival. A direct role for NPM-ALK in cellular transformation has been shown in vitro with immortalized cell lines and in vivo using retroviral transfer experiments. Nonetheless, there is no direct evidence of its oncogenic potential in T lymphocytes, which represent the most common target of ALK chimeras. Here, we describe a new mouse model of lymphomagenesis in which human NPM-ALK transcription was targeted to T cells. NPM-ALK transgenic (Tg) mice were born with the expected mendelian distribution, normal lymphoid organs, and a normal number and proportion of helper and suppressor T cells. However, after a short period of latency, all NPM-ALK Tg mice developed malignant lymphoproliferative disorders (mean survival, 18 weeks). NPM-ALK Tg thymic lymphomas displayed a T-cell phenotype characteristic of immature thymocytes and frequently coexpressed surface CD30. A subset of the NPM-ALK Tg mice also developed clonal B-cell plasma cell neoplasms. These tumors arose in peripheral lymphoid organs (plasmacytomas) or within the bone marrow and often led to peripheral neuropathies and limb paralysis. Our NPM-ALK Tg mice are a suitable model to dissect the molecular mechanisms of ALK-mediated transformation and to investigate the efficacy of new therapeutic approaches for the treatment of human ALCL in vivo.
Introduction
Human Anaplastic LargeCell Lymphomas (ALCLs) are a unique subset of lymphomas partly distinguished by their coexpression of the CD30 antigen.1 Classical cytogenetic studies demonstrated that ALCLs carry unique translocations within the p23 region of chromosome 2.2-4 In 1994, Morris et al5 cloned the t(2;5) translocation and discovered that a novel tyrosine kinase gene, the anaplastic lymphoma kinase (ALK), was fused to theNPM/B23 gene. NPM participates in nucleocytoplasmic trafficking6,7 and has been recently shown to regulate the duplication of centrosomes.8 The ALK gene encodes a tyrosine kinase receptor whose physiologic expression is largely limited to neuronal cells.9,10 However, the physiologic role of the ALK receptor remains largely unknown because ALK−/− mice appear normal.11 Nonetheless,ALK is phylogenetically highly conserved,9,10suggesting that it might have an important role in neuronal cellular function. In fact when constitutively activated in the rat pheocromocytoma cells PC12, ALK leads to neuronal differentiation and provides antiapoptotic signals in stress conditions12(data not shown). Recently, Stoica et al13 have also demonstrated that pheotrophin binds to ALK receptor,3but other additional ligands might exist.
In the past 5 years, several groups have successfully cloned new ALCL translocations and demonstrated that the ALK gene can fuse to multiple targets, which include the TFG, TPM3, ATIC, CLTCL, RanBP2, and MSN genes.11 Proteins fused to ALK largely determine the subcellular localization of the derived fusion proteins, being cytoplasmic (ATIC-, TGF-ALK, etc), cytoplasmic and nuclear (NPM-ALK), or membranous (MSN-ALK).11Moreover, ALK translocations can also be detected in nonlymphoid neoplasms such as inflammatory myofibroblastic tumors,14 and ALK expression has been described in neuroblastomas15 as well as in a unique subtype of immunoglobulin A (IgA)–positive plasmacytoid tumors.16
Cellular transformation by NPM-ALK has been demonstrated in immortalized rodent fibroblasts17 and confirmed in studies that have shown that ALK protects Ba/F3 and PC12 cells from interleukin-3 or growth factor withdrawal13,17 (data not shown). Transfer of NPM-ALK–transduced bone marrow cells into irradiated host recipient mice resulted in the generation in vivo of large cell B-cell lymphomas.18 In the past few years, the molecular mechanisms of NPM-ALK–mediated cellular transformation have also been partially elucidated.11 It has been shown that the ALK portion of the fusion protein, corresponding to the cytoplasmic tail of the ALK receptor and containing the catalytic domain, is absolutely required for transformation,17 whereas all the N-terminal regions of the ALK chimeras function as dimerization domains.11,19 As a result of spontaneous dimerization, ALK undergoes autophosphorylation and becomes catalytically active. Constitutively active ALK fusion proteins can bind multiple adaptor proteins and activate a series of pathways involved in cell proliferation, transformation, and survival. These include the phospholipase cγ (PLC-γ) phosphatidylinositol 3 kinase (PI3K)/Akt and the Janus kinase 3/signal transducer and activator of transcription 3 (Jak3-Stat3) pathways.17,20-22 All these molecules and their putative roles were identified by using either nonhematopoietic cells or immortalized B cells, leaving the molecular mechanisms of T-cell transformation by ALK chimeras still unknown. Moreover, the recent discovery of healthy individuals carrying lymphocytes with NPM-ALK or ATIC-ALK translocations23suggests that ALK-mediated T-cell transformation is a complex event that requires multiple and still unknown steps.
To unveil the role of ALK in T-cell transformation, we generated a mouse model in which expression of NPM-ALK was targeted to T lymphocytes. All NPM-ALK transgenic (Tg) mice developed clonal lymphoproliferative disorders after a short period of latency. In addition to T-cell lymphomas, a sizable fraction of our mice also acquired plasma cell neoplasms. Studies using these NPM-ALK Tg mice will allow a better understanding of the molecular mechanisms and genetic defects leading to ALK-mediated transformation.
Materials and methods
NPM-ALK Tg mice, cell lines, and statistical analysis
NPM-ALK transgenic mice were generated by injecting Swiss-Webster blastocysts with a construct in which the full-length cDNA of NPM-ALK chimera was placed under the control of the murine CD4 promoter. The transgenic cassette (CD4 cassette) included the minimal CD4 enhancer (339 base pair [bp]), the minimal murine CD4 promoter (487 bp), the transcription initiation site, and 70 bp of the untranslated first exon and part of the first intron of the murineCD4 gene but lacked the CD8 silencer.24 The NPM-ALK founders were back-crossed into Balb/c and C57B/6 strains and housed in a germ-free facility (Skirball Institute of Biomolecular Medicine, New York University School of Medicine, NY).
Positive NPM-ALK mice were detected by polymerase chain reaction (PCR) by using genomic DNA obtained from mouse tail biopsies as previously described.25 All experiments presented in this study were derived from mice (C57BL/6 and Balb/c backgrounds) obtained from 2 independent transgenic lines (N1 and N16). Δcul1 Tg mice were obtained by placing (SacI/SalI) the human Cul1-N252 cDNA (encoding 1 to 252 [N252] amino terminal residues) into the CD4 cassette Tg.26 Screening of founder animals and their corresponding offspring was performed by PCR and confirmed by Southern hybridization on genomic DNA from tail biopsies. Rag2−/− mice were purchased from Taconic (Germantown, NY).
Primary NPM-ALK cells were obtained from fresh thymic tumors after being cultured in complete RPMI-1640 medium in vitro.
Survival curves were performed by using the nonparametric model of Kaplan-Meier.
Immunoprecipitation and Western blot analysis
Tissue samples and cell lines were lysed (50 mM Tris-HCl (tris(hydroxymethyl)aminomethane) pH7.4, 150 mM NaCl, 0.1% Triton X-100, 5 mM EDTA (ethylenediaminetetraacetic acid), 1 mM Na3VO4, and 1 mM phenylmethylsulfonyl fluoride (PMSF), and protease inhibitors), and supernatants were then used for immunoprecipitation and Western blotting analysis. For immunoprecipitations, 0.2 to 0.5 mg total proteins were incubated for 1 hour at 4°C with 3 μg rabbit anti-ALK antibody (Ab) or a cocktail of mouse anti-ALK monoclonal antibodies (Mabs; Zymed, South San Francisco, CA), or with anti–Grb-2 Ab (SC255; Santa Cruz Biotechnology, Santa Cruz, CA), anti-PI3K Ab (UBI Biotechnology, Waltham, MA); then 30 μL protein G–Sepharose beads (1:1) were added for 30 minutes. Immunocomplexes were washed (3 times with the lysis buffer) and subsequently loaded onto a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel. For Western blotting, 30 μg proteins were run in SDS-PAGE gels and transferred onto nitrocellulose membranes. Membranes were blocked (5% low-fat milk in phosphate-buffered saline [PBS] with 0.1% Tween 20 for 1 hour at room temperature [RT]) and subsequently incubated with the primary antibodies for 1 hour at RT (rabbit anti-ALK [1:4000; Zymed]; mouse anti-ALK Mab [1:5000; Zymed]; antiphosphotyrosine [PY20; 1:1000; Transduction Lab-Becton Dickinson, Mountain View, CA]; anti–Stat3 Mab [1:1000, Zymed]; anti–Jak-1, Jak2, Jak3, Tyk-2 Ab [1:500; Zymed]; anti-Shc Ab [1:500; Santa Cruz]). Filters were washed 3 times and then incubated with horseradish peroxidase (HRPO)–conjugated goat antimouse or antirabbit (1:2000; Amersham, Arlington Heights, IL, for 1 hour at RT) antibodies. Immunocomplexes were detected by using a chemiluminescence system (ECL; Amersham, Piscataway, NJ).27
Southern blotting
Southern blotting was performed as previously described.25 Briefly, high-molecular-weight genomic DNAs (10 μg) were digested by EcoRI, HindIII, orPvu endonucleases, and then digested fragments were separated by electrophoresis. DNAs were subsequently transferred onto nitrocellulose. Radiolabeled cDNA probes were used to study the genomic configuration of T-cell receptor β (TCRβ) and heavy-chain immunoglobulin loci.28 Human NPM-ALK genomic sequences were investigated using BamHI-digested DNAs using a specific ALK cDNA probed (BamHI-BamHI).
Flow cytometry, histology, and immunohistochemistry
Single-cell suspensions were obtained from isolated tissue samples. Cells were washed, counted, and stained with the following murine primary fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, or Tricolor-conjugated antibodies: Thy-1, CD4, CD8, B220, CD25, CD3, TCR α/β, TCR γ/δ (Caltag Laboratories, Burlingame, CA), CD30, CD44, and CD45RB (Pharmingen-BD Biosciences, San Jose, CA). After staining (30 minutes at 4°C), cells were washed and analyzed by using a fluorescence-activated cell sorter scan (FACScan; Becton Dickinson) flow cytometer as described.25
For the histologic and immunohistochemistry analyses, tissue samples were fixed in PBS-buffered formalin (10%) and subsequently embedded in paraffin. Dewaxed 4-μm–thick tissue sections were stained with hematoxylin and eosin or after microwave retrieval (citrate buffer, pH 6.6, 15 minutes) incubated with anti-ALK primary antibody (1:1000; Zymed), anti–Ki-67 (Novacastra), anti-CD45R (B220; 1:100; Caltag), and anti-CD138 (1:20; Pharmingen-BD). Bound complexes were revealed by using the avidin biotin peroxidase complex and a semiautomated immunostainer (DAKO, Carpinteria, CA; or Ventana ES Medical Systems, Tucson, AZ). Mouse light- and heavy-chain expression was performed by using alkaline-conjugated rabbit antimouse antibodies (Southern Biotechnology Associates, Birmingham, AL). For immunofluorescence stains, paraffin-embedded tissue sections were treated as described earlier. Sections were then incubated with rabbit anti-ALK Ab. After washing, tissue sections were incubated with biotin-conjugated antirabbit Ab (1:200; Vector) and then FITC-Avidin (1:200; Sigma-Aldrich, St Louis, MO). Sections were subsequently incubated with normal rabbit serum (1:10, 30 minutes at RT) and then stained with PE-conjugated anti-B220 (1:20; Caltag) in presence of rabbit serum (1:10). After washing, slides were briefly dried and coverslipped with antifade (Vysis, Downers Grove, IL). Fluorescence staining was visualizes by using the 2.7 Cytovision software (Applied Imaging, Santa Clara, CA).
Tissue culture
The rate of spontaneous and in vitro–induced cell death was evaluated according to DNA content and propidium iodide or Annexin V (Pharmingen-BD) stainings.25 Briefly, thymocytes were cultivated with immobilized anti-CD3 antibody (10 μg/mL; 2C11; a gift of J. Bluestone) and soluble anti-CD28 antibody (5 μg/mL; Pharmingen-BD). Alternatively thymocytes were cocultured with dexamethasone (0.1 μM), tumor necrosis factor (TNF; 15 ng/mL), cycloheximide (75 μg/mL; Sigma-Aldrich), anti-Fas Ab (0.5 μg/mL; Pharmingen-BD), phorbol 12-myristate 13-acetate (PMA; 10 ng/mL; Sigma-Aldrich), or Ionomycin (1 μM; Sigma-Aldrich). At the indicated times, cells were harvested, washed, and stained.
Purified peripheral T cells were obtained by magnetic-bead separation. Briefly, 1 × 107 lymph node cells were first incubated (for 30 minutes at RT) with a cocktail of antibodies (0.2-0.5 μg each antibody/106 cells) against B cells (B220; Caltag), macrophages (CD11c; Caltag), and natural killer (NK) cells (Anti-NK; Caltag). At the end of incubation, 70 μL antirat-conjugated magnetic beads (Dynabeads; Dynal, Lake Success, NY) were added. Bead-coated cells were separated in a magnetic field, and unbound cells were washed in cold PBS (3 times). Negatively selected T cells were first stained with FITC-conjugated anti-CD3 mAb and analyzed by FACS to determine their purity (always > 95%). Highly purified T cells (5 × 104) were cultivated in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS), streptomycin and penicillin, and 10−5 M 2-mercaptoethanol in 96-well plate coated with anti-CD3 (0.1-10 μg/mL) and soluble anti-CD28 (0.5 μg/mL) antibody for 48 hours. Alternatively purified T cells were cocultured with PMA (10 ng/mL) or concanavalin A (ConA; 5 μg/mL). 3H-thymidine (0.037 MBq/well [1 μCi/well]; New England Nuclear, Boston, MA) was added for the last 18 hours of culture. Cells were harvested and counted in a 5000TD scintillation counter (Beckman, Fullerton, CA).
Electrophoretic methods
Semiatutomated agarose electrophoresis and immunofixation were performed on HYDRASYS and HYRYS systems (Sebia, Norcross, GA) according to the manufacturer's instructions. For protein electrophoresis, 10 μL sample was applied manually to the sample template. The subsequent sample application, electrophoresis (pH 8.6, 20 W, at 20°C), gel drying, and staining were performed automatically. The resulting electrophoretic profiles were scanned using the Hyris densitometer (Sebia). For immunofixation, each sample was applied in 6 different positions on agarose gels (Hydragel Immunofixation; Sebia), and the electrophoretic separation performed automatically under identical conditions as earlier. Either fixative or monospecific antisera to mouse immunoglobulins (κ, λ, IgG, IgM, and IgA; Southern Biotechnology Associates) were applied to the electrophoresis lanes to allow for fixation and immunoprecipitation, respectively. Detection of monoclonal bands was assessed by visual inspection of stained gels.
Results
NPM-ALK is expressed in normal T cells
To study the influence of NPM-ALK in T cells of mice, the full-length cDNA of NPM-ALK fusion gene was cloned in vector under the control of the murine CD4 promoter (Figure1A). Injection of this construct into blastocysts yielded 6 different NPM-ALK founders that were identified from 3 foster mothers. The copy number of the NPM-ALK transgene varied considerably among the different lines (Figure 1B). With the exception of one mouse (N8), all founders and their corresponding NPM-ALK progenies (N1, N14, N16) expressed the expected ALK fusion protein with a molecular weight of 80 kDa (Figure 1C). This protein corresponded to the NPM-ALK of human cell lines carrying the t(2;5) translocation and was expressed at levels similar to those of human ALCL-derived cell lines (Figure 1C). All 5 NPM-ALK–expressing founders were crossed to generate 5 different mouse lines. However, N5 and N15 died, before mating, of bilateral posterior limb paralysis and thymic tumor, respectively.
Generation of NPM-ALK Tg mice.
(A) NPM-ALK cDNA was cloned into a construct containing the CD4 enhancer and promoter as described in “Materials and methods.” (B) Southern blotting of representative animals obtained from different foster mothers. BamHI-digested DNAs were hybridized with a radio-labeled ALK cDNA probe (1 = N1, 2 = N16, 4 = N15, and 8 = N8. Lane 3, 5, 6, and 7 correspond to correspondent normal littermate). (C) The expression and size of the fusion protein was characterized by Western blot. Proteins were extracted from thymi of Tg (N1, N14, and N16) and wild-type (WT) mice and loaded onto SDS-PAGE gel. The expression of the NPM-ALK chimera was detected with polyclonal rabbit anti-ALK antibody. The protein extract from the human ALCL-derived cell line DHL was used as a control. The loading was checked by Western blot for the ubiquitous CDK2 protein. (D) Histology of NPM-ALK Tg mice. Tg thymus (left panels) or spleen (right panels) were fixed in formalin and embedded in paraffin. Hematoxylin and eosin stains (top panels, × 100) showed normal thymus and spleen architecture in the preneoplastic tissue. Immunostaining with anti-ALK antibody (bottom panels) demonstrated a diffuse positivity in Tg thymocytes with stronger signal in medullary lymphocytes (× 100). In Tg spleen the ALK positivity was localized in the periarteriolar T cell areas of the white pulp (× 400). Left insert shows a nuclear and cytoplasmic staining in Tg lymphocytes (× 400); right insert shows a lower magnification of the spleen (× 100).
Generation of NPM-ALK Tg mice.
(A) NPM-ALK cDNA was cloned into a construct containing the CD4 enhancer and promoter as described in “Materials and methods.” (B) Southern blotting of representative animals obtained from different foster mothers. BamHI-digested DNAs were hybridized with a radio-labeled ALK cDNA probe (1 = N1, 2 = N16, 4 = N15, and 8 = N8. Lane 3, 5, 6, and 7 correspond to correspondent normal littermate). (C) The expression and size of the fusion protein was characterized by Western blot. Proteins were extracted from thymi of Tg (N1, N14, and N16) and wild-type (WT) mice and loaded onto SDS-PAGE gel. The expression of the NPM-ALK chimera was detected with polyclonal rabbit anti-ALK antibody. The protein extract from the human ALCL-derived cell line DHL was used as a control. The loading was checked by Western blot for the ubiquitous CDK2 protein. (D) Histology of NPM-ALK Tg mice. Tg thymus (left panels) or spleen (right panels) were fixed in formalin and embedded in paraffin. Hematoxylin and eosin stains (top panels, × 100) showed normal thymus and spleen architecture in the preneoplastic tissue. Immunostaining with anti-ALK antibody (bottom panels) demonstrated a diffuse positivity in Tg thymocytes with stronger signal in medullary lymphocytes (× 100). In Tg spleen the ALK positivity was localized in the periarteriolar T cell areas of the white pulp (× 400). Left insert shows a nuclear and cytoplasmic staining in Tg lymphocytes (× 400); right insert shows a lower magnification of the spleen (× 100).
The CD4 transgene cassette allows the expression of the target protein in all T cells, including early progenitor thymocytes (CD4+/CD8+) and single positive T cells (CD4+/CD8− and CD4−/CD8+).25 As predicted, the transgenic NPM-ALK protein was localized to cortical and medullary thymocytes, lymphocytes within the interfollicular areas of lymph nodes, and in the T-cell areas of the splenic white pulp (Figure 1D). NPM-ALK was detected in the cytoplasm and nucleus, a pattern similar to that observed in human NPM-ALK+ cells.
Stat3 and Jak3 are constitutively phosphorylated in NPM-ALK Tg mice
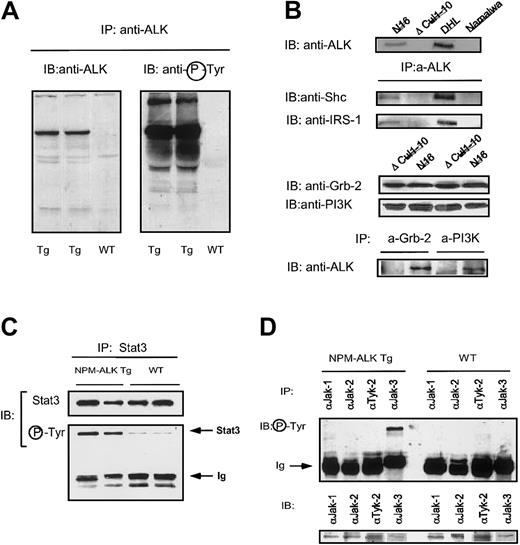
Because NPM-ALK is constitutively autophosphorylated in human ALCL cells, we analyzed the phosphorylation status of NPM-ALK in transgenic cells and observed that in normal, as well as neoplastic NPM-ALK cells, it is constitutively phosphorylated (Figure2A). Because activated ALK fusion proteins can efficiently bind Shc, PLC-γ, Grb-2, and PI3K,29 we studied whether the transgenic NPM-ALK fusion protein could efficiently bind the corresponding mouse proteins as well. As shown in Figure 2B, mouse Shc, IRS-1, Grb-2, and PI3K proteins efficiently bound NPM-ALK in normal as well as in neoplastic cells. Moreover, we were able to demonstrate that phosphorylated Stat3 could be coprecipitated with ALK (data not shown). Because NPM-ALK leads to the constitutive activation of Stat3,21,22 and Jak3,22 we further investigated the activation status of these molecules in our NPM-ALK Tg mice. As shown in Figure 2C-D, NPM-ALK Tg thymocytes, but not control cells, displayed constitutively phosphorylated Stat3 and Jak3. Overall, these findings demonstrate that the NPM-ALK transgene is constitutively activated in T cells and binds to the same adaptor proteins as in humans. Thus, our transgenic model mimics the molecular features of human NPM-ALK+lymphomas.
Molecular characterization of NPM-ALK Tg mice.
(A) Expression and constitutive activation of NPM-AK in Tg mice. Thymocytes from Tg and WT mice were lysed and immunoprecipitated with anti-ALK Ab as described in “Materials and methods.” Western blot with anti-ALK revealed the presence of the protein in Tg but not WT mice (left panel). NPM-ALK protein was constitutively phosphorylated in Tg mice as revealed by the antiphosphotyrosine Ab (right panel). (B) NPM-ALK protein expressed in murine T cells coprecipitates with Shc, IRS-1, Grb-2, and PI3K. Lysed from ALK+samples were immunoprecipitated with anti-ALK (upper panel) or with anti–Grb-2 or anti-PI3K (lower panel). Immunocomplexes were gel electrophoresed and, after transfer, incubated with the indicated antibodies. Direct Western blotting were also performed as indicated. All data are representative of at least 3 different experiments. (C) NPM-ALK Tg mice activate Stat3. Proteins extracted from Tg and WT thymocytes were immunoprecipitated with anti-Stat3 Ab and loaded onto a SDS-PAGE gel. Stat3 protein was similarly immunoprecipitated from both Tg and WT thymocytes. Antiphosphotyrosine Ab revealed the presence of higher levels of activated Stat3 in NPM-ALK Tg mice. (D) NPM-ALK Tg mice activate Jak3. Jak-family of proteins were immunoprecipitated from Tg and WT mice and detected with antiphosphotyrosine Ab. Only Jak3 was constitutively phosphorylated in Tg but not in WT mice. The Jak-family of proteins were equally immunoprecipitated in Tg and WT mice.
Molecular characterization of NPM-ALK Tg mice.
(A) Expression and constitutive activation of NPM-AK in Tg mice. Thymocytes from Tg and WT mice were lysed and immunoprecipitated with anti-ALK Ab as described in “Materials and methods.” Western blot with anti-ALK revealed the presence of the protein in Tg but not WT mice (left panel). NPM-ALK protein was constitutively phosphorylated in Tg mice as revealed by the antiphosphotyrosine Ab (right panel). (B) NPM-ALK protein expressed in murine T cells coprecipitates with Shc, IRS-1, Grb-2, and PI3K. Lysed from ALK+samples were immunoprecipitated with anti-ALK (upper panel) or with anti–Grb-2 or anti-PI3K (lower panel). Immunocomplexes were gel electrophoresed and, after transfer, incubated with the indicated antibodies. Direct Western blotting were also performed as indicated. All data are representative of at least 3 different experiments. (C) NPM-ALK Tg mice activate Stat3. Proteins extracted from Tg and WT thymocytes were immunoprecipitated with anti-Stat3 Ab and loaded onto a SDS-PAGE gel. Stat3 protein was similarly immunoprecipitated from both Tg and WT thymocytes. Antiphosphotyrosine Ab revealed the presence of higher levels of activated Stat3 in NPM-ALK Tg mice. (D) NPM-ALK Tg mice activate Jak3. Jak-family of proteins were immunoprecipitated from Tg and WT mice and detected with antiphosphotyrosine Ab. Only Jak3 was constitutively phosphorylated in Tg but not in WT mice. The Jak-family of proteins were equally immunoprecipitated in Tg and WT mice.
Cellular phenotype and lymphoid organ development in NPM-ALK transgenic mice
To characterize the putative effects resulting from the constitutive activation of NPM-ALK in T lymphocytes, we analyzed the morphologic and phenotypic features of T-cell lymphoid populations and their activation and differentiation states. Overall, the relative and absolute numbers of T and B lymphocytes, within primary and secondary lymphoid organs, were similar in Tg and control littermate mice. Microscopic evaluation demonstrated a normal lymphoid organization with the physiologic preservation of all lymphoid microenvironments. Finally, the histologic surveys of lung, kidney, stomach, intestine, testis, ovaries, and brain did not reveal any morphologic anomalies.
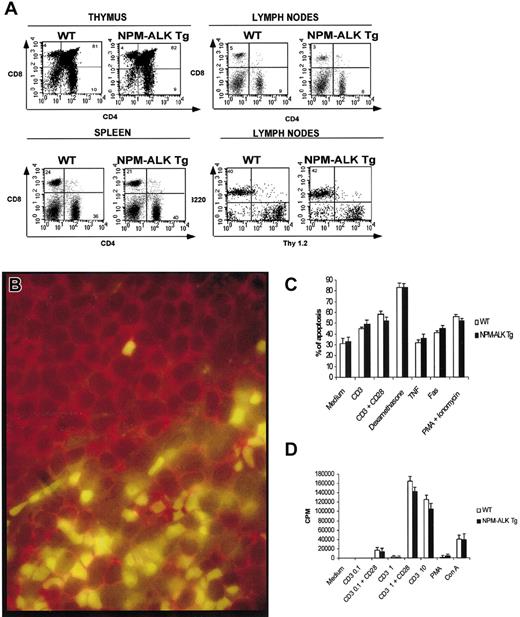
Flow cytometry of NPM-ALK Tg thymocytes showed a normal distribution of CD4−/CD8− and CD4+/CD8+ cells as well as of single positive CD4+ or CD8+ lymphocytes (Figure3A). No significant differences were observed in the expression of other T cell-associated and/or -restricted markers. A normal percentage and expression of Vβ chains and/or the CD3 complexes were also documented in transgenic T lymphocytes, demonstrating that T-cell commitment and maturation proceed normally in these mice (data not shown). The peripheral lymphoid organs showed a normal proportion of B and T lymphocytes and a normal ratio of the CD4+ and CD8+ populations (Figure 3A). Finally, the percentage of activated peripheral T lymphocytes was similar in Tg and WT mice as demonstrated by expression of CD25 and CD69 antigens (3%-5% of the total cells). Flow cytometry of spleen showed no significant differences in the distribution of myeloid, erythroid, or granulocytic lineages. Double immunofluorescence studies were also performed to address whether mature T and/or B cells could express NPM-ALK. As shown in Figure 3B, NPM-ALK expression (nuclear green staining) was restricted to B220/CD45R−cells (B220/CD45R+ cells showed only a red membrane staining) present in the T-cell areas of splenic germinal centers, suggesting that NPM-ALK was largely restricted to T lymphocytes.
Normal phenotype of NPM-ALK Tg mice.
(A) Single cell suspensions obtained from thymocytes, spleen, and lymph nodes were stained with the indicated antibodies and analyzed as described in “Materials and methods.” Tg and WT mice had comparable phenotype in both immature and mature T cells and B lymphocytes. (B) NPM-ALK expression in mature T and B lymphocytes: NPM-ALK expression is restricted only to T cells. Paraffin-embedded tissue section from a preoplastic of NPM-ALK Tg spleen mouse was stained with anti-ALK (green) and anti-B220 (red) Abs. Normal response of Tg lymphocytes to apoptotic and proliferative stimuli. (C) Tg and WT thymocytes were isolated and stimulated for 24 hours with the indicated reagents. The spontaneous and induced apoptotic rate was comparable in Tg and WT mice. (D) Peripheral T lymphocytes were purified from lymph nodes as described in “Materials and methods” and cultured for 72 hours in the presence of the indicated reagents. 3H-thymidine was added for the last 18 hours of culture. Proliferative responses of WT and Tg mice were comparable. The data are representative of at least 2 independent experiments.
Normal phenotype of NPM-ALK Tg mice.
(A) Single cell suspensions obtained from thymocytes, spleen, and lymph nodes were stained with the indicated antibodies and analyzed as described in “Materials and methods.” Tg and WT mice had comparable phenotype in both immature and mature T cells and B lymphocytes. (B) NPM-ALK expression in mature T and B lymphocytes: NPM-ALK expression is restricted only to T cells. Paraffin-embedded tissue section from a preoplastic of NPM-ALK Tg spleen mouse was stained with anti-ALK (green) and anti-B220 (red) Abs. Normal response of Tg lymphocytes to apoptotic and proliferative stimuli. (C) Tg and WT thymocytes were isolated and stimulated for 24 hours with the indicated reagents. The spontaneous and induced apoptotic rate was comparable in Tg and WT mice. (D) Peripheral T lymphocytes were purified from lymph nodes as described in “Materials and methods” and cultured for 72 hours in the presence of the indicated reagents. 3H-thymidine was added for the last 18 hours of culture. Proliferative responses of WT and Tg mice were comparable. The data are representative of at least 2 independent experiments.
To determine whether the constitutive expression of NPM-ALK could possibly modify the survival and/or proliferative potential of T lymphocytes, NPM-ALK Tg thymocytes were incubated in vitro with different apoptotic stimuli. As shown in Figure 3C, both Tg and controls had similar rates of spontaneous and induced apoptosis. The in vitro proliferative rates of purified peripheral T lymphocytes, stimulated with suboptimal and to “ad hoc” concentrations of mitogens, were also similar in transgenic and control mice (Figure 3D). These findings indicate that NPM-ALK alone is not capable of significantly modifying the survival and cell growth of T lymphocytes from young mice in vitro.
NPM-ALK transgenic mice develop spontaneous lymphoid tumors
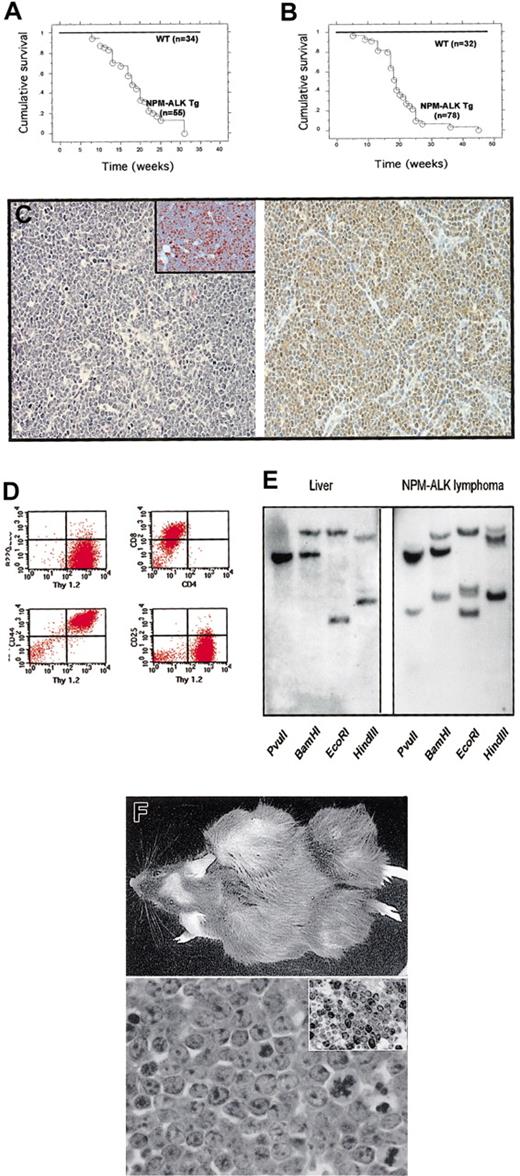
Mice from N1, N14, and N16 lines were healthy up to 5 to 7 weeks of life. After the fifth week, Tg animals started to develop tumors. Survival curves obtained from 86 mice for the N16 line and 110 mice for the N1 line showed a mean survival of 18.5 (Figure4A), and 17 weeks (Figure 4B), respectively, with a overall incidence of 100% for both lines. Tumors were mainly represented by thymic lymphomas or plasma cell neoplasms (Figures 5 and6), and all 3 lines developed, albeit with different frequencies, both thymic lymphomas and/or plasma cell tumors. Mice belonging to the N1 line showed, in fact, a prevalence of plasma cell tumors (> 80%), in contrast to N16 mice that more often developed thymic lymphomas (> 90%). Thymic and plasma cell tumors occurred with a similar frequency (50%) in mice of the N14 line. In rare cases (< 5% overall), we also found neoplasms characterized by atypical, spindle cells within a dense connective tissue. In addition, we also documented rare tumors (< 1%) characterized by immature cells with abundant cytoplasm lacking either T- or B-cell markers, but expressing CD11b. These tumors involved central and peripheral lymphoid tissues and were often observed infiltrating the liver, kidneys, lungs, and other internal organs.
NPM-ALK Tg mice develop lymphomas.
Survival curves NPM-ALK Tg lines N16 (A) and N1(B). (C) Thymic lymphomas. Thymic lymphomas were composed of a homogeneous population of medium-sized lymphoid cells. Numerous mitosis and apoptotic bodies were present (left panel, × 100) (Ki67+ cells were documented by immunohistochemistry, insert; × 200). Immunohistochemical staining with anti-ALK Ab demonstrated a nuclear and cytoplasmic expression of the NPM-ALK fusion protein (right panel, × 100). (D) Typical phenotype of thymic lymphomas. Tumor cells obtained from neoplastic thymus were stained with the indicated Abs and analyzed (Thy1+, B220−, CD44+, CD8+, CD4+/−, CD25−). (E) Southern blot analysis of NPM-ALK lymphomas showing a rearranged pattern of the T-cell receptor with all the enzymes used for digestion. Germline liver DNA was used as control. (F) NPM-ALK T-cell lines established tumors in immunodeficient mice. Tumor cells (2 × 106) (NPM-ALK-Ova) were injected subcutis, and animals were followed daily for 4 weeks (upper panel). Tumors were composed of medium-sized blasts (lower panel, × 400) with high proliferation index (anti-Ki67 staining in the insert, × 200).
NPM-ALK Tg mice develop lymphomas.
Survival curves NPM-ALK Tg lines N16 (A) and N1(B). (C) Thymic lymphomas. Thymic lymphomas were composed of a homogeneous population of medium-sized lymphoid cells. Numerous mitosis and apoptotic bodies were present (left panel, × 100) (Ki67+ cells were documented by immunohistochemistry, insert; × 200). Immunohistochemical staining with anti-ALK Ab demonstrated a nuclear and cytoplasmic expression of the NPM-ALK fusion protein (right panel, × 100). (D) Typical phenotype of thymic lymphomas. Tumor cells obtained from neoplastic thymus were stained with the indicated Abs and analyzed (Thy1+, B220−, CD44+, CD8+, CD4+/−, CD25−). (E) Southern blot analysis of NPM-ALK lymphomas showing a rearranged pattern of the T-cell receptor with all the enzymes used for digestion. Germline liver DNA was used as control. (F) NPM-ALK T-cell lines established tumors in immunodeficient mice. Tumor cells (2 × 106) (NPM-ALK-Ova) were injected subcutis, and animals were followed daily for 4 weeks (upper panel). Tumors were composed of medium-sized blasts (lower panel, × 400) with high proliferation index (anti-Ki67 staining in the insert, × 200).
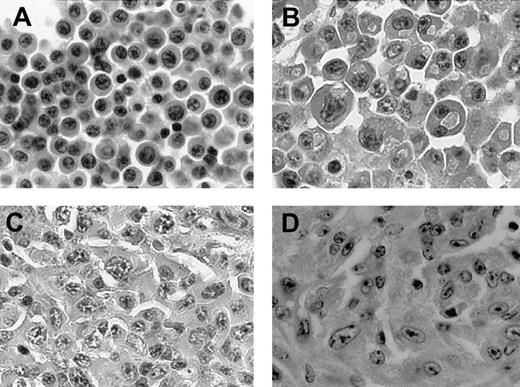
NPM-ALK Tg mice develop plasma cell tumors.
(A-D) Histologic sections of 4 representative plasma cell neoplasms (×400).
NPM-ALK Tg mice develop plasma cell tumors.
(A-D) Histologic sections of 4 representative plasma cell neoplasms (×400).
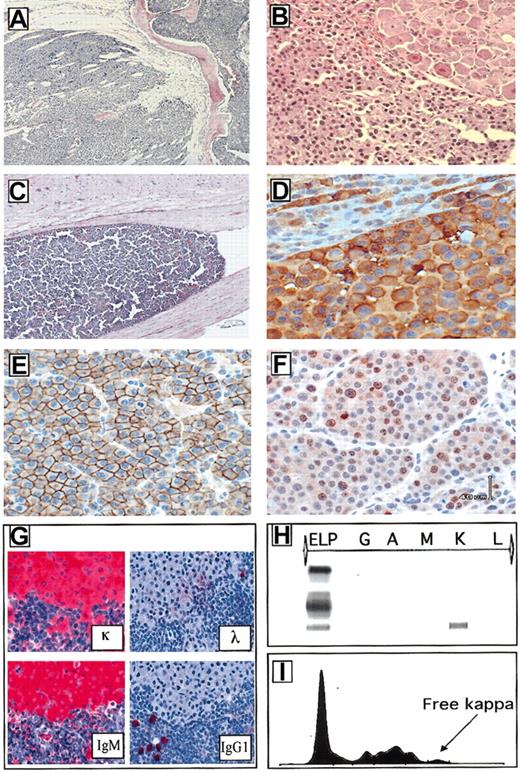
Plasma cell immunophenotype and clonality.
Plasma cell involving the bone marrow replaced the normal bone marrow and disrupted the bone trabeculae (A, × 200). Neoplastic plasma cells often infiltrated the perispinal tissues and ganglions (B, × 200) and in some cases invaded the central nervous system (C, × 200). NPM-ALK was largely confined within the cytoplasm of the neoplastic plasma cells (D, × 400). Tumor cells were invariably CD138+ (E, × 400), displayed a variable number of Ki-67+ cells (F, × 400) and they expressed clonotype heavy and light immunoglobulin determinants (G, × 100). The serum analysis also demonstrated the presence of free light chain immunoglobulin (H-I).
Plasma cell immunophenotype and clonality.
Plasma cell involving the bone marrow replaced the normal bone marrow and disrupted the bone trabeculae (A, × 200). Neoplastic plasma cells often infiltrated the perispinal tissues and ganglions (B, × 200) and in some cases invaded the central nervous system (C, × 200). NPM-ALK was largely confined within the cytoplasm of the neoplastic plasma cells (D, × 400). Tumor cells were invariably CD138+ (E, × 400), displayed a variable number of Ki-67+ cells (F, × 400) and they expressed clonotype heavy and light immunoglobulin determinants (G, × 100). The serum analysis also demonstrated the presence of free light chain immunoglobulin (H-I).
The mediastinal T-cell lymphomas were composed of medium-sized lymphoblasts, with a relatively high mitotic index (10-15 mitosis/10 high power field [hpf]) and high proliferation index as demonstrated by anti–Ki-67 staining (Figure 4C). These immature thymocytes were always Thy-1+ and CD44+ but B220−. The expression of other antigens was variable. Most of the tumors lacked CD4, CD8, CD3, and TCR, but a fraction was CD4+/−/CD8+, CD3+/−, and CD30+ (Figure 4D). Their clonal nature was documented by Southern blot analysis (Figure 4E). Moreover, in a limited number of cases we performed classical cytogenetic analysis, which documented a normal karyotype (data not shown). Finally from a representative group of these tumors, we established 9 different cell lines, whose immunophenotypes matched those of the corresponding primary tumors. All these tumor cell lines grew efficiently in soft agar and in immunodeficient mice (Rag2−/−) after subcutis or intravenous injections (Figure 4F).
Plasma cell tumors could be categorized into 3 major groups, based on their cytologic features. The first group included tumors composed primarily of mature plasma cells characterized by a large cytoplasm and eccentric and sometime binucleated nuclei with evident nucleoli. The second group included tumors with large, atypical cells with irregular nuclei and conscious nucleoli. Finally, a subset of these neoplasms displayed very atypical, pleomorphic/anaplastic cells (Figure 5A-D). Plasmacytomas occurring in lymph nodes, spleen, and very rarely the thymus often completely replaced these lymphoid organs and invariably invaded the surrounding tissues. Furthermore, in a substantial subset of the transgenic mice (20%), the neoplastic plasma cells occupied the bone marrow spaces and invaded into the vertebral bones, compressing and often destroying spinal ganglia and nerves (Figure 6A,B). In rare instances the neoplastic cells, growing within the perispinal spaces, even reached the central nervous system (Figure 6C). These histologic findings corroborated the frequent gross limb paralysis of these mice and other postural and behavioral (spinning and rotational) habits. Notably, these plasma cell tumors occurred with the same frequency in mice crossed in C57BL/6 and Balb/c backgrounds. Immunophenotypic analysis of these neoplasms demonstrated that these tumors rarely expressed B220/CD45R but were invariably NPM-ALK+ (Figure6D) and CD138+ (Figure 6E). The proliferation rate as measured by the Ki-67 staining was variable ranging from 10% to 40% (Figure 6F). The B-cell origin of these tumors was further confirmed by the Southern blotting (Figure 7A) and by enzyme-linked immunosorbent assay (ELISA; data not shown). Furthermore, immunohistochemical staining performed on paraffin-embedded tissue samples demonstrated the clonotypic expression of heavy and light chain of these tumors (Figure 6G). Moreover, free light chain immunoglobulin was demonstrated in animals carrying plasma cell neoplasms (Figure 6H-I). Collectively, these findings demonstrate that these neoplasms express clonal immunoglobulin, which can be secreted and detected in the serum.
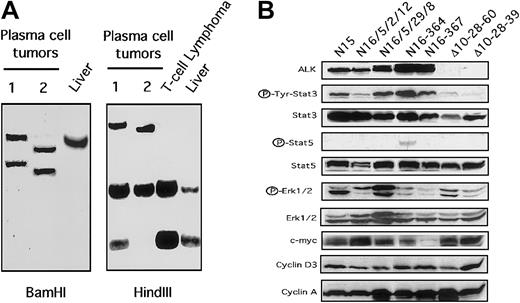
Plasma cell and NPM-ALK tumors.
(A) Southern blot analysis of plasma cell tumors showing rearranged pattern of the immunoglobulin gene. Germline liver DNA was used as control. (B) Constitutive expression of Stat3 in NPM-ALK tumor cells. Total cell extracts from NPM-ALK+ cell lines (lanes 1,2) and from fresh tumors were immunoblotted with the indicated antibodies. Thymic tumor derived from Δcul1 transgenic mice served as controls.
Plasma cell and NPM-ALK tumors.
(A) Southern blot analysis of plasma cell tumors showing rearranged pattern of the immunoglobulin gene. Germline liver DNA was used as control. (B) Constitutive expression of Stat3 in NPM-ALK tumor cells. Total cell extracts from NPM-ALK+ cell lines (lanes 1,2) and from fresh tumors were immunoblotted with the indicated antibodies. Thymic tumor derived from Δcul1 transgenic mice served as controls.
Finally, we investigated the expression profiles of several cell cycle regulators and Stat3 and Stat5 in fresh tumor samples and in 3 NPM-ALK T-cell lines. All NPM-ALK+ samples showed the constitutive expression of phosphorylated Stat3 (Figure 7B). However, a single NPM-ALK case displayed very low levels of phosphorylated Stat5, despite the relatively high levels of Stat5. Interestingly the expressions of c-myc, phospho-Erk-1/2, and cyclin A and D3 were similar in NPM-ALK and in Δcul1 tumors (Figure 7B). We decided to use Δcul1 tumors as control because these tumors show a phenotype similar to that observed in NPM-ALK mice and because the Δcul1 expression was achieved using the same transgenic cassette. Overall, these findings confirm that NPM-ALK Tg neoplastic T cells express high levels of phosphorylated Stat3 and parallel the findings in human ALCL.21 22
Discussion
We have produced and characterized a new mouse model of NPM-ALK–induced lymphomagenesis and have demonstrated that human NPM-ALK leads invariably to the generation of T-cell lymphomas and plasma cell tumors. Our findings show that ALK can efficiently bind a series of mouse adaptor proteins and result in the constitutive activation of Jak3 and Stat3. Nonetheless, in normal cells NPM-ALK alone does not increase cell proliferation and/or promote the survival of normal thymocytes to proapototic agents.
Even though several groups have demonstrated the transforming potential of ALK chimeric proteins in vitro, and Kuefer et al18 have shown that NPM-ALK–containing retrovirus can lead to the transformation of hematopoietic cells toward B-cell large cell lymphomas, the ability of NPM-ALK to induce the transformation of T lymphocytes in vivo is still under debate. Indeed, understanding the pathogenetic role of NPM-ALK in T-cell transformation is important because most of the ALK+ human ALCLs are T cell in origin30 and ALK+ lymphocytes have been detected in healthy individuals.23
Our in vivo studies demonstrated that the constitutive activation of ALK can successfully prompt, with a relatively short latency, spontaneous lymphomagenesis in all mice. This is particularly interesting considering that in other murine transgenic models T-cell tumors occur in only a subset of the animals.28,31,32 The efficient ability of activated ALK to induce transformation may be due to the diversity and complexity of the ALK-signaling pathway. In fact, we and others have shown that PI3K, PLC-γ, Ras, and Jak3-Stat3 pathways can be simultaneously activated.17,21,22,29Interestingly, these pathways not only enhance cell growth but also provide antiapoptotic signals, in some cases by synergistically augmenting their individual effects. Toward this end, the activation of Stat3 and PI3K provide synergistic survival signals via Bcl-xL22 and AKT,29 respectively. Growth signals can be also achieved via Stat3 and its downstream effectors, mainly cyclinD1 and c-myc (data not shown), and Ras via Erk1/2.
In our model the T-cell tumors were exclusively lymphoblastic lymphomas. The precise mechanism(s) for this prevalence is unclear. Because thymocytes proliferate rapidly, it is possible that cell proliferation may be an important requirement for the ALK-mediated transformation observed in our model. A relatively high rate of cell division may allow the acquisition of a sufficient number of genetic alterations capable of cooperating with ALK, thereby spurring transformation. Alternatively, the transformation of NPM-ALK thymocytes may occur because immature T cells express unique genes, which may promote the activation of unique pathways or facilitate genetic aberrations. For example, immature thymocytes actively undergo gene rearrangement of their TCR loci, and they are likely to undergo erroneous genetic recombination.33 These errors might lead to cell death but in the presence of ALK may fortuitously encourage or promote transformation.
Immunophenotypic analysis showed that a fraction (approximately 50%) of ALK T cells coexpressed the CD30 antigen, a molecule that is invariably expressed by human ALCL.1 Because a very small subpopulation of normal thymocytes express detectable surface CD30,34 it is unclear whether the NPM-ALK CD30+ tumors may simply derive from these normal CD30+ thymocytes or whether CD30 could be up-regulated as a result of ALK expression or cell transformation. If NPM-ALK directly regulated the expression of CD30, one could predict the overexpression of CD30 in normal NPM-ALK Tg thymocytes. However, the analysis of normal transgenic cells did not show any CD30 up-regulation. Thus, transformed NPM-ALK+ cells may undergo nonspecific changes, such as chromatin remodeling, etc, which facilitate the transcription of other genes, including CD30, via transcription factors whose expression might be ALK mediated. These hypotheses require additional studies.
Together with T-cell lymphomas, NPM-ALK Tg mice also developed ALK+ plasma cell tumors. The B-cell origin of these tumors was confirmed by the presence of specific heavy-chain immunoglobulin gene rearrangements and by expression of B-cell/plasma cell–associated antigens CD45R and CD138. Our results exclude the possibility that ALK tumors may develop as a result of the unique insertion of the transgene in proximity of genes expressed in B cells and/or responsible for the activation of target genes that facilitate B-cell transformation, because all the 3 transgenic lines developed such tumors. The constitutive expression of ALK in these neoplastic plasma cells in addition to their high incidence in NPM-ALK Tg mice strongly suggest that the occurrence of plasma cell neoplasms is due to the forced expression of ALK. Nevertheless, our CD4 cassette should allow the expression of the desired transgene only in T cells.24 The aberrant expression of ALK in the neoplastic plasma cells suggests that this cassette may be transcriptionally active in some B cells that may be committed to plasma cell differentiation and/or or in plasma cells. The expression of CD4 in normal B cells has not been described. However, the absence of the CD8 silencer in our construct may result in the inappropriate expression of the driven transgene. The low level of expression of CD30 in some non–T cells in CD30 Tg mice tends to support this hypothesis (data not shown). Alternatively, plasma cells may aberrantly transcribe CD4 and thus might have the appropriate transcription machinery to induce the expression of NPM-ALK in our transgenic B cells.35-37 Finally, Delsol et al16 have described a group of IgA+plasmacytoid B cell tumors that overexpresses ALK and CD4 antigens.16
Regardless of the precise mechanisms leading to the aberrant expression of ALK in Tg plasma cells, NPM-ALK Tg mice are a suitable model to study plasma cell tumors and, in particular, multiple myeloma. In fact, in addition to peripheral plasma cell tumors, 20% of our Tg mice displayed primary neoplasms within the bone marrow, often involving the dorsal vertebrae. These tumors led to the compression and/or infiltration of ganglia and spinal nerves and ultimately resulted in the paralysis of the posterior legs. The clinical presentation and histologic features of these tumors closely recapitulated those of human multiple myelomas. Therefore, NPM-ALK transgenic mice represent the only murine model for multiple myeloma. In fact because the first study of Anderson et al,38 who demonstrated that after injection of pristane, Balb/c mice were prone to develop plasmacytomas, several investigators have described several other plasma cell models that do not display the features of human multiple meylomas.32 39-41
The discovery of rare plasmacytoid tumors overexpressing ALK in humans16 and the high frequency of plasma cell neoplasms in our mice strongly indicate that the forced expression of ALK could transactivate crucial pathways for the development of plasma cell dyscrasias. We and others have shown that ALK can constitutively transactivate Stat3. Stat3 is known to play an important role in the pathogenesis of multiple myelomas, and its activation is required for the maintenance and survival of neoplastic plasma cells.42Interestingly, the activation of Stats might be achieved in multiple ways. This often occurs via the interleukin 6 receptor (IL-6R) engagement, but recently it was also shown that the inappropriate activation of fibroblast growth factor receptor 3 (FGFR3) can efficiently lead to Stat1 and Stat3 activation.43Collectively, these findings strongly suggest that the transactivation of Stats, and in particular of Stat3, plays a crucial role in the pathogenesis of plasma cell tumors. Finally, activated ALK via Grb-2, Shc, and other adaptors leads to the activation of Ras and Erk1/2 (data not shown), and Ras has been demonstrated to have an important role in the pathogenesis of plasma cell neoplasms.44 45
In conclusion our findings have confirmed the tumorigenic activity of ALK in vivo and have shown that ALK can efficiently transform T lymphocytes and lead to the development of plasma cell neoplasms. Our model will provide a valuable tool to dissect the signaling of ALK and to identify new putative recurrent aberrations cooperating with ALK in promoting T-cell transformation. The NPM-ALK mice are the first in vivo murine model for multiple myeloma and represent a unique model in which to investigate the efficacy of new therapeutic approach for the treatment of both ALCL and multiple myelomas.
We thank Drs A. Rostagno and E. Zhu for their technical assistance.
Prepublished online as Blood First Edition Paper, November 7, 2002; DOI 10.1182/blood-2002-05-1343.
Supported by grant RO1-CA64033 from the National Institutes of Health and by an Associazione Italiana per la Ricerca sul Cancro (AIRC) grant.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Giorgio Inghirami, New York University, Department of Pathology and Kaplan Cancer Center, 550 First Ave, New York, NY 10016; e-mail: inghig01@med.nyu.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal