Some cells undergo apoptosis in response to DNA damage, whereas others do not. To understand the biochemical pathways controlling this differential response, we have studied the intracellular localization of cyclin B1 in cell types sensitive or resistant to apoptosis induced by DNA damage. We found that cyclin B1 protein accumulates in the nucleus of cells that are sensitive to γ radiation–induced apoptosis (thymocytes, lymphoid cell lines), but remains cytoplasmic in apoptosis-resistant cells (primary and transformed fibroblasts). Treatment of both cell types with leptomycin B, an inhibitor of CRM1-dependent cyclin B1 nuclear export, induces apoptosis. Furthermore, ectopic expression of cyclin B1-5xE, a protein that preferentially localizes to the nucleus, is sufficient to trigger apoptosis. Conversely, expression of cyclin B1-5xA, a predominantly cytoplasmic protein, fails to induce apoptosis. This suggests that nuclear accumulation is necessary for cyclin B1–dependent apoptosis. Our observations are consistent with the idea that localization of cyclin B1 is among the factors determining the cellular decision to undergo apoptosis in response to DNA damage.
Introduction
DNA damage causes both cell cycle arrest and apoptosis.1 However, the response to genotoxic stress varies between tissues and some cells rapidly undergo apoptosis in response to DNA damage, whereas others do not. For example, hematopoietic cells undergo apoptosis after exposure to as little as 100 to 200 cGy γ radiation,2,3 whereas fibroblastlike cells are resistant to these doses.4,5 The biochemical basis for this difference has yet to be clearly established. In this report, we have investigated the role that the cyclin B1 cell cycle regulator has in controlling the apoptotic response to γ radiation. Cyclin B1 has important roles in both mitosis6 and γ radiation–induced apoptosis.7
B-type cyclins are binding partners of the cdc2 (cdk1) serine/threonine kinase.8 The cyclin B/cdc2 heterodimer is referred to as the M phase–promoting factor (MPF) because of its ability to induce mitosis by phosphorylating and activating enzymes regulating chromatin condensation, nuclear membrane breakdown, and mitotic microtubule reorganization.9 Of the B-type cyclins, B1 is likely to be most important in mitotic regulation because mice lacking cyclin B2 develop normally and are fertile, whereas mice lacking cyclin B1 die during embryonic development.10 The kinase activity of the cyclin B1/cdc2 complex is regulated by the abundance of cyclin B, the association kinetics of cyclin B and cdc2, and by the phosphorylation state of cdc2.8 For mitosis to proceed, an active cyclin B/cdc2 complex must also accumulate in the nucleus in late prophase.11 Nuclear accumulation of cyclin B1 at the onset of mitosis is dependent on phosphorylation within its cytoplasmic retention signal (CRS)12,13 InXenopus, cyclin B1 nuclear accumulation is controlled, at least in part, through phosphorylation by the pololike kinase.14 Phosphorylation of the CRS may enhance association with cyclin F or importin β, proteins that enhance nuclear import of cyclin B1.15,16 Alternatively, phosphorylation may decrease association with CRM1,17 a nuclear exporter of cyclin B1.
Cyclin B1 is the target of multiple mitotic checkpoints. In human HeLa cells, DNA damage causes a decrease in cyclin B1 mRNA abundance, a decrease in cyclin B1 mRNA half-life, and an inhibition of cyclin B1 nuclear accumulation.18,19 The repression of cyclin B1 transcription is also one mechanism by which the p53 tumor suppressor inhibits G2/M transition.20
Cyclin B1 is a key regulator of apoptosis in some cell types. Cyclin B1 protein is both necessary and sufficient for the induction of γ radiation–induced apoptosis in hematopoietic cells.7Cyclin B1 accumulation is also involved in the apoptosis caused by nerve growth factor (NGF) withdrawal21 and T-cell–receptor activation.22 Because of the importance of cyclin B1 nuclear localization in mitosis, we have investigated the possibility that cyclin B1 nuclear accumulation may also be a key regulator in the cellular decision to undergo apoptosis in response to DNA damage. In this report, we have found that cyclin B1 rapidly accumulates in the nucleus in cells sensitive to γ radiation–induced apoptosis, but not in the nucleus of cells resistant to radiation-induced apoptosis. Inhibition of CRM1, which leads to cyclin B1 nuclear accumulation, is sufficient to induce apoptosis in both the radiation-sensitive and -resistant cells. Furthermore, expression of a cyclin B1 protein that is predominantly nuclear (cyclin B1-5xE) is sufficient to induce apoptosis, whereas a cytoplasmic cyclin B1 allele (cyclin B1-5xA) is not capable of inducing apoptosis. This study provides evidence that localization of cyclin B1 is important in the cellular decision to undergo apoptosis in response to DNA damage.
Materials and methods
Cell culture
Ramos is a human Burkitt lymphoma line; NIH3T3 is an immortalized mouse fibroblast line; Cos-1 is a SV40-transformed African green monkey kidney cell line. All these lines were obtained from the American Type Culture Collection (ATCC; Manassas, VA). HF172 cells are primary human fibroblast cells (gift of Gurmit Singh, McMaster University, Hamilton, ON, Canada). HF172, Cos-1, and NIH3T3 cells were maintained in Dulbecco modified minimal essential medium supplemented with 10% fetal bovine serum (FBS; Gibco BRL, Burlington, ON, Canada) and 2% penicillin-streptomycin (Sigma, St Louis, MO). Ramos cells were maintained in RPMI 1640 medium (Gibco BRL), 10% FBS, and 2% penicillin-streptomycin. Thymocytes were obtained from 8-week-old C57Bl/6 mice, washed in phosphate-buffered saline (PBS) and suspended in RPMI supplemented with 10% FBS. All cell lines were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Plasmid DNA, oligonucleotides, and transfections
Control pCMX and pCMX-cyclin B1Δ151 were received from Tony Hunter, cyclin B-5xA/5xE from Anja Hagting (Wellcome/CRC Institute), and dominant-negative cdc2 (pCMVcdc2D146N) and cdk2 (pCMVcdk2D145N) from Ed Harlow (Massachusetts General Hospital Cancer Center). Plasmids used in the transfections were prepared via Qiagen maxi-prep plasmid purification kits (Qiagen, Mississauga, ON, Canada) according to the manufacturer's protocol. For transfections NIH3T3, Cos-1, and HF172 cells were seeded at 2 × 106 cells/60-mm plate and cultured overnight. Cells were then transiently transfected with Superfect (Qiagen) using a total of 7 μg pDNA and 20 μL Superfect reagent.
Sense and antisense oligonucleotides were prepared by the McMaster University sequencing center; 2 μg of each oligonucleotide was delivered to cells with a 1:4 ratio (wt/wt) of Superfect (Qiagen) for 3 hours. Transfection efficiencies exceeded 80%, as determined using fluorescein isothiocyanate (FITC)–labeled oligonucleotides. Cyclin A sense: CGG CGC AGA GTT GCC CAA CAT; cyclin A antisense: ATG TTG GGC AAC TCT GCG CCG; cyclin B1 antisense: CAT CGG CTT GGA GAG GGA TT; cyclin B1 sense GAT GCC CGA ACC TCT AAA TAA.
Annexin V staining
Redistribution of phosphatidylserine to the outer plasma membrane was visualized by incubating the cells with FITC-conjugated human recombinant annexin V (Immunotech, Mississauga, ON, Canada). Cells were rinsed with PBS containing Ca++and Mg++ and resuspended in 490 μL ice-cold annexin V–binding buffer to 105 to 106 cells/mL. Annexin V/FITC and propidium iodide were added to the cell suspensions on ice as specified by the manufacturer and incubated on ice in the dark for 10 minutes. Cell cycle profiles of no less than 20 000 cells were generated on a Coulter (Mississauga, ON, Canada) EPICS XL Profile flow cytometer. Data were collected and analyzed using Coulter Epics System II Software Version 3.0. Cells were irradiated using a calibrated Cesium 137 gamma cell irradiator (Nordica Instruments, Toronto, ON, Canada).
Immunohistochemistry
To prepare cells for immunohistochemistry, adherent cells were seeded onto glass coverslips. Following treatment, cells were fixed overnight at 4°C in 4% paraformaldehyde in PBS. Cells were then rinsed once with PBS, permeabilized for 3 minutes with 0.2% Triton X-100, washed once with PBS, and then blocked for 1 hour at room temperature with 1:20 FBS or normal goat serum (Vector Laboratories, Burlingame, CA) in PBS. Cells were incubated with cyclin B1 or D1 primary antibody (1:500 or 1:1000 depending on batch; Medicorp, Montreal, QC, Canada), diluted in PBS for 1 hour at room temperature, washed once with PBS, and incubated with Alexa-conjugated 488-nm secondary goat antimouse antibody (Molecular Probes, Eugene, OR). For laminin staining, we used lamin A/C primary antibody (Santa Cruz Biotechnologies, Santa Cruz, CA) diluted 1:250 in PBS for 1 hour, rinsed once in PBS, and incubated with Alexa-conjugated 546-nm secondary goat antimouse antibody (Molecular Probes). DNA was detected with 1 μg/mL Hoechst dye 33342 (Sigma) or Yo-Pro3 (Molecular Probes; no. Y-3607). Staining for adherent cell lines was visualized by fluorescence microscopy using a Leitz Metallux 3 microscope, a Wild MPS46 Photoautomat camera (Leica). Staining for the suspension cell line Ramos and for primary thymocytes was visualized by Zeist Axiovert 100M confocal system with both and argon ion 488-nm line and a He-Ne 543 line. Serial sections (0.5 μM) in the z-axis of the cell were analyzed using LSM 510 software version 2.3. Deconvolution microscopy was performed using a Leitz DM1RB and the computer program Open Lab (Improvision, Lexington, MA).
Results
Cyclin B1 accumulates in the nucleus of cell types sensitive to apoptosis but remains cytoplasmic in radiation-resistant cell types
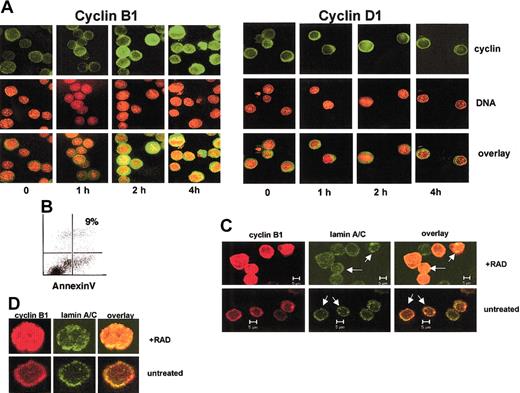
Some cells undergo apoptosis in response to DNA damage, whereas others do not. We used primary mouse thymocytes and human Ramos Burkitt lymphoma cells as apoptosis-sensitive cells and NIH3T3, Cos-1, and primary human HF172 fibroblast cell lines as archetypal resistant cells. As shown in Figure 1A, exposure to 400 cGy γ radiation is sufficient to induce apoptosis in mouse thymocytes and Ramos cells, as measured by annexin V staining.23 At these doses, no apoptosis is detected in the HF172, NIH3T3, and Cos-1 cell lines. Radiation doses up to 1000 cGy did not induce apoptosis in HF172, NIH3T3, or Cos-1 cells (not shown).
Cell type differences in the sensitivity to radiation-induced apoptosis correlates with localization differences in cyclin B1 protein.
(A) A dose of 200 cGy γ radiation causes apoptosis in mouse primary thymocytes and Ramos cells but not human HF172 fibroblasts, Cos-1, and NIH3T3 cell lines. Apoptosis is determined flow cytometrically by annexin V staining 12 hours after irradiation. (B) A dose of 200 cGy γ radiation causes the nuclear localization of cyclin B1 protein in mouse primary thymocytes and Ramos cells but not human HF172 fibroblasts, Cos-1, and NIH3T3 cell lines. Green fluorescence indicates cyclin B1 staining; red fluorescence indicates nuclear staining with the dye Yo-Pro3 (thymocytes, Ramos) or Hoechst 33342 (HF172, Cos-1, NIH3T3); yellow indicates where the stains overlap. Staining was performed 4 hours after irradiation. Ramos and thymocyte images are confocal, whereas those of HF172, Cos-1, and NIH3T3 are conventional fluorescence microscopy. Original magnification B, × 630.
Cell type differences in the sensitivity to radiation-induced apoptosis correlates with localization differences in cyclin B1 protein.
(A) A dose of 200 cGy γ radiation causes apoptosis in mouse primary thymocytes and Ramos cells but not human HF172 fibroblasts, Cos-1, and NIH3T3 cell lines. Apoptosis is determined flow cytometrically by annexin V staining 12 hours after irradiation. (B) A dose of 200 cGy γ radiation causes the nuclear localization of cyclin B1 protein in mouse primary thymocytes and Ramos cells but not human HF172 fibroblasts, Cos-1, and NIH3T3 cell lines. Green fluorescence indicates cyclin B1 staining; red fluorescence indicates nuclear staining with the dye Yo-Pro3 (thymocytes, Ramos) or Hoechst 33342 (HF172, Cos-1, NIH3T3); yellow indicates where the stains overlap. Staining was performed 4 hours after irradiation. Ramos and thymocyte images are confocal, whereas those of HF172, Cos-1, and NIH3T3 are conventional fluorescence microscopy. Original magnification B, × 630.
We used confocal and fluorescence microscopy to determine whether there might be differences in the intracellular localization of cyclin B1 between apoptosis-sensitive and apoptosis-resistant cell lines. The spherical structure of the suspension growing Ramos and thymocytes requires confocal imaging to clearly determine intracellular localization of a given protein. On the other hand, protein localization of the thinner adherent HF172, NIH3T3, and Cos-1 cells can be more optimally visualized using conventional fluorescence microscopy. As shown in Figure 1B, cyclin B1 localizes to the nucleus in the radiation-sensitive thymocytes and Ramos cells within 4 hours of radiation exposure. On the other hand, cyclin B1 remains predominantly cytoplasmic in the radiation-resistant HF172, NIH3T3, and Cos-1 cells. As shown in Figure 2A, the appearance of cyclin B1 in the nucleus of Ramos cells is first visible at 2 hours after irradiation, when approximately 30% of the cells shows evidence of nuclear cyclin B1. Less than 5% of the untreated cells or cells 1 hour after irradiation show nuclear cyclin B1. By 4 hours, 70% to 80% of the irradiated cells show evidence of nuclear cyclin B1. To determine whether other cyclins might also accumulate in the nucleus after irradiation, we visualized the intracellular localization of cyclin D1 in irradiated cells. Cyclin D1 is a regulator of the mammalian G1/S transition24 and undergoes cell cycle–dependent accumulation in the nucleus.25 However, as shown in Figure 2A, cyclin D1 does not accumulate in the nucleus of irradiated Ramos cells. This is consistent with the idea that radiation specifically induces nuclear accumulation of cyclin B1.
Radiation induces cyclin B1 nuclear accumulation in a time-dependent manner.
(A) Ramos cells subjected to 400 cGy γ radiation and the indicated times after irradiation stained for cyclin B1 or cyclin D1 (green) or DNA (red). The overlay of the images is in yellow. (B) Ramos cells 4 hours after irradiation do not show evidence for apoptosis, as measured by annexin staining. (C) The irradiated Ramos cells have an intact nuclear membrane. Untreated cells or those 4 hours following irradiation were stained with antibodies specific for cyclin B1 (red) or lamins A/C (green). The nuclear lamin is intact in both treatments as evidenced by a circular lamin-staining ring (arrows). An overlay of the 2 colors indicates that cyclin B1 protein is within the nuclear lamina in irradiated Ramos cells. (D) Higher magnification of irradiated and unirradiated Ramos stained for cyclin B1 (red) or lamin A/C (green). Original magnification A,D, × 630.
Radiation induces cyclin B1 nuclear accumulation in a time-dependent manner.
(A) Ramos cells subjected to 400 cGy γ radiation and the indicated times after irradiation stained for cyclin B1 or cyclin D1 (green) or DNA (red). The overlay of the images is in yellow. (B) Ramos cells 4 hours after irradiation do not show evidence for apoptosis, as measured by annexin staining. (C) The irradiated Ramos cells have an intact nuclear membrane. Untreated cells or those 4 hours following irradiation were stained with antibodies specific for cyclin B1 (red) or lamins A/C (green). The nuclear lamin is intact in both treatments as evidenced by a circular lamin-staining ring (arrows). An overlay of the 2 colors indicates that cyclin B1 protein is within the nuclear lamina in irradiated Ramos cells. (D) Higher magnification of irradiated and unirradiated Ramos stained for cyclin B1 (red) or lamin A/C (green). Original magnification A,D, × 630.
Cyclin B1 nuclear accumulation occurs prior to apoptosis, and Figure 2B shows that at 4 hours after irradiation, a time point at which there is considerable nuclear cyclin B1, there is little annexin staining. Moreover, Ramos cells with nuclear cyclin B1 in Figure 2A do not show apoptosis-specific chromatin condensation, as evidenced by the diffuse nature of the DNA stain in the irradiated cells. Similar results were obtained with thymocytes (not shown). These results suggest that cyclin B1 accumulation in the nucleus is an early event in radiation-induced apoptosis.
Because apoptosis involves breakdown of nuclear structures,26 we wished to exclude the possibility that the apparent nuclear localization of cyclin B1 in irradiated Ramos cells might have resulted from cyclin B1 diffusing throughout a cell lacking an intact nucleus. To this end, we stained Ramos cells with an antibody specific for human lamins A and C, integral protein components of the nuclear lamina.27 As shown in Figure 2C, irradiated Ramos cells maintain an intact lamin-staining ring (arrows) that contains cyclin B1 protein within it. On the other hand, cyclin B1 protein in unirradiated Ramos cells does not show detectable cyclin B1 within the lamin ring. A higher magnification of a representative irradiated and unirradiated Ramos cell is shown in Figure 2D. This is consistent with the idea that cyclin B1 protein is indeed nuclear during the early stages of radiation-induced apoptosis.
Treatment with LMB induces apoptosis and sensitizes cells to radiation
We have previously reported that cyclin B1 has an important role in radiation-induced apoptosis in Ramos and other hematopoietic cells.7 Because only the radiation-sensitive cells in Figure 1 showed evidence of radiation-induced cyclin B1 nuclear accumulation, we hypothesized that this difference might account for differences in radiation sensitivity. We reasoned that increasing cyclin B1 nuclear levels might itself be sufficient to sensitize the radiation-resistant cells. Nuclear accumulation of cyclin B1 is regulated in part by the CRM1 (exportin 1) protein, which exports cyclin B1 from the nucleus into the cytoplasm.28 29 To determine whether nuclear accumulation of cyclin B1 might sensitize radiation-resistant cells, we first determined whether the CRM1 inhibitor leptomycin B (LMB) could trigger apoptosis in NIH3T3 cells. As shown in Figure3A, LMB treatment is sufficient to activate apoptosis, as measured by the large increase in annexin staining. This dose of LMB is sufficient to cause nuclear accumulation of cyclin B1 (Figure 3B). Because CRM1 has multiple cellular targets other than cyclin B1, we used cyclin B1 antisense to determine whether LMB-induced apoptosis required cyclin B1. As shown in Figure3C, cyclin B1 antisense, but not sense, oligonucleotides inhibit LMB-induced apoptosis. Treatment with antisense inhibits cyclin B1 protein production in the presence and absence of LMB as determined by Western blotting (Figure 3D). Control sense oligonucleotides had little effect on cyclin B1 protein levels. Antisense to cyclin A does not affect LMB-induced apoptosis (Figure 3E) but does decrease cyclin A but not cyclin B protein levels (Figure 3F). As shown in Figure 3G, doses of LMB that do not induce appreciable apoptosis alone (0.05 ng/mL) sensitize NIH3T3 cells to radiation-induced apoptosis. Similar results were also obtained with Cos-1 cells. This is consistent with the idea that modulating cyclin B1 nuclear levels through LMB can sensitize radiation-resistant cells to radiation-induced apoptosis. As shown in Figure 3H, LMB also induces apoptosis in Ramos cells. However, LMB induces apoptosis in Ramos cells at a dose 500 times less than in NIH3T3 cells.
Leptomycin B (LMB) induces apoptosis in a cyclin B1–dependent manner.
(A) LMB (3 ng/mL) induces apoptosis in NIH3T3 cells as measured by annexin V staining. (B) LMB (3 ng/mL) causes nuclear accumulation of the cyclin B1 protein. Original magnification × 400. Cyclin B1 antibody, green; Hoechst 33342 DNA, blue. (C) Treatment of NIH3T3 cells with cyclin B1 antisense oligonucleotides inhibits LMB-induced apoptosis. Sense oligonucleotides had no effect on apoptosis. Apoptosis is measured in the presence and absence of 3 ng LMB. Error bars represent the SEM of no less than 20 000 cells and is a representative of triplicate experiments. (D) Cyclin B1 protein levels indicating that the antisense treatment from Figure 2C lowers cyclin B1 protein levels in the presence or absence of LMB. Protein (10 μg/well) is loaded and cdc2 staining is used as a loading control. (E) Cyclin A antisense has no affect on LMB-induced apoptosis, as measured by annexin staining. (F) Cyclin A antisense reduces cyclin A but not cyclin B1 protein levels. (G) LMB sensitizes NIH3T3 and Cos-1 cells to radiation-induced apoptosis. Cells were treated with 0.05 ng/mL LMB and annexin V binding measured after 200 cGy γ radiation. Errors are the mean of triplicate experiments. (H) LMB (0.05 ng/mL) induces apoptosis in Ramos cells (0.05 ng/mL), as measured by annexin V staining.
Leptomycin B (LMB) induces apoptosis in a cyclin B1–dependent manner.
(A) LMB (3 ng/mL) induces apoptosis in NIH3T3 cells as measured by annexin V staining. (B) LMB (3 ng/mL) causes nuclear accumulation of the cyclin B1 protein. Original magnification × 400. Cyclin B1 antibody, green; Hoechst 33342 DNA, blue. (C) Treatment of NIH3T3 cells with cyclin B1 antisense oligonucleotides inhibits LMB-induced apoptosis. Sense oligonucleotides had no effect on apoptosis. Apoptosis is measured in the presence and absence of 3 ng LMB. Error bars represent the SEM of no less than 20 000 cells and is a representative of triplicate experiments. (D) Cyclin B1 protein levels indicating that the antisense treatment from Figure 2C lowers cyclin B1 protein levels in the presence or absence of LMB. Protein (10 μg/well) is loaded and cdc2 staining is used as a loading control. (E) Cyclin A antisense has no affect on LMB-induced apoptosis, as measured by annexin staining. (F) Cyclin A antisense reduces cyclin A but not cyclin B1 protein levels. (G) LMB sensitizes NIH3T3 and Cos-1 cells to radiation-induced apoptosis. Cells were treated with 0.05 ng/mL LMB and annexin V binding measured after 200 cGy γ radiation. Errors are the mean of triplicate experiments. (H) LMB (0.05 ng/mL) induces apoptosis in Ramos cells (0.05 ng/mL), as measured by annexin V staining.
Nuclear but not cytoplasmic forms of cyclin B1 induce apoptosis
To determine directly if accumulation of cyclin B1 in the nucleus is sufficient to induce apoptosis, we used cyclin B1Δ151, a cyclin B1 variant that is expressed predominantly in the nucleus.13Cyclin B1Δ151 lacks the amino-terminal 151 amino acids and the CRS but is able to bind cdc2 and form a functional cyclin B/cdc2 complex.13 As shown in Figure4A, NIH3T3 cells transfected with cyclin B1Δ151 become apoptotic relative to control cells transfected with the empty myc-tag vector. As shown in Figure 4B, cyclin B1Δ151 is expressed predominantly in the nucleus.
A cyclin B1 allele lacking CRM1-binding sites induces apoptosis.
(A) Transient transfection of NIH3T3 cells with cyclinB1Δ151 (myc epitope tagged) causes apoptosis, as measured by annexin staining, whereas transfection with the parental pCDNA3-myc vector does not. The figure is representative of 3 experiments. (B) Cells transfected with cyclinB1Δ151 express the protein predominantly in the nucleus as visualized by anti-myc antibody staining. Original magnification × 1000.
A cyclin B1 allele lacking CRM1-binding sites induces apoptosis.
(A) Transient transfection of NIH3T3 cells with cyclinB1Δ151 (myc epitope tagged) causes apoptosis, as measured by annexin staining, whereas transfection with the parental pCDNA3-myc vector does not. The figure is representative of 3 experiments. (B) Cells transfected with cyclinB1Δ151 express the protein predominantly in the nucleus as visualized by anti-myc antibody staining. Original magnification × 1000.
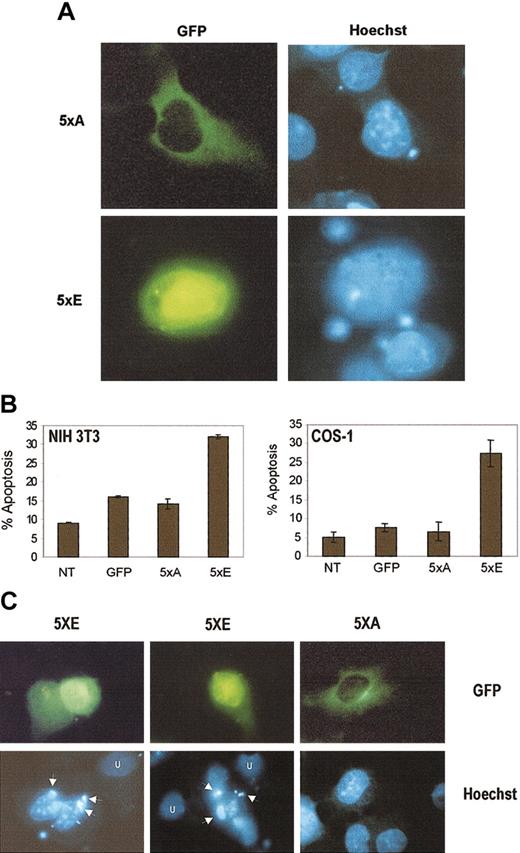
Nuclear accumulation of cyclin B1 is regulated, at least in part, by the phosphorylation state of 5 serine residues (Ser116, Ser126, Ser128, Ser133, Ser147) within the CRS. Phosphorylation of the serines enhances nuclear accumulation.28 To determine whether nuclear localization of cyclin B1 is necessary for apoptosis, we made use of 2 altered cyclin B1 alleles: cyclin B-5xA, where the CRS serine residues have been altered to nonphosphorylatable alanines, and cyclin B-5xE, where the CRS serine residues have been altered to phosphomimetic glutamic acid.28 Both cyclin B-5xA and cyclin B-5xE are green fluorescent protein (GFP) tagged at the amino terminus and the cytomegalovirus (CMV) promoter controls their expression. As shown in Figure 5A, cyclin B-5xE protein expression is primarily nuclear, whereas cyclin B-5xA expression is cytoplasmic. As shown in Figure 5B, cyclin B-5xE is able to induce apoptosis, as measured by annexin staining, in NIH 3T3 and Cos-1 cells. Cyclin B1-5xA, however, is not capable of inducing apoptosis. The levels of apoptosis in 5xA-transfected cells is similar to GFP-transfected cells, but is slightly higher than in untransfected cells because of the transfection procedures. In addition, Figure 5C shows that cyclin B-5xE expression induces the formation of apoptotic bodies in NIH3T3 cells that are not seen in 5xA-transfected cells. This suggests that to induce apoptosis, cyclin B1 must accumulate in the nucleus.
Nuclear, but not cytoplasmic, cyclin B1 alleles induce apoptosis.
(A) Cyclin B-5xE (labeled 5xE) localizes predominantly to the nucleus, whereas cyclin B-5xA (labeled 5xA) is predominantly cytoplasmic (both GFP tagged). Hoechst nuclear counterstain is indicated. (B) The level of apoptosis in cyclin B-5xE–transfected cells is substantially higher than that induced by cyclin B-5xA. The apoptosis induced by cyclin B-5xA is comparable to control pCDNA3 transfected cells. Apoptosis in pCDNA3 control transfected cells is likely due to the transfection and staining protocol. Percent apoptosis was measured by annexin V staining and flow cytometry analysis. Total DNA (5 μg) was constant in all transfections. Error bars represent the mean and SD of 3 independent transfections. (C) Cyclin B-5xE expression induces apoptotic bodies (arrows) not seen in untransfected cells (U) or cells transfected with cyclin B-5xA. Original magnification A,C, × 1000.
Nuclear, but not cytoplasmic, cyclin B1 alleles induce apoptosis.
(A) Cyclin B-5xE (labeled 5xE) localizes predominantly to the nucleus, whereas cyclin B-5xA (labeled 5xA) is predominantly cytoplasmic (both GFP tagged). Hoechst nuclear counterstain is indicated. (B) The level of apoptosis in cyclin B-5xE–transfected cells is substantially higher than that induced by cyclin B-5xA. The apoptosis induced by cyclin B-5xA is comparable to control pCDNA3 transfected cells. Apoptosis in pCDNA3 control transfected cells is likely due to the transfection and staining protocol. Percent apoptosis was measured by annexin V staining and flow cytometry analysis. Total DNA (5 μg) was constant in all transfections. Error bars represent the mean and SD of 3 independent transfections. (C) Cyclin B-5xE expression induces apoptotic bodies (arrows) not seen in untransfected cells (U) or cells transfected with cyclin B-5xA. Original magnification A,C, × 1000.
Discussion
We have found that hematopoietic cells (primary mouse thymocytes and Ramos human B cells) undergoing γ radiation–induced apoptosis accumulate cyclin B1 in their nucleus. Increasing nuclear cyclin B1 levels in these cells with LMB induces apoptosis, suggesting that nuclear accumulation of cyclin B1 is itself a signal for apoptotic initiation. Cyclin B1 is the regulatory subunit of the cdc2 kinase and other groups have previously found that cdc2 activation is required for the apoptosis induced by granzyme B, T-cell activation, and multiple anticancer agents.22,30,31 However, cdc2 activation is not necessary for all forms of apoptosis.32-34 Thus, it is likely that some pathways of apoptosis require cyclin B1/cdc2, whereas others do not. Our data are consistent with the idea that cyclin B1 is involved in apoptosis induced by DNA damage.
We find that cells sensitive to γ radiation–induced apoptosis accumulate cyclin B1 in their nucleus, whereas apoptosis-resistant cells do not. Inducing nuclear cyclin B1 accumulation with LMB sensitizes these cells to radiation-induced apoptosis. This observation is consistent with the idea that differential cytoplasmic localization of cyclin B1 may be why some cell types, generally hematopoietic cell types, readily undergo apoptosis to DNA damage, whereas others, generally epithelial cells, do not. Exclusion of cyclin B1 from the nucleus and inactivation of cyclin B1/cdc2 kinase activity in response to DNA damage causes a G2/M arrest allowing DNA repair.18 19 It is possible that apoptosis-sensitive cells lack the biochemical mechanisms that prevent cyclin B1 from entering the nucleus following DNA damage. Resistant cells are capable of undergoing apoptosis when transfected with nuclear-specific isoforms of cyclin B1 or when treated with LMB. Thus, these cells remain competent for apoptosis induction by nuclear cyclin B1.
We found that in addition to sensitizing cells to DNA damage–induced apoptosis, LMB is itself capable of inducing apoptosis. We observed that cyclin B1 antisense prevents LMB from inducing apoptosis in NIH3T3 cells. This suggests that though CRM1 controls the nuclear export of multiple proteins, cyclin B1 is likely to be the important CRM1 target for apoptosis. The ability of LMB to sensitize NIH3T3 cells to radiation-induced apoptosis suggests that this fungal metabolite may find use as an anticancer agent in conjunction with other apoptosis-inducing stimuli. LMB1 has antitumor activity against some solid tumors xenografted into mice,35 but it has yet to be tried in vivo with radiation or chemotherapeutic drugs. Because cyclin B1 is critical in controlling the mitosis in normal cells, LMB is likely to have some toxicity in normal cells, a characteristic that LMB would share with other chemotherapeutics. However, it is possible that a rapidly proliferating tumor cell may display more sensitivity to LMB than normal quiescent cells due to the increased abundance of total cyclin B1 in an actively proliferating cell population. This idea remains to be tested, but LMB could hold some promise as strategy for anticancer therapy.
The mechanisms by which nuclear localization of cyclin B1 in apoptosis-sensitive cells leads to apoptosis is unclear. Because the pololike kinase is important in controlling cyclin B1 nuclear accumulation during mitosis in Xenopus,14 it may also be involved in regulating DNA damage–induced apoptosis. Because nuclear accumulation of cyclin B1 is a trigger for mitosis, it is possible that DNA damage–induced cyclin B1 nuclear accumulation may lead to apoptosis through an inappropriate triggering of mitosis.36 Cyclin B1/cdc2 could affect that apoptotic machinery directly or apoptosis may proceed through entry into a G2/M-like cell state. Because sensitivity to DNA damage–induced apoptosis controls the efficacy of many anticancer therapies,37 it is important to elucidate the biochemical pathways that govern cyclin B1 nuclear localization in radiation-sensitive and -resistant cells.
We thank our colleagues Steven Innocente, Nisha Anand, and Patricia Collins for their insight into this project. We are grateful to John Hassell, Michael Rudnicki, Maria Rozakis-Adcock, and Gurmit Singh for many helpful discussions. We thank Tony Hunter, Jonathan Pines, Anja Hagting, Ed Harlow, and Sander van den Heuvel for vectors. LMB was generously donated by Dr M. Yosida. We appreciate the microscopy expertise and advise provided by Larry Arseault, Marnie Timlek, Marcia West, and Sharka Llotak.
Prepublished online as Blood First Edition Paper, November 7, 2002; DOI 10.1182/blood-2002-04-1103.
Supported by the National Cancer Institute of Canada with funds from the Canadian Cancer Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jonathan Lee, Hamilton Regional Cancer Center, 699 Concession St, Hamilton, ON, Canada L8V 5C2; e-mail:jonathan.lee@hrcc.on.ca.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal