The granulocyte colony-stimulating factor (G-CSF) and Flt-3 receptor agonist progenipoietin-1 (ProGP-1) has potent effects on dendritic cell (DC) expansion and may be an alternative to G-CSF for the mobilization of stem cells for allogeneic stem cell transplantation (SCT). We studied the ability of stem cell grafts mobilized with this agent to induce graft-versus-host disease (GVHD) to minor and major histocompatibility antigens in the well-described B6 → B6D2F1 SCT model. ProGP-1, G-CSF, or control diluent was administered to donor B6 mice. ProGP-1 expanded all cell lineages in the spleen, and unseparated splenocytes from these animals produced large amounts of interleukin 10 (IL-10) and transforming growth factor beta (TGFβ) whereas the expression of T-cell adhesion molecules was diminished. Transplantation survival was 0%, 50%, and 90% in recipients of control-, G-CSF–, and ProGP-1–treated allogeneic donor splenocytes, respectively (P < .0001). Donor pretreatment with ProGP-1 allowed a 4-fold escalation in T-cell dose over that possible with G-CSF. Donor CD4 T cells from allogeneic SCT recipients of ProGP-1 splenocytes demonstrated an anergic response to host antigen, and cytokine production (interferon gamma [IFNγ], IL-4, and IL-10) was also reduced while CD8 T-cell cytotoxicity to host antigens remained intact. Neither CD11chi DCs nor CD11cdim/B220hi DCs from ProGP-1–treated animals conferred protection from GVHD when added to control spleen. Conversely, when equal numbers of purified T cells from control-, G-CSF–, or ProGP-1–treated allogeneic donors were added to allogeneic T-cell–depleted control spleen, survival at day 60 was 0%, 15%, and 90%, respectively (P < .0001). The improved survival in recipients of ProGP-1 T cells was associated with reductions in systemic tumor necrosis factor alpha generation and GVHD of the gastrointestinal tract. We conclude that donor pretreatment with ProGP-1 is superior to G-CSF for the prevention of GVHD after allogeneic SCT, primarily due to incremental affects on T-cell phenotype and function.
Introduction
Allogeneic stem cell transplantation (SCT) is currently indicated in the treatment of a number of malignant and nonmalignant diseases. However, use of the procedure is limited by its serious complications, the most common of which is graft-versus-host disease (GVHD). Recently, the use of granulocyte colony-stimulating factor (G-CSF)–mobilized stem cell grafts has improved rates of immune and hematopoetic reconstitution, reduced transplantation-related mortality, and improved leukemia eradication after SCT.1 G-CSF has been shown to induce TH2 differentiation in donor T cells prior to SCT and this has been suggested to be a major protective mechanism from GVHD in this setting.2 In support of this, administration of other cytokines that induce TH2 differentiation either in vivo3 or in vitro4 also protect from GVHD. However, G-CSF may also reduce GVHD through effects on monocytes,5-7 natural killer (NK) cells,8 and natural killer T (NKT) T cells.9 The mechanism of TH2 differentiation induced by G-CSF is obscure. The lack of surface expression of G-CSF receptors by T cells supports the notion that this is an indirect effect and in vitro data have implicated monocytes as the responsible immunomodulatory cell.6,7Recently, a subset of dendritic cells (DCs), known as plasmacytoid DCs, has been identified in humans that appears to preferentially induce TH2 differentiation following activation. The proportion of plasmacytoid DCs is increased in the peripheral blood of healthy donors receiving G-CSF.10 Although G-CSF may induce TH2 differentiation before SCT, similar T-cell effects have not been demonstrated in patients undergoing SCT. The suggestion that increased numbers of donor plasmacytoid DCs mediate changes in T-cell function and reductions in GVHD10following allogeneic SCT therefore remains highly speculative.
In the mouse, splenic DCs (characterized by high CD11c and class II expression) have been shown to consist of CD8hi and CD8lo subtypes.11 The high levels of interferon alpha (IFNα) and interleukin 12 (IL-12) produced by the CD8hi DCs predict a potential TH1 response after SCT. On the other hand, these DCs have a reduced capacity to induce proliferative responses in T cells relative to the CD8lo DC subtypes.12 Murine plasmacytoid DCs were recently described as CD11cdimB220+ that are predominantly CD8lo.13
Flt-3 ligand (Flt-3L) is a hematopoietic cytokine that increases DC numbers 20- to 30-fold in mice14 and humans.15 In murine bone marrow transplantation (BMT) models, Flt-3 ligand given days 25 to 40 after BMT enhances donor DC and T-cell expansion with a resultant increase in both GVHD and graft-versus-leukemia (GVL).16 In contrast, treatment of the donor with Flt-3L alone prior to BMT has no apparent effect on subsequent GVHD.16 Progenipoietin-1 (ProGP-1) is a synthetic chimeric molecule that stimulates both G-CSF and Flt-3 receptors and appears to be significantly more potent in the expansion of stem cells and DCs than the combination of both native cytokines due to higher binding affinity.17 18 We reasoned that ProGP-1 may offer an attractive system to study the effect of DC subsets on GVHD after allogeneic SCT.
We therefore compared the ability of stem cell grafts mobilized by either ProGP-1 or G-CSF to induce GVHD. Our data confirm that ProGP-1 expands large numbers of DCs in donor animals. Recipient animals are protected from GVHD in association with an altered T-cell adhesive phenotype and a reduced response to alloantigen after SCT. This confers a secondary inability to augment inflammatory cytokine production and GVHD of the gastrointestinal (GI) tract after SCT, thereby preventing the induction of GVHD.
Materials and methods
Mice
Female C57BL/6 (B6, H-2b, Ly-5.2+), B6 PTRCA Ly-5a (H-2b, Ly-5.1+), and B6D2F1 (H-2b/d, Ly-5.2+)19 mice were purchased from the Australian Research Centre (Perth, Western Australia, Australia). The age of mice used as BM transplant recipients ranged between 8 and 14 weeks. Mice were housed in sterilized microisolator cages and received filtered water and normal chow, or autoclaved drinking water for the first 2 weeks after BMT.
Cytokine treatment
Recombinant human G-CSF (Amgen, Thousand Oaks, CA), progenipoietin (Pharmacia, St Louis, MO), or control diluent was diluted in 1 μg/mL of murine serum albumin in phosphate buffered saline (PBS) before injection. Mice were injected subcutaneously with G-CSF (10 μg/animal per day), ProGP-1 (20 μg/animal per day), or diluent once daily from day −10 to day −1.
Bone marrow transplantation
Mice underwent transplantation according to a standard protocol as has been described previously.2 20 Briefly, on day −1, B6D2F1 mice received 1100 cGy total body irradiation (137Cs source at 108 cGy/min), split into 2 doses separated by 3 hours to minimize GI toxicity. Donor spleens were chopped, digested in collagenase and DNAse, then whole unseparated spleen cells were resuspended in 0.25 mL of Leibovitz L-15 media (Gibco BRL, Gaithersburg, MD) and injected intravenously into recipients. In most experiments, Ly-5a (H-2b, Ly-5.1+) animals were used as donors. Survival was monitored daily, recipient's body weight and GVHD clinical score were measured weekly. Donor cell engraftment was determined by examining the proportion of Ly-5.1+ per (Ly-5.2+ + Ly-5.1+) cells in peripheral blood or spleen after transplantation.
Assessment of GVHD
The degree of systemic GVHD was assessed by a scoring system that sums changes in 5 clinical parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity (maximum index = 10).3 21-23 Individual mice were ear-tagged and graded weekly from 0 to 2 for each criterion without knowledge of treatment group. Animals with severe clinical GVHD (scores > 6) were killed according to ethical guidelines and the day of death deemed to be the following day.
Splenocyte and DC preparation
DC purification was undertaken as previously described.24 Briefly, spleens were chopped and digested in collagenase and DNAse. Light-density cells were selected by nycodenz density (1.077 g/L) centrifugation. Non–DC-lineage cells were depleted by coating with rat immunoglobulin G (IgG) antibodies to B cells (CD19), T cells (CD3, Thy1), granulocytes (Gr-1), and erythroid cells (Ter-119). The coated cells were then removed by magnetic beads coupled to anti–rat IgG (Dynal ASA, Oslo, Norway). At the end of this procedure, 65% to 85% of these cell populations were DCs (class II/CD11chi). DCs were presorted to remove autofluorescent macrophages (prior to phenotypic analysis) and then purified by fluorescence-activated cell-sorting (FACS) (FACS Vantage, Becton Dickinson, San Jose, CA) to more than 98% purity using phycoerythrin (PE) CD11c and PE-Cy5 B220 staining.
T-cell depletion
Splenocytes were depleted of T cells by incubation for 40 minutes (4 degrees) with hybridoma supernatants containing anti–CD4 (GK1.5), CD8 (3.155), and Thy1.2 (HO-13-4) monoclonal antibodies (mAbs). Cell suspensions were then incubated with rabbit complement (Cederlane Laboratories, Ontario, ON, Canada) for 30 minutes at 37 degrees and the process repeated. Resulting cell suspensions had less than 1% contamination of viable CD3 T cells.
FACS analysis
Fluorescein isothiocyanate (FITC)–conjugated mAbs to mouse Ly 5.1 and Ly 5.2 antigens, CD4, CD8, CD11c, class II, CD3, GR-1, CD11b, and B220, and identical PE-conjugated mAbs were purchased from PharMingen (San Diego, CA). In DC analysis, CyChrome CD4 and CD8 antibodies were also used from Pharmingen. Cells were first blocked with mAb 2.4G2 for 15 minutes at 4°C, followed by the relevant conjugated mAb for 30 minutes at 4°C. Finally, cells were washed twice with PBS/0.2% bovine serum albumin (BSA), fixed with PBS/1% paraformaldehyde, and analyzed by FACS Calibur (Becton Dickinson). Propidium iodide was added in the final wash to label dead cells. DC staining was undertaken on presorted cell populations in which autofluorescent cells were removed by high speed presorting (FACS Vantage) and subsequent analysis was performed the same day on unfixed cells.
Cell cultures
Culture media additives were purchased from Gibco BRL and medium was purchased from Sigma (St Louis, MO). Peritoneal macrophages were lavaged and pooled from individual animals within a treatment group before culture at 1 × 105 cells per well in flat-bottom 96-well Falcon plates (Lincoln Park, NJ) with or without lipopolysaccharide (LPS). Cell culture was performed in 2% fetal calf serum (FCS)/Dulbecco modified Eagle medium (DMEM) (day 7 cultures) supplemented with 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acid, 0.02 mM β-mercaptoethanol, and 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.75, at 37°C in a humidified incubator supplemented with 5% CO2. Supernatants were collected at 5 hours for tumor necrosis factor alpha (TNFα) analysis by enzyme-linked immunosorbent assay (ELISA). Peritoneal macrophages lavaged from animals 7 days after transplantation were more than 95% donor as determined by Ly5.1 staining. All other cell cultures were performed in 10% FCS/DMEM. In in vitro experiments, purified B6 T cells were cultured in round-bottom 96-well plates (Falcon) with 105 irradiated (2000 R) B6D2F1 peritoneal macrophages (primary mixed lymphocyte culture [MLC]) and supernatants harvested at 72 hours. Cultures were then pulsed with 3H-thymidine (1 μCi [0.037 MBq] per well) and proliferation was determined 16 hours later on a 1205 Betaplate reader (Wallac, Turku, Finland). In secondary MLC, purified T cells were cultured in flat-bottom 24-well plates (Falcon) with irradiated (2000 R) B6D2F1 splenocytes. 6 days later, cells were removed and restimulated with irradiated (2000 R) B6D2F1 peritoneal macrophages. Supernatants were removed 24 hours later and3H-thymidine added as above. In experiments of T-cell function ex vivo, splenocytes were removed from animals 7 to 10 days after transplantation and 3 to 6 spleens combined from each group. These cells were plated in 96-well flat-bottom plates with platebound anti–CD3 and –CD28 mAbs (both 10 μg/mL) or 105irradiated (2000 R) peritoneal macrophages lavaged from naive B6D2F1 (allogeneic) animals. At 40 hours, cultures were pulsed with3H-thymidine (1 μCi [0.037 MBq] per well) and proliferation was determined 16 hours later. In separation experiments, CD4+ cells were positively selected from splenocyte populations using the mini-MACS system (Miltenyi Biotech, Bergisch Gladbach, Germany) or FACS (FACS Vantage). Following selection, positive and negative fractions were FACS stained and each fraction had less than 1% contamination of opposing CD4+ or CD8+ cells. Purified CD4+ or CD8+populations were then plated and analyzed as above.
51Cr release assays
P815 (H-2d) or EL4 (H-2b) tumor targets (2 × 106) were labeled with 100 μCi (3.7 MBq) of 51Cr for 2 hours. After washing 3 times, labeled targets were plated at 104 cells per well in U-bottom plates (Costar, Cambridge, MA). CD8+ splenocytes from allogeneic BM transplant recipients (prepared by magnetic selection as described in “Cell cultures”) were added to triplicate wells at varying effector-to-target ratios and incubated for 5 hours in the presence of IL-2 (10 U/mL). Maximal and background release were determined by the addition of Triton-X (Sigma) or media alone to targets, respectively. 51Cr activity in supernatants taken 5 hours later was determined in a scintillation counter and lysis was expressed as a percentage of maximum. Lysis was expressed in lytic units (1000/effector-to-target ratio that induced 10% and 20% lysis).
Cytokine ELISAs
The antibodies used in the TNFα, IFNγ, IL-10, TGFβ, and IL-4 assays were purchased from PharMingen. All assays were performed according to the manufacturer's instructions. Supernatants were collected after 4 to 5 hours of culture for TNFα, and 40 hours for IL-4, IL-10, and IFNγ analysis. Serum was stored at −70°C until analysis. Briefly, samples were thawed and diluted 1:3 to 1:24 and TNFα, IFNγ, IL-10, and IL-4 proteins were captured by the specific primary mAb, and detected by biotin-labeled secondary mAb followed by horseradish peroxidase (HRP)–conjugated streptavidin. The biotin-labeled assays were developed with TMB substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD). Plates were read at 450 nm using a microplate reader (Bio-Rad, Hercules, CA). Recombinant cytokines (PharMingen) were used as standards for ELISA assays. Samples and standards were run in duplicate and the sensitivity of the assays was 16 pg/mL to 20 pg/mL for TNFα, 0.063 U/mL for IFNγ, and 15 pg/mL for IL-10 and IL-4. TNFα from LPS-stimulated peritoneal cells is expressed as picograms per 105macrophages, as previously described.22
Histology
Formalin-preserved distal small bowel was embedded in paraffin, and 5 μm-thick sections were stained with hematoxylin and eosin for histologic examination. Slides were coded and examined in a blinded fashion by one individual (A.D.C.) using a semiquantitative scoring system for abnormalities known to be associated with GVHD.3,22,25 Specifically, 7 parameters each were scored for small bowel (villous blunting, crypt regeneration, crypt epithelial cell apoptosis, crypt loss, luminal sloughing of cellular debris, lamina propria inflammatory cell infiltrate, and mucosal ulceration). The scoring system for each parameter denoted 0 as normal; 0.5 as focal and rare; 1 as focal and mild; 2 as diffuse and mild; 3 as diffuse and moderate; and 4 as diffuse and severe, as previously published in human26,27 and experimental3,22 25 GVHD histology. Scores were added to provide a total score of 28.
Statistical analysis
Survival curves were plotted using Kaplan-Meier estimates and compared by log-rank analysis. The Mann-Whitney U test was used for the statistical analysis of cytokine data and clinical scores.P < .05 was considered statistically significant.
Results
ProGP-1 pretreatment results in a marked expansion of CD8hi DCs
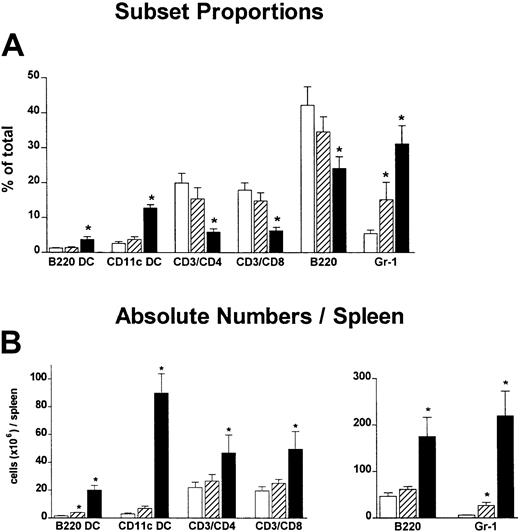
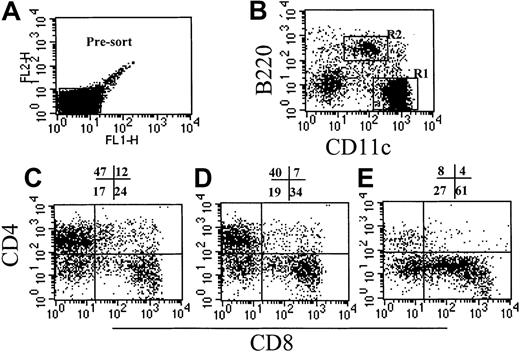
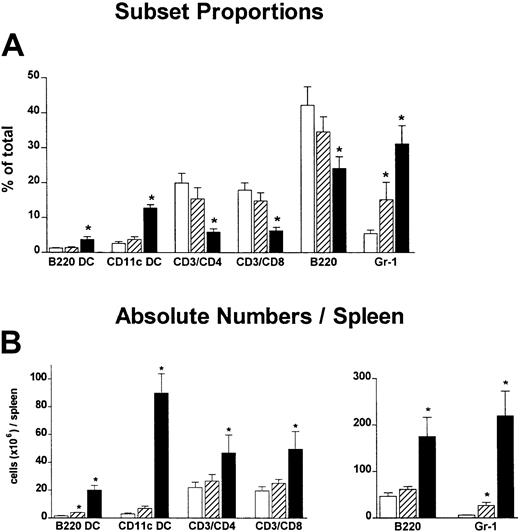
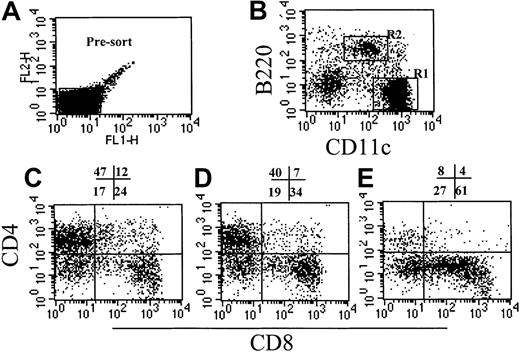
In these studies we compared the effect on GVHD of donor pretreatment with ProGP-1 and G-CSF, the latter being the current cytokine used for the mobilization of allogeneic stem cells in clinical practice. Injections of control diluent, ProGP-1 (20 μg/animal), or G-CSF (10 μg/animal) were administered daily for 10 days. This administration regimen for ProGP-1 was shown in preliminary studies to result in the greatest expansion of stem cells and DCs, consistent with recent reports.28 The G-CSF dose was half that of the chimeric G-CSF/FLT-3L molecule ProGP-1. At the end of this treatment period, absolute numbers of splenocytes increased by 65% in the G-CSF group and 700% in the ProGP-1 group. As shown in Figure 1, the percentages of DCs, CD4 and CD8 T cells, and B cells were similar in control- and G-CSF–treated animals, consistent with our previous findings.20 In contrast, ProGP-1 resulted in a 10-fold increase in the percentage of CD11chi and CD11cdim/B220hi DCs (Figure 1) and a 65% reduction in the percentage of T cells. Significant (30%) reductions in the overall proportion of B220+/CD19+ cells were also noted following ProGP-1 administration. As expected, the proportion of granulocytes was significantly increased in both G-CSF– and ProGP-1–treated animals. To examine the profound effect of ProGP-1 on DC expansion more closely, DCs (CD11chi) were purified and phenotyped according to the expression of CD8 and CD4, as previously published.24 The percentage of CD11chi/CD8hi DCs increased in animals treated with either ProGP-1 or G-CSF compared with those treated with control diluent (Figure 2). This was most dramatic following ProGP-1 administration, which resulted in a 14-fold increase in the proportion of splenic CD8 DCs and a 100-fold increase in the absolute number of these cells. The DCs in the ProGP-1–treated donors included a CD8dim subset in which all DCs were CD11blo(relative to CD4 DC CD11b expression) and a larger CD8hisubset, in which the majority of DCs were also CD11blo (75%). The remaining 25% were CD11bneg. Identical cellular proportions and expansion were seen in the peripheral blood of ProGP-1–treated animals, confirming that the spleen phenotype was representative of that in the blood.
Effect of donor pretreatment on spleen phenotype.
Naive B6 mice were treated with control diluent (■), G-CSF (10 μg/animal per day for 10 days, ▨), or ProGP-1 (20 μg/animal per day for 10 days, ▪). Spleens were harvested on day 11, chopped, digested, and phenotyped. DCs were either CD11cdim/B220hi or CD11chi. (A) Proportion of lineage cells per spleen. (B) Absolute numbers of lineage cells per spleen. *P < .05 compared with controls.
Effect of donor pretreatment on spleen phenotype.
Naive B6 mice were treated with control diluent (■), G-CSF (10 μg/animal per day for 10 days, ▨), or ProGP-1 (20 μg/animal per day for 10 days, ▪). Spleens were harvested on day 11, chopped, digested, and phenotyped. DCs were either CD11cdim/B220hi or CD11chi. (A) Proportion of lineage cells per spleen. (B) Absolute numbers of lineage cells per spleen. *P < .05 compared with controls.
Effect of cytokine pretreatment on splenic DC phenotype.
Naive B6 mice were treated with control diluent, G-CSF, or ProGP-1 as described in “Materials and methods.” DCs were enriched as described, presorted to remove autofluorescent macrophages (A), and stained with CD11c and B220 (B). The CD11chi DCs (R1) from control spleen (C), G-CSF spleen (D), and ProGP-1 spleen (E) were further analyzed for CD4 and CD8 expression.
Effect of cytokine pretreatment on splenic DC phenotype.
Naive B6 mice were treated with control diluent, G-CSF, or ProGP-1 as described in “Materials and methods.” DCs were enriched as described, presorted to remove autofluorescent macrophages (A), and stained with CD11c and B220 (B). The CD11chi DCs (R1) from control spleen (C), G-CSF spleen (D), and ProGP-1 spleen (E) were further analyzed for CD4 and CD8 expression.
Donor pretreatment with ProGP-1 is superior to G-CSF in reducing the severity of GVHD
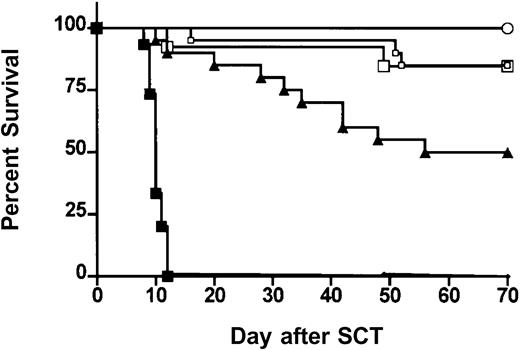
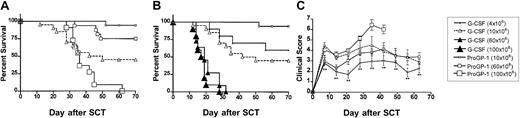
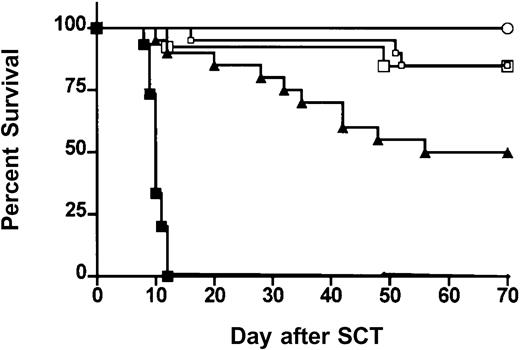
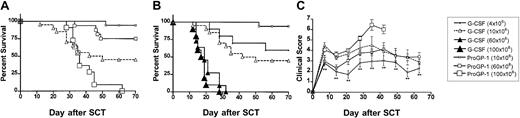
We next examined the effects of ProGP-1 mobilization in a well-established murine SCT model (B6 Ly5a → B6D2F1) that induces GVHD to major and minor histocompatibility antigens. Although this model utilizes spleen as a stem cell source rather than peripheral blood, its validity has been proven by the informative data indicating the beneficial effects of G-CSF on both GVHD and GVL2,20that has since been confirmed clinically.1 In preliminary experiments, we confirmed that the prolonged 10-day course of G-CSF was at least equivalent in preventing GVHD as the standard 6-day course used in clinical practice. Survival at day 70 was 50% versus 30% in recipients of splenocytes from donors treated with 10 days (n = 10) versus 6 days (n = 10) of G-CSF (P = .49). In addition, clinical scores were not statistically different in the first 50 days after transplantation (P > .12) although there was a trend to less GVHD in recipients of the 10-day course of G-CSF at later time points. Pretreatment of donors with 10 days of G-CSF was therefore used as the relevant control to ProGP-1 in subsequent experiments. In these experiments, allogeneic donor B6 animals received daily injections of either control diluent, G-CSF, or ProGP-1 and splenocytes were harvested on day 11. B6D2F1 recipient mice were irradiated with 1100 cGy of total body irradiation (TBI) and received transplants of 107 splenocytes from respective donors. To compensate for the reduced T-cell dose in the ProGP-1 recipients, a further cohort of recipients received transplants of ProGP-1 splenocytes in which additional purified ProGP-1 T cells were added, so as to equilibrate T-cell dose (3 × 106 T cells) across groups. As shown in Figure 3 and as previously described,20 GVHD induced in this model is severe with all recipients of control splenocytes dying in 2 weeks with characteristic features of GVHD (weight loss, hunching, fur ruffling, etc). In contrast, 100% of non-GVHD controls that received transplants of T-cell–depleted allogeneic splenocytes survived, confirming that this splenocyte dose contained sufficient stem cells to rescue lethally irradiated recipients and that GVHD is mediated by donor T cells. Mice undergoing allogeneic SCT using G-CSF splenocytes had a significantly improved survival at day 70 compared with recipients of allogeneic control splenocytes (50% versus 0%,P < .001). Recipients of ProGP-1 splenocytes had a survival at day 70 in excess of 90% which was significantly better (P < .05) than recipients of G-CSF splenocytes. The addition of more T cells to ProGP-1 splenocytes did not increase GVHD mortality (Figure 3), although clinical GVHD scores were significantly increased (data not shown). In order to further establish the magnitude of protection afforded by ProGP-1 mobilization over that seen with G-CSF, cohorts of animals received transplants of escalating doses of splenocytes from either ProGP-1– or G-CSF–treated donors. As demonstrated in Figure 4A, survival in recipients of 106 splenocytes (1.2 × 106 T cells) from ProGP-1–treated donors was superior to that in recipients of splenocyte doses from G-CSF donors that ranged from 4 × 106 to 100 × 106(1.2 × 106 to 30 × 106 T cells). As expected, GVHD mortality was dependent on splenocyte dose in both groups. As shown in Figure 4B, survival in recipients of 60 × 106 ProGP-1–treated splenocytes (7.2 × 106 T cells) was superior to that seen in recipients of 10 × 106 G-CSF splenocytes (3 × 106 T cells; 75% versus 40%,P < .03). Survival was similar, however, when a dose of 100 × 106 ProGP-1 splenocytes (12 × 106 T cells) was compared with 10 × 106 G-CSF splenocytes (3 × 106 T cells; P = .26). In addition, GVHD clinical scores (Figure 4C) were similar in surviving recipients of 10 × 106 G-CSF splenocytes (3 × 106 T cells) and 60 × 106 ProGP-1–treated splenocytes (7.2 × 106 T cells). Given the differences in T-cell doses that this represents, these data suggest that donor pretreatment with ProGP-1 allows a 2- to 4-fold escalation in T-cell dose over that possible with G-CSF.
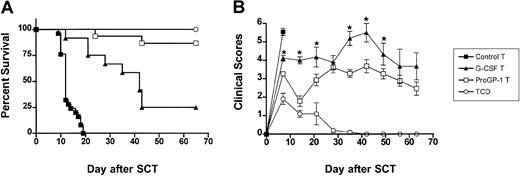
Donor pretreatment with ProGP-1 attenuates GVHD severity.
Survival curves by Kaplan-Meier analysis, pooled from 2 similar experiments. Donor B6 mice were treated with G-CSF (10 μg/animal per day for 10 days), ProGP-1 (20 μg/animal per day for 10 days), or control diluent. Splenocytes (107) from control– (•, control allogeneic, n = 15), G-CSF– (▴, G-CSF allogeneic, n = 20), and ProGP-1–treated (□, ProGP-1 allogeneic, n = 15) donors were harvested on day 11 and transplanted into lethally irradiated (1100 cGy) B6D2F1 recipient mice. Additional ProGP-1 T cells were added to a ProGP-1 (■, ProGP-1 allogeneic adjusted, n = 13) cohort to equilibrate the T-cell dose across the groups. Control-treated T-cell–depleted (○, TCD allogeneic, n = 8) spleens were transplanted as non-GVHD controls. Survival,P < .0001 for control allogeneic versus all others,P = .05 for G-CSF allogeneic versus ProGP-1 allogeneic.
Donor pretreatment with ProGP-1 attenuates GVHD severity.
Survival curves by Kaplan-Meier analysis, pooled from 2 similar experiments. Donor B6 mice were treated with G-CSF (10 μg/animal per day for 10 days), ProGP-1 (20 μg/animal per day for 10 days), or control diluent. Splenocytes (107) from control– (•, control allogeneic, n = 15), G-CSF– (▴, G-CSF allogeneic, n = 20), and ProGP-1–treated (□, ProGP-1 allogeneic, n = 15) donors were harvested on day 11 and transplanted into lethally irradiated (1100 cGy) B6D2F1 recipient mice. Additional ProGP-1 T cells were added to a ProGP-1 (■, ProGP-1 allogeneic adjusted, n = 13) cohort to equilibrate the T-cell dose across the groups. Control-treated T-cell–depleted (○, TCD allogeneic, n = 8) spleens were transplanted as non-GVHD controls. Survival,P < .0001 for control allogeneic versus all others,P = .05 for G-CSF allogeneic versus ProGP-1 allogeneic.
Donor pretreatment with ProGP-1 allows escalation of graft cell dose above that possible with donor pretreatment with G-CSF.
(A-B) Survival curves by Kaplan-Meier analysis, pooled from 3 similar experiments. Donor B6 mice were treated as in Figure 3. Splenocytes were harvested on day 11 and transplanted into lethally irradiated B6D2F1 recipient mice at doses of 4 × 106/animal (for G-CSF group only, n = 10), 10 × 106/animal (n = 10-20), 60 × 106/animal (n = 10-15), and 100 × 106/animal (n = 10). This equates to T-cell doses of 1.2 × 106, 3.0 × 106, 18 × 106, and 30 × 106 in G-CSF–treated donors and 1.2 × 106, 7.2 × 106, and 12 × 106 in ProGP-1–treated donors.P < .03 for all G-CSF versus ProGP-1 (10 × 106 and 60 × 106). *P = .26 for G-CSF (10 × 106) versus ProGP-1 (100 × 106). (C) GVHD clinical scores as described in “Materials and methods” were determined as a measure of GVHD severity in surviving animals. *P < .05 for G-CSF (10 × 106) versus ProGP-1 (60 × 106) and **P < .01 for G-CSF (10 × 106) versus ProGP-1 (10 × 106) at the time points indicated.
Donor pretreatment with ProGP-1 allows escalation of graft cell dose above that possible with donor pretreatment with G-CSF.
(A-B) Survival curves by Kaplan-Meier analysis, pooled from 3 similar experiments. Donor B6 mice were treated as in Figure 3. Splenocytes were harvested on day 11 and transplanted into lethally irradiated B6D2F1 recipient mice at doses of 4 × 106/animal (for G-CSF group only, n = 10), 10 × 106/animal (n = 10-20), 60 × 106/animal (n = 10-15), and 100 × 106/animal (n = 10). This equates to T-cell doses of 1.2 × 106, 3.0 × 106, 18 × 106, and 30 × 106 in G-CSF–treated donors and 1.2 × 106, 7.2 × 106, and 12 × 106 in ProGP-1–treated donors.P < .03 for all G-CSF versus ProGP-1 (10 × 106 and 60 × 106). *P = .26 for G-CSF (10 × 106) versus ProGP-1 (100 × 106). (C) GVHD clinical scores as described in “Materials and methods” were determined as a measure of GVHD severity in surviving animals. *P < .05 for G-CSF (10 × 106) versus ProGP-1 (60 × 106) and **P < .01 for G-CSF (10 × 106) versus ProGP-1 (10 × 106) at the time points indicated.
Donor T-cell engraftment in the spleen 7 days after SCT was 94.7% ± 1.4% in recipients of control splenocytes, 95.4% ± 0.7% in recipients of G-CSF splenocytes and 96.5% ± 0.1% in recipients of ProGP-1 splenocytes. The proportion of donor cells in the peripheral blood of recipients of G-CSF and ProGP-1 splenocytes at day 75 after SCT was 99.4% ± 0.6% and 99.2% ± 0.4%, respectively. In recipients of T-cell–depleted splenocytes, 81% ± 3.2% of peripheral blood cells were donor (P < .05 versus G-CSF and ProGP-1), confirming that the prolonged survival following allogeneic G-CSF– and ProGP-1–treated splenocytes was not due to the presence of stable mixed donor-host chimerism.
Donor pretreatment with ProGP-1 results in a T-cell phenotype with reduced capacity to induce GVHD
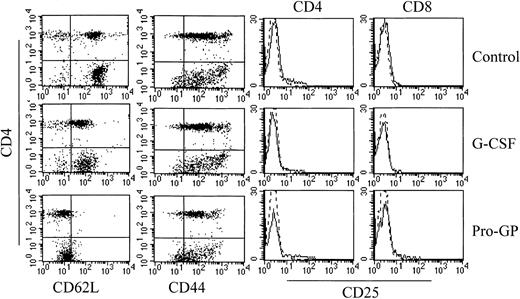
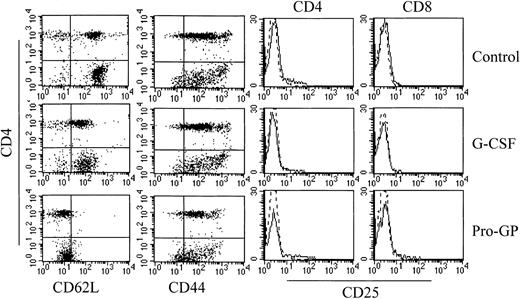
The GVHD induced in these models is dependent on T-cell function20 29 and we therefore examined the effect of G-CSF and ProGP-1 administration on T-cell phenotype and function. CD3+CD4+ and CD3+CD8+ T cells from ProGP-1–treated donors demonstrated an almost complete loss of L-selectin expression whereas T cells from G-CSF–treated animals demonstrated an intermediate pattern of L-selectin loss (Figure5). A similar pattern of expression was demonstrated in T cells from the peripheral blood of G-CSF– and ProGP-1–treated donors (data not shown). The reduction in L-selectin expression did not coincide with an increase in the proportion of CD44hi T cells (Figure 5), suggesting that the loss of L-selectin was not due to an expansion of memory T cells. In this regard, ProGP-1 and G-CSF did not induce T-cell activation as assessed by CD25 (Figure 5) and CD69 expression (data not shown). L-selectin and CD44 expression on splenic T cells from recipients of control and ProGP-1 splenocytes 4 days after transplantation was equivalent (40% and 90%, respectively), indicating that the loss of expression of these molecules prior to transplantation was transient. In studies of T-cell function, CD3+CD4+ T cells were purified as described and stimulated in vitro with mitogen. As shown in Table1, cytokine treatment did not alter proliferative responses although both ProGP-1 and G-CSF significantly increased the production of the type 2 cytokines IL-4 and IL-10 whereas IFNγ production was unchanged. To study T-cell responses to alloantigen in vivo after SCT, animals received transplants of splenocytes from control-, G-CSF–, or ProGP-1–treated donors as in Figure 1. Donor CD4 and CD8 T cells were purified from the spleens of animals 7 days later. As shown in Table2, CD4 T cells isolated from mice undergoing allogeneic SCT with ProGP-1–treated (and to a lesser extent G-CSF–treated) splenocytes failed to proliferate to host antigen. Cytokine generation (IFNγ, IL-4, and IL-10) was also impaired. This impairment in proliferation was not corrected by the addition of exogenous IL-2 (50 U/mL) to MLC (control, 123 963 cpm ± 11 289 cpm; G-CSF, 31 382 cpm ± 1991 cpm; ProGP-1, 28 832 cpm ± 2368 cpm). However, cytotoxicity to host antigens in the donor CD8 population was not significantly altered (Table 2). The difference in T-cell responses before and after SCT suggests that ProGP-1 and G-CSF modulate T-cell function predominantly in vivo, perhaps due to impaired T-cell homing and/or the effects of additionally expanded donor cellular fractions or the products thereof.
Effect of cytokine pretreatment on splenic T-cell phenotype.
Naive B6 mice were treated with control diluent, G-CSF, or ProGP-1 as described in the legend to Figure 3. Splenocytes were harvested and digested, and CD3+ T cells were examined for their expression of CD4, L-selectin, CD44, and CD25 by 3-color flow cytometry.
Effect of cytokine pretreatment on splenic T-cell phenotype.
Naive B6 mice were treated with control diluent, G-CSF, or ProGP-1 as described in the legend to Figure 3. Splenocytes were harvested and digested, and CD3+ T cells were examined for their expression of CD4, L-selectin, CD44, and CD25 by 3-color flow cytometry.
Neither CD11chi nor CD11cdim/B220hi donor DCs from ProGP-1–treated animals provide protection from GVHD
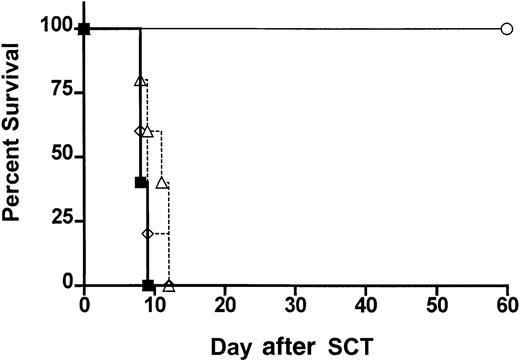
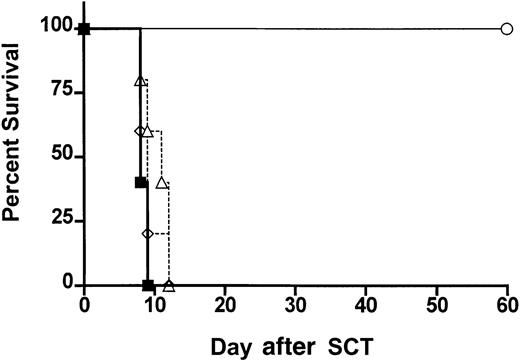
Protection from GVHD has been associated with the expansion of host CD8+CD11chi DCs by Flt-3L administration.30 This DC subset has been defined as the antigen-presenting cells (APCs) primarily responsible for antigen cross presentation31 whereas the human CD11c−CD123+ plasmacytoid DCs have been associated with G-CSF–mediated transplant tolerance.10 We therefore studied the ability of highly purified donor DCs to provide protection from GVHD. Animals received transplants of control-treated B6 spleen supplemented with FACS-sorted (> 98% pure) CD11chi splenic DCs (predominantly CD8+) or CD11cdim/B220hi DCs from ProGP-1–treated donors in numbers that reflected the proportion present in whole ProGP-1–treated spleen (Figure 1). As shown in Figure6, all animals that received these cell populations died at a similar rate to control animals, suggesting that neither population provided protection from GVHD in isolation.
ProGP-1–expanded donor DC populations fail to confer protection from GVHD.
Survival curves by Kaplan-Meier analysis, pooled from 2 similar experiments. Donor B6 mice were treated with ProGP-1 or control diluent. Splenocytes were harvested on day 11 and control splenocytes were transplanted into lethally irradiated B6D2F1 recipient mice at doses of 107/animal (▪, allogeneic, n = 10). ProGP-1–expanded CD11chi DCs (▵, n = 10) or CD11cdim/B220hi DCs (⋄, n = 5) were added to control allogeneic splenocytes in numbers equivalent to those in unseparated ProGP-1 spleen (106CD11chi and 2.5 × 105CD11cdim/B220hi). Syngeneic spleen (○, syngeneic, n = 3) was transplanted as a non-GVHD control.
ProGP-1–expanded donor DC populations fail to confer protection from GVHD.
Survival curves by Kaplan-Meier analysis, pooled from 2 similar experiments. Donor B6 mice were treated with ProGP-1 or control diluent. Splenocytes were harvested on day 11 and control splenocytes were transplanted into lethally irradiated B6D2F1 recipient mice at doses of 107/animal (▪, allogeneic, n = 10). ProGP-1–expanded CD11chi DCs (▵, n = 10) or CD11cdim/B220hi DCs (⋄, n = 5) were added to control allogeneic splenocytes in numbers equivalent to those in unseparated ProGP-1 spleen (106CD11chi and 2.5 × 105CD11cdim/B220hi). Syngeneic spleen (○, syngeneic, n = 3) was transplanted as a non-GVHD control.
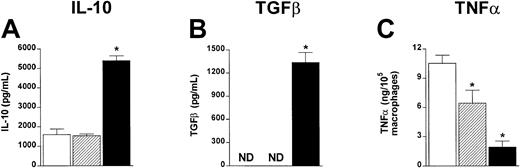
ProGP-1 augments IL-10 and TGFβ and reduces TNFα production in vitro
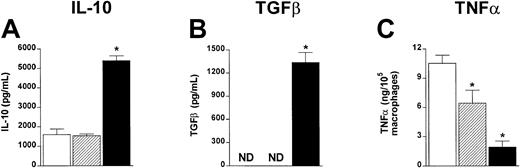
T-cell function may be altered in vivo by both proinflammatory and anti-inflammatory cytokines, which are known to play critical roles in GVHD.22 32 To examine cytokine production from control-, G-CSF–, or ProGP-1–treated donors, unseparated spleen cells were stimulated in vitro with LPS and IL-10 and TGFβ determined in culture supernatants. As shown in Figure 7, ProGP-1 spleens produced high amounts of IL-10 and TGFβ relative to control and G-CSF spleens. After transplantation using these cell populations, a major reduction in TNFα was demonstrated in cultures of macrophages from recipients of ProGP-1–treated splenocytes (Figure7C). Macrophages from recipients of G-CSF–treated splenocytes produced intermediate quantities of TNFα. These data confirm that donor pretreatment with ProGP-1 results in a graft composition favoring anti-inflammatory cytokine production.
ProGP-1–expanded spleen cells produce increased IL-10 and TGFβ and inhibit TNFα production after allogeneic SCT.
Unfractionated spleen cells from control- (■), G-CSF– (▨) or ProGP-1–treated (▪) donors were stimulated in vitro with LPS. IL-10 (A) and TGFβ (B) were determined in 48-hour culture supernatants by ELISA. Results are mean ± SD of triplicate wells and represent one of 3 identical experiments. (C) Animals received transplants of whole control spleen (■, n = 4), G-CSF spleen (▨, n = 4), or ProGP-1 spleen (▪, n = 4) as in Figure 1. Peritoneal macrophages were harvested from animals 7 days after SCT and stimulated with LPS. TNFα was determined in 5-hour culture supernatants by ELISA. Results are normalized to production per 105 macrophages based on CD11b staining. * P < .05 versus control spleen. ND indicates not detected.
ProGP-1–expanded spleen cells produce increased IL-10 and TGFβ and inhibit TNFα production after allogeneic SCT.
Unfractionated spleen cells from control- (■), G-CSF– (▨) or ProGP-1–treated (▪) donors were stimulated in vitro with LPS. IL-10 (A) and TGFβ (B) were determined in 48-hour culture supernatants by ELISA. Results are mean ± SD of triplicate wells and represent one of 3 identical experiments. (C) Animals received transplants of whole control spleen (■, n = 4), G-CSF spleen (▨, n = 4), or ProGP-1 spleen (▪, n = 4) as in Figure 1. Peritoneal macrophages were harvested from animals 7 days after SCT and stimulated with LPS. TNFα was determined in 5-hour culture supernatants by ELISA. Results are normalized to production per 105 macrophages based on CD11b staining. * P < .05 versus control spleen. ND indicates not detected.
The inhibition of GVHD following donor pretreatment with ProGP-1 is mediated through effects on the T cell
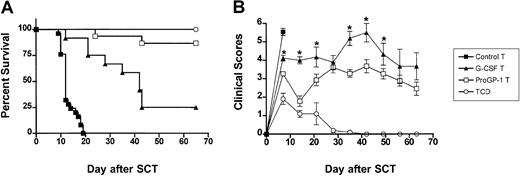
Since GVHD is a T-cell–dependent process, we next studied whether the ability of ProGP-1 to reduce GVHD was mediated through effects on the donor T cell. To compare the capacity of the T cells from treated animals to induce GVHD in isolation, all transplant recipients received T-cell–depleted control splenocytes together with equivalent numbers of purified splenic T cells from either control-, G-CSF–, or ProGP-1–treated donors. As shown in Figure8A, 100% of recipients of control T-cell–depleted splenocytes survived whereas 100% of recipients of control T-cell–depleted splenocytes supplemented with purified control T cells died of GVHD by day 20. In contrast, the median survival was increased in recipients of control T-cell–depleted splenocytes supplemented with purified G-CSF–treated T cells to 40 days and survival at day 70 increased to 25% (P < .001 versus recipients of control T cells). Recipients of control T-cell–depleted splenocytes supplemented with purified ProGP-1–treated T cells had significantly less GVHD than either group, with 90% survival at day 70 (P < .0001 versus recipients of G-CSF and control T cells). GVHD severity in surviving animals, as determined by clinical score, was also significantly reduced in recipients of purified ProGP-1–treated T cells relative to purified G-CSF–treated T cells (Figure 8B). These data indicate that both G-CSF and ProGP-1 alter the capacity of donor T cells to induce GVHD and that the enhanced survival of recipients of ProGP-1–treated allografts relates to T-cell effects.
Donor pretreatment with ProGP-1 abrogates T-cell alloreactivity in vivo.
(A) Survival curves by Kaplan-Meier analysis, pooled from 2 similar experiments. Donor B6 mice were treated as in Figure 3. Splenocytes were harvested on day 11 and control spleen was T-cell–depleted. T cells from control (control allogeneic T, n = 25), G-CSF (G-CSF allogeneic T, n = 12), and ProGP-1 spleen (ProGP-1 allogeneic T, n = 15) were purified and added back in equal numbers (3 × 106) to T-cell–depleted control spleen (7 × 106). T-cell–depleted control spleen (TCD allogeneic, n = 5) was transplanted as a non-GVHD control. These grafts were transplanted into lethally irradiated B6D2F1 recipient mice. Survival, P < .001, G-CSF B6 T cells versus control B6 T cells; P < .0001, G-CSF allogeneic T cells versus ProGP-1 allogeneic T cells. (B) GVHD clinical scores as described in “Materials and methods” were determined as a measure of GVHD severity in surviving animals. *P < .05 between G-CSF T cells and ProGP-1 T cells curves at the time points indicated.
Donor pretreatment with ProGP-1 abrogates T-cell alloreactivity in vivo.
(A) Survival curves by Kaplan-Meier analysis, pooled from 2 similar experiments. Donor B6 mice were treated as in Figure 3. Splenocytes were harvested on day 11 and control spleen was T-cell–depleted. T cells from control (control allogeneic T, n = 25), G-CSF (G-CSF allogeneic T, n = 12), and ProGP-1 spleen (ProGP-1 allogeneic T, n = 15) were purified and added back in equal numbers (3 × 106) to T-cell–depleted control spleen (7 × 106). T-cell–depleted control spleen (TCD allogeneic, n = 5) was transplanted as a non-GVHD control. These grafts were transplanted into lethally irradiated B6D2F1 recipient mice. Survival, P < .001, G-CSF B6 T cells versus control B6 T cells; P < .0001, G-CSF allogeneic T cells versus ProGP-1 allogeneic T cells. (B) GVHD clinical scores as described in “Materials and methods” were determined as a measure of GVHD severity in surviving animals. *P < .05 between G-CSF T cells and ProGP-1 T cells curves at the time points indicated.
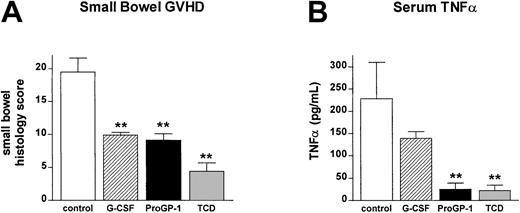
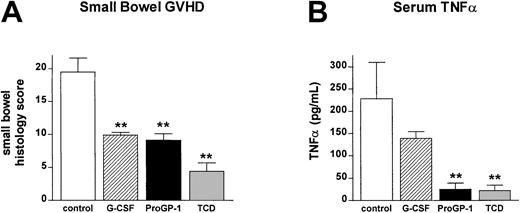
T cells from ProGP-1–treated donors fail to induce GI tract injury and systemic TNFα production after allogeneic SCT
In order to confirm that the ex vivo data in Table 2 were representative of T-cell function in vivo, IFNγ levels were determined in the sera of animals 5 days after transplantation. IFNγ levels were significantly reduced in recipients of both G-CSF– and ProGP-1–treated T cells (63 ± 6.4 U/mL versus 46.4 ± 8.0 U/mL and 44.4 ± 5.3 U/mL), consistent with the ex vivo data. GVHD mortality in this transplantation model is TNFα dependent22 and IFNγ primes mononuclear cells to produce high TNFα levels following stimulation with bacterial-derived antigens that are primarily derived from the GI tract.33As shown in Figure 9A, T cells from ProGP-1– and G-CSF–treated donors failed to induce severe GVHD of the GI tract relative to recipients of control-treated T cells. In vivo, TNFα levels in the sera of recipients of ProGP-1 T cells were 10-fold lower than those in recipients of control T cells and were indistinguishable from non-GVHD controls (Figure 9B). Recipients of G-CSF–treated T cells had TNFα levels intermediate between recipients of control and ProGP-1 T cells, consistent with the mortality seen in this group.
Donor pretreatment with ProGP-1 reduces GI tract injury and inflammatory cytokine generation after SCT.
Recipient mice received transplants as in Figure 6. (A) GI tract histology was determined by semiquantitative histology as described in “Materials and methods” in recipients of control T cells (■, n = 6), G-CSF T cells (▨, n = 5), ProGP-1 T cells (▪, n = 5), or T-cell–depleted spleen (░, n = 4). (B) At 10 days after transplantation, TNFα was determined in the sera of recipients of control T cells (■, n = 7), G-CSF T cells (▨, n = 5), ProGP-1 T cells (▪, n = 5), or T-cell–depleted spleen (░, n = 3) by ELISA as described in “Materials and methods.” **P < .01 versus control B6 T.
Donor pretreatment with ProGP-1 reduces GI tract injury and inflammatory cytokine generation after SCT.
Recipient mice received transplants as in Figure 6. (A) GI tract histology was determined by semiquantitative histology as described in “Materials and methods” in recipients of control T cells (■, n = 6), G-CSF T cells (▨, n = 5), ProGP-1 T cells (▪, n = 5), or T-cell–depleted spleen (░, n = 4). (B) At 10 days after transplantation, TNFα was determined in the sera of recipients of control T cells (■, n = 7), G-CSF T cells (▨, n = 5), ProGP-1 T cells (▪, n = 5), or T-cell–depleted spleen (░, n = 3) by ELISA as described in “Materials and methods.” **P < .01 versus control B6 T.
Discussion
We have shown that donor pretreatment with ProGP-1 significantly reduces GVHD in an experimental SCT model and that this reduction is greater than that seen following donor G-CSF pretreatment. Our data confirm that donor pretreatment with ProGP-1 results in a significantly greater expansion of CD11chi DCs and CD11cdim/B220hi DCs than G-CSF pretreatment, although these DCs do not appear to protect from GVHD in isolation. We demonstrate that T cells from ProGP-1–treated donor animals have an altered adhesive phenotype and an impaired capacity to induce GVHD. This occurs in association with impaired CD4 T-cell proliferation and function although cytolytic capacity remains intact. Finally, ProGP-1 impairs TNFα generation after SCT to a greater extent than G-CSF alone and prevents GVHD of the GI tract, which is critical for the amplification of systemic GVHD.
ProGP-1 pretreatment results in an anergic proliferative response to alloantigen after SCT. In conjunction with this, both TH1 and TH2 cytokine responses (including IL-10) to mitogen were reduced, suggesting this effect was not associated with changes to T-cell differentiation or the induction of a regulatory cell population. Donor pretreatment with ProGP-1 results in the production of large amounts of IL-10 and TGFβ from stem cell grafts, and donor T-cell anergy would be consistent with the effects of these cytokines. In support of this, Zeller and colleagues have previously demonstrated that IL-10 and TGFβ confer T-cell anergy when added to MLC and that these T cells fail to induce GVHD after SCT,34 consistent with the findings of this study. The cellular origin of IL-10 and TGFβ following ProGP-1 administration, and their effect on APC and T-cell function after SCT are important issues that are currently being studied.
The ability of G-CSF to alter T-cell cytokine profiles has been previously documented although the changes to T-cell differentiation have varied.2,5,20 In contrast to a previous study,2,20 we found that G-CSF and ProGP-1 increased the generation of TH2 cytokines, although there was not a clear reduction in TH1 cytokine production. This study differed from the former in that we used purified CD4+ T cells rather than an enriched T-cell preparation that contained CD4+ and CD8+ T cells and nonadherent contaminating cells. In addition, we studied T-cell effects after a more prolonged schedule of G-CSF, to match that for ProGP-1. To date, there have been no experimental or clinical studies demonstrating that donor pretreatment with G-CSF induces TH2 differentiation after SCT, which represents the most informative period for analysis. TH1 cells produce cytokines (especially IFNγ) that prime mononuclear cells to produce cytopathic quantities of inflammatory cytokines (TNFα and IL-1) following stimulation with LPS.35 This is a major mechanism by which TH1 differentiation results in TNFα production and gut injury during GVHD.33 It is now clear that TH2 cytokines are also important in the induction and pathology of GVHD since the prevention of TH2 cytokine generation after SCT using STAT-4 knockout donors and the elimination of tyrosine kinase–transduced donor TH2 clones after SCT ameliorates experimental GVHD.36,37 In addition, the prevention of IL-4 generation after SCT provides protection from GVHD.38Therefore, both TH1 and TH2 cytokines are important in the induction of GVHD, and the inhibition of both types of cytokines by donor pretreatment with ProGP-1 and G-CSF in this study is consistent with the observed reduction in GVHD.
Markedly reduced L-selectin expression was observed on both CD4 and CD8 T cells following ProGP-1 administration. Since L-selectin is an essential molecule in the homing of naive T cells to lymphoid organs, it is possible that reduced expression may play a role in delaying the initiation of immune responses during the induction of GVHD. Furthermore, the effect of ProGP-1 on L-selectin was far more dramatic than that of G-CSF (Figure 2) and this may represent an additional mechanism by which ProGP-1 results in greater amelioration of GVHD than G-CSF alone. Changes to L-selectin expression have been previously described following G-CSF administration, as was the case in this study, and L-selectinlo cells have a reduced capacity to respond to alloantigen in vitro.39 Furthermore, the blockade of L-selectin is known to prevent experimental GVHD.40 Since reciprocal increases were not seen in CD44 expression, we hypothesize that the loss of L-selectin is likely to represent enzymatic cleavage or conformational distortion of this molecule (rather than expansion of memory T cells), perhaps due to products released from the expanded myelomonocytic lineage in G-CSF– and ProGP-1–treated donors.
The T-cell cytotoxic pathways (involving perforin, granzymes, Fas-FasL, and other members of the TNFα superfamily) all play a role in GVHD.41,42 Much of the therapeutic benefit of allogeneic SCT is due to the GVL effect which is mediated by these cytotoxic pathways of donor cytotoxic T lymphocytes (CTLs) and NK cells. Donor pretreatment with ProGP-1 (and G-CSF) appeared to have little effect on CTL activity against host antigens. It is therefore tempting to postulate that ProGP-1 may further separate GVHD and GVL relative to that already demonstrated for G-CSF in both clinical1 and experimental studies.20 In addition, the large numbers of DCs transplanted following donor pretreatment with ProGP-1 may increase antigen presentation of leukemia- and hematopoietic-specific peptides and enhance GVL after SCT. This concept is currently being tested in these models. Another potential benefit of the use of ProGP-1 as a stem cell–mobilizing agent relative to G-CSF is the ability to collect large numbers of donor DCs for immunotherapy purposes after transplantation at a single time point. The latter has been proven effective in preclinical models utilizing Flt-3L–expanded DCs.43
T cells from ProGP-1–treated donors clearly have a reduced capacity to induce systemic TNFα production and GVHD of the GI tract relative to those from G-CSF– and control-treated donors. The reduction in TNFα is likely to be due to a number of factors. First, both G-CSF– and ProGP-1–treated T cells result in less IFNγ production than control-treated T cells and so reduce mononuclear cell priming. In addition, T cells from both G-CSF– and ProGP-1–treated donors result in similar reduction in GVHD of the GI tract and so maintain GI tract integrity, limiting the leak of LPS and other immunomodulatory molecules that stimulate TNFα generation. The fact that T cells from ProGP-1–treated donors reduce TNFα generation to a greater extent than T cells from G-CSF–treated donors, despite similar degrees of IFNγ generation and GI tract injury, suggesting that additional T-cell–dependent mechanisms are invoked. We postulate that this is the result of an increased reduction in CD62L expression, resulting in an adhesive phenotype that may prevent T-cell migration, activation, and TNFα generation in primary lymphoid organs. Further inhibition of mononuclear cell inflammatory responses would also be predicted by the generation of higher levels of anti-inflammatory cytokines (IL-10 and TGFβ) in ProGP-1– compared with G-CSF–treated donors.
G-CSF remains the mainstay of donor pretreatment in current allogeneic stem cell protocols. ProGP-1 is an extremely active molecule for the purpose of stem cell mobilization and appears to be 8 to 10 times more potent than G-CSF and 2 to 3 times more potent than the combination of the native G-CSF and Flt-3L molecules.17 ProGP-1 has a higher affinity for the G-CSF receptor than the native G-CSF molecule and the differences observed in GVHD in this study may relate to downstream effects as a consequence of increased potency through the G-CSF receptor-ligand pair alone. In support of this, we have previously demonstrated improved protection from GVHD following a 10-fold increase in G-CSF dose in this system.2,20Alternatively, the additional agonist activity through the Flt-3L receptor may confer increased protection, presumably via the expansion of donor APCs. Clearly, pretreatment of the donor with Flt-3L alone does not result in a reduction in GVHD16 as may have been expected if donor DCs were important in controlling GVHD. Furthermore, treatment of recipients with FLT-3L augments both GVHD and GVL.44 Conversely, the recent report of reduced GVHD following recipient treatment with FLT-3L prior to SCT is intriguing and may relate to a change in host DC phenotype, activation status, or both,30 and is consistent with the established paradigm that host APCs are important in the induction of GVHD.45Thus, unlike host APCs, the relative role of donor APCs (and DCs in particular) in the induction of GVHD remains unclear at this time.
Human studies have demonstrated that G-CSF expands “lymphoplasmacytoid” DCs, which can induce TH2 differentiation in vitro10 and this has been suggested as a mechanism by which G-CSF can prevent acute GVHD. We confirm that the counterpart murine CD11cdim/B220hi DC is also expanded after G-CSF and ProGP-1 treatment. However, we could not find any evidence that this cell provides protection from GVHD in isolation, nor was the T-cell phenotype after SCT consistent with this theory. This does not exclude the possibility that these cells may confer protection from acute GVHD if transferred in higher numbers or in conjunction with other cell populations modulated by G-CSF or ProGP-1. Recent studies suggest that increased DC2 doses in the BMT setting are associated with reduced chronic GVHD46 and so a causal relationship between high DC2 doses, TH2 differentiation, and the increased incidence of chronic GVHD in the clinical SCT setting also seems unlikely. G-CSF is known to expand monocytes capable of inhibiting T-cell responses, in part through IL-10 production.6,7 Although IL-10 is capable of ameliorating GVHD,47 48 the ability of a monocyte population or DC precursor to prevent GVHD has not been tested in experimental models and now deserves further study.
Allogeneic stem cell transplantation with G-CSF–mobilized stem cells represents a major advance in the management of hematologic malignancies due to the increased separation of GVHD and GVL compared with that following traditional BMT. Despite this, GVHD remains a major limitation of allogeneic SCT and further advances are required to improve the safety of this procedure. The data presented here suggest that in addition to enhancing stem cell mobilization, the administration of progenipoietin rather than G-CSF to stem cell transplant donors is likely to further reduce GVHD and deserves study in carefully conducted clinical trials.
G.R.H. is a Wellcome Trust Senior Overseas Research Fellow.
Prepublished online as Blood First Edition Paper, October 17, 2002; DOI 10.1182/blood-2002-05-1529.
Supported in part by grants from the National Health and Medical Research Council (NHMRC) and Queensland Cancer Fund (QCF).
J.K.W. is employed by Pharmacia, whose (potential) product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Geoffrey Hill, Immunoregulation Laboratory, The Queensland Institute of Medical Research, 300 Herston Rd, Herston, QLD 4006, Australia; e-mail: geoffh@qimr.edu.au.