Intracellular trafficking of chemokine receptors plays an important role in fine-tuning the functional responses of neutrophils and lymphocytes in the inflammatory process and HIV infection. Although many chemokine receptors internalize through clathrin-coated pits, regulation of the receptor trafficking is not fully understood. The present study demonstrated that CXCR2 was colocalized with transferrin and low-density lipoprotein (LDL) after agonist treatment for different periods of time, suggesting 2 intracellular trafficking pathways for this receptor. CXCR2 was colocalized with Rab5 and Rab11a, which are localized in early and recycling endosomes, respectively, in response to agonist stimulation for a short period of time, suggesting a recycling pathway for the receptor trafficking. However, overexpression of a dominant-negative Rab5-S34N mutant significantly attenuated CXCR2 sequestration. The internalized CXCR2 was recycled back to the cell surface after removal of the agonist and recovery of the cells, but receptor recycling was inhibited by overexpression of a dominant-negative Rab11a-S25N mutant. After prolonged (4-hour) agonist treatment, CXCR2 exhibited significantly increased colocalization with Rab7, which is localized in late endosomes. The colocalization of CXCR2 with LDL and LAMP-1 suggests that CXCR2 is targeted to lysosomes for degradation after prolonged ligand treatment. However, the colocalization of CXCR2 with Lamp1 was blocked by the overexpression of a dominant-negative Rab7-T22N mutant. In cells overexpressing Rab7-T22N, CXCR2 was retained in the Rab5- and Rab11a-positive endosomes after prolonged (4-hour) agonist treatment. Our data suggest that the intracellular trafficking of CXCR2 is differentially regulated by Rab proteins.
Introduction
Chemokines are a family of small proteins that regulate leukocyte trafficking and are therefore important in lymphoid organogenesis, inflammation, and host defense. Based on the presence of the conserved cysteines near the N-terminus of chemokines, chemokines can be classified into CC, CXC, CX3C, and C subfamilies.1-4 The effects of chemokines on target cells are mediated by a family of G-protein–coupled 7-transmembrane receptors (GPCRs). Chemokine receptors are expressed predominantly in neutrophils and lymphocytes. To date, 19 chemokine receptors have been identified, including 11 CC chemokine receptors (CCR1-11), 6 CXC chemokine receptors (CXCR1-6), and one for each C and CX3C chemokine receptor.4 These receptors play important roles in inflammatory response, chemotaxis, immune cell development, leukocyte homing, and angiogenesis.5,6 Some of the chemokine receptors (eg, CCR5 and CXCR4) act as coreceptors in HIV infection.7
Agonist occupancy of chemokine receptors, like other types of GPCRs, activates the coupled heterotrimeric G proteins that mobilize intracellular second messengers such as inositol triphosphates, cyclic adenosine monophosphate, and intracellular free calcium.8The receptors are rapidly phosphorylated by G-protein–coupled receptor kinases or protein kinase C, followed by receptor desensitization and internalization.9-11 Many chemokine receptors internalize through clathrin-coated pits, a process facilitated by adaptor proteins such as β-arrestin and adaptin 2.12-17Internalized receptors may be dephosphorylated in early endosomes by protein phosphatases (PPs) such as PP2A.18Dephosphorylated receptors are either recycled back to the cell surface or transmitted to lysosomes for degradation.13 Recycling and degradation rates might vary among different kinds of chemokine receptors.
Intracellular trafficking of chemokine receptors plays an important role in regulation of the functional responses of the chemokine receptor expressing cells in inflammatory processes and HIV infection. Increasing lines of evidence show the critical role of receptor internalization in receptor-mediated chemotaxis. Studies on CXCR2, a major chemokine receptor expressed in neutrophils, have demonstrated that blocking the receptor internalization reduces receptor-mediated chemotaxis.12,13 The responses of neutrophils, the major infiltrate in the course of acute inflammation, could be regulated in part by the processes involving chemokine receptor internalization.18-20 Moreover, chemokine-mediated block to HIV entry is associated with CCR5 internalization efficiency.21 However, the mechanisms underlying intracellular trafficking of chemokine receptors are not fully understood.
Intracellular trafficking of membrane receptors is tightly regulated by a subfamily of ras-like small GTPases (Rab GTPases). Approximately 40 members of Rab GTPases have been identified, and each is believed to be specifically associated with a particular organelle or pathway. Among them, Rab4 is considered to be localized in early and recycling endosomes.22 Rab5 and Rab15 are localized in early endosomes,22-25 Rab11 and Rab25 are localized in recycling endosomes,23,26-28 and Rab7 and Rab9 are localized in late endosomes.29,30 Because Rab9 is also present in the trans-Golgi network and regulates transport from late endosomes to the trans-Golgi network,30 the more likely candidate responsible for the continuous fusion events between late endosomes and lysosomes is Rab7. Although the potential role of these Rab GTPases in the trafficking of GPCRs is largely unknown, some GPCRs, such as the dopamine D2 receptor and the β2-adrenergic receptor (β2-AR), have been shown to be regulated by certain Rab GTPases.31-33 A prototypical GPCR, β2-AR internalizes into Rab5- and Rab4-positive endosomes,31 and the overexpression of dominant-negative Rab5 and Rab4 blocks receptor internalization and recycling, respectively.31 However, because of the enormous number of GPCRs and the diverse cellular function evoked by each receptor, it is conceivable that multiple mechanisms may be used for receptor trafficking. In contrast to the available data on Rab GTPases and the β2-AR, little is known about the role of these Rab GTPases in regulating the intracellular trafficking of chemokine receptors. In this paper, we demonstrate that after agonist-induced internalization, the CXCR2 chemokine receptor becomes progressively associated with Rab5-, Rab11a-, and Rab7-positive endosomes, and overexpression of the dominant-negative mutants of Rab5, Rab11a, and Rab7 specifically block receptor internalization, recycling, and lysosomal sorting, respectively.
Materials and methods
Plasmids
CXCR2 in pRC/CMV was constructed previously.34 The plasmids of enhanced green fluorescence protein (EGFP)-Rab5, EGFP-Rab5-Q79L, and EGFP-Rab5-S34N were generous gifts from Dr Stephan S. G. Ferguson.31 The plasmids of EGFP-Rab11a, EGFP-Rab11a-S20V, and EGFP-Rab11a-S25N were constructed as described previously.35 The plasmids of Rab7, Rab7-Q67L, Rab7-T22N, EGFP-Rab7, EGFP-Rab7-Q67L, and EGFP-Rab11-T22N were generous gifts from Angela Wandinger-Ness.36 Wild-type or mutant Rab11a in pCDNA3.1 was constructed by amplifying the fragments from the EGFP-Rab11 plasmids using polymerase chain reaction (PCR) and insertion into the BamHI and XhoI sites.
Cell culture and transfection
Human embryonic kidney (HEK293) cells and rat basophilic leukemia (RBL-2H3) cells were grown in Dulbecco modified Eagle medium (DMEM), containing 10% fetal bovine serum and a 1:100 dilution of penicillin/streptomycin, at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells were transfected with plasmids encoding CXCR2 using Lipofectamine Plus reagent (Life Technologies, Carlsbad, CA). Stably transfected cells were selected with 560 μg/mL Geneticin (G418) and were evaluated for receptor expression using sodium iodide 125I-CXCL1 binding assay (NEX-321; Dupont NEN, Boston, MA). Cells stably expressing CXCR2 were transiently transfected with EGFP-tagged Rab GTPases using Lipofectamine Plus reagent (Life Technologies). For the stable cell lines of HEK293 cells coexpressing CXCR2 and wild-type or mutant Rab7, cells were transfected using Lipofectamine Plus reagent (Life Technologies), and the stable clones were selected with 560 μg/mL G418. Transfection efficiency for the EGFP-Rab protein expression was analyzed by calculating the percentage of the transfected cells (showing green fluorescence) from the total cells in one field under the microscope. Unless otherwise indicated, approximately 80% of HEK293 cells were transfected by the plasmids of these Rab proteins, and approximately 20% of RBL-2H3 cells were transfected.
Confocal visualization of agonist-induced internalization of CXCR2
Confocal microscopy was performed on a Zeiss LSM-410 laser scanning microscope using a Zeiss 63 × 1.3 numerical aperture (NA) oil immersion lens. HEK293 cells coexpressing CXCR2 and EGFP-Rab proteins were grown on coverslips. Cells were treated with carrier buffer or CXCL8 for different times and were fixed with methanol. Cells were washed with phosphate-buffered saline (PBS) and were incubated with a mouse monoclonal CXCR2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 30 minutes. Cells were washed and incubated with a rhodamine-conjugated antimouse antibody (Molecular Probes) for 30 minutes. Confocal microscopy was performed using dual excitation (488 nm, EGFP; 568 nm, rhodamine or Cy3) and emission (515-540 nm, EGFP; 590-610 nm, rhodamine or Cy3) filter sets. To study the colocalization of CXCR2 with Lamp-1, cells were treated with CXCL8 for 4 hours, fixed in methanol. Cells were incubated with an antibody mixture containing a rabbit CXCR2 antibody (Santa Cruz Biotechnology) and a mouse monoclonal Lamp-1 antibody (PharMingen, San Jose, CA) for 30 minutes. Cells were washed and incubated with an antibody mixture containing a rhodamine-conjugated antirabbit antibody (Molecular Probes, Eugene, OR) and a fluorescein isothiocyanate (FITC)-conjugated antimouse antibody (Molecular Probes) for 30 minutes. Confocal microscopy was performed using dual-excitation (488 nm, FITC; 568 nm, rhodamine) and emission (515-540 nm, FITC; 590-610 nm rhodamine) filter sets. Specificity of labeling and absence of signal crossover were established by examination of single-labeled samples. To determine the colocalization of CXCR2 with transferrin or LDL, HEK293 cells stably expressing CXCR2 were incubated with transferrin–Texas Red (25 μg/mL) (Molecular Probes) or with Dil-LDL (40 μg/mL) (Molecular Probes) for 1 hour or 4 hours at 37°C in the presence of 200 nM CXCL8. Cells were washed with ice-cold serum-free medium, fixed in methanol, and processed for further immunostaining of CXCR2. Cells were incubated with a monoclonal CXCR2 antibody for 30 minutes followed by incubation with an FITC-conjugated antimouse antibody for 30 minutes. Incubation with antibodies was performed at room temperature. Colocalization of CXCR2 with transferrin or LDL was visualized by confocal microscopy. The percentage of colocalized CXCR2 was analyzed by calculating the colocalized receptor fluorescence (yellow) from the total internalized receptor fluorescence (yellow and green).
Ligand-receptor complex internalization assay
The acid-wash technique12 was used to determine the kinetics of CXCL8-induced internalization of CXCR2. HEK293 cells expressing CXCR2 were grown to confluence on 24-well plates precoated with 0.1 mg/mL poly-l-lysine (Sigma; MWt 30 000-70 000) for 1 hour and washed once with distilled water before use. Cells were incubated at 4°C in 0.5 mL serum-free DMEM containing 75 nCi/mL (2.775 KBq/mL) 125I-CXCL8 for 1 hour. The medium was subsequently removed; 1 mL ice-cold serum-free DMEM was added carefully to each well and aspirated, and then another 1-mL aliquot of ice-cold serum-free DMEM was added before incubation at 37°C for the indicated time. The medium was removed, and the cells were incubated with 1 mL ice-cold 0.2 M acetic acid with 0.5 M NaCl for 6 minutes. After incubation, the cells were washed once with 1-mL ice-cold serum-free DMEM and were lysed with 1 mL 1% sodium dodecyl sulfate (SDS) with 0.1 N NaOH (lysis solution). The radioactive cell lysate was then counted on a γ-counter (Gamma 5500; Beckman, Muskegon, MI). Total cell surface receptor binding was measured after incubation with 125I-CXCL8 medium followed by washing the cells with ice-cold serum-free DMEM. Nonspecific binding was measured by adding 200 mM acetic acid with 0.5 M NaCl after incubation with125I-CXCL8 in binding medium. Calculation of the percentage of internalized receptor was performed as described before.12
Fluorescence-activated cell sorting analysis
HEK293 cells expressing CXCR2 and Rab11a proteins were incubated in 20 mM HEPES (N-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)-buffered DMEM at 37°C for 30 minutes in the presence or absence of CXCL8 (100 nM). Cells were then washed in ice-cold medium and incubated in medium at 37°C for different times as indicated. Cells were incubated with a monoclonal CXCR2 antibody (Santa Cruz Biotechnology) at 4°C for 1 hour, then washed with ice-cold medium followed by incubation with FITC-conjugated antimouse immunoglobulin G (IgG) at 4°C for 30 minutes. Cells were washed and fixed in 2% formaldehyde in PBS and were analyzed in FACScan equipped with CellQuest software (Becton Dickinson, Franklin Lakes, NJ).
Results
Time-dependent internalization of CXCR2 into different endosomal compartments
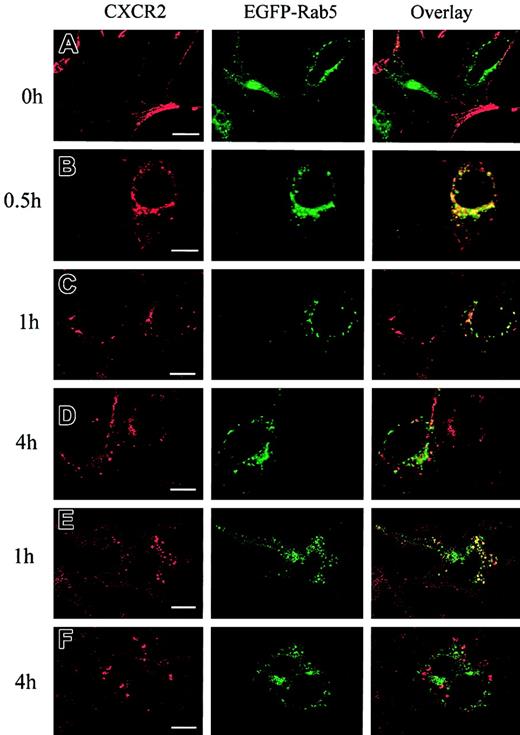
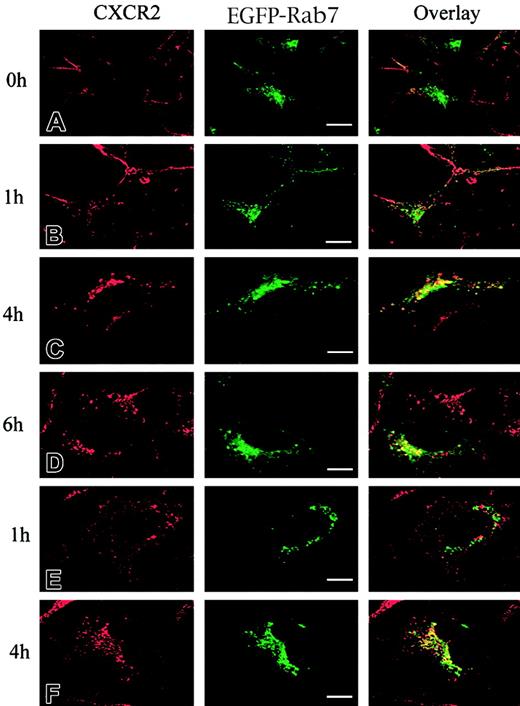
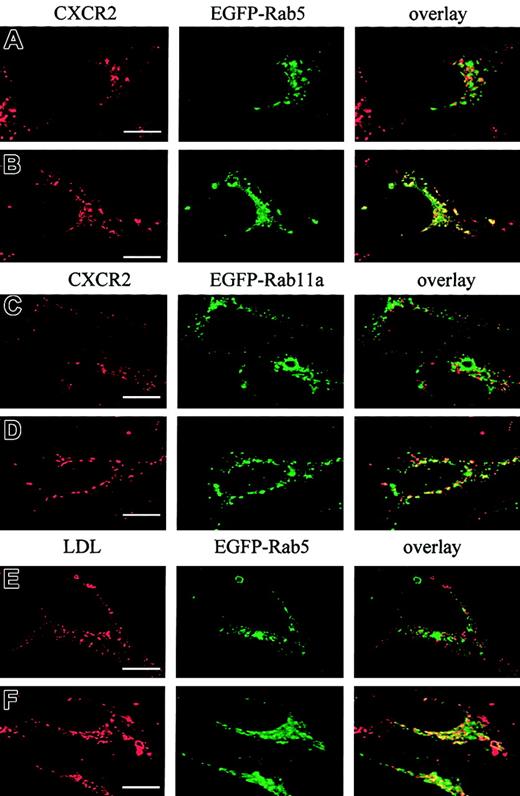
It has been postulated that CXCR2 is internalized into endosomal compartments in response to agonist stimulation.13 The small GTPases Rab5, Rab11a, and Rab7 are localized in early, recycling, and late endosomes, respectively, and they broadly regulate the intracellular trafficking and membrane fusion of endocytic vesicles.37 Here we determined whether agonist induced the internalization of CXCR2 into these small GTPase-containing endosomes. To visualize the colocalization of CXCR2 with these EGFP-Rab proteins, HEK293 cells and RBL-2H3 cells stably expressing CXCR2 were transiently transfected with plasmids encoding EGFP-tagged Rab5, Rab11a, and Rab7, respectively. HEK293 cells were treated with CXCL8 (200 nM) for different times, and the localization of CXCR2 and EGFP-Rab proteins was determined by confocal microscopy. As shown in Figures1-3, before agonist stimulation, CXCR2 was expressed predominantly on the cell surface, whereas EGFP-tagged Rab5, Rab11a, and Rab7 were located largely in the cytoplasm (Figures1A,2A,3A). In response to agonist stimulation, the internalized CXCR2 was found to be partially colocalized with EGFP-Rab5 (Figure 1B-C) and EGFP-Rab11a (Figure 2B-C), and colocalization lasted for at least 1 hour (Figures 1C,2C). After prolonged (4-hour) agonist exposure, CXCR2 was retained in the cytoplasm, but colocalization of the internalized receptor with EGFP-Rab5 and EGFP-Rab11a was significantly decreased (Figures 1D,2D). These data suggest that CXCR2 might have been transported to late endosomes after prolonged agonist stimulation. This is supported by the observation that after agonist treatment for 1 hour, little colocalization of CXCR2 with EGFP-Rab7 was observed (Figure3B), whereas after treatment for 4 hours and 6 hours, there was significant colocalization of CXCR2 with EGFP-Rab7 (Figure 3C-D). Similarly, in RBL-2H3 cells coexpressing CXCR2 and the EGFP-tagged Rab proteins, CXCL8 treatment for 1 hour resulted in significant colocalization of the receptor with EGFP-Rab5 (Figure1E) and EGFP-Rab11a (Figure 2E) but little colocalization with EGFP-Rab7 (Figure 3E). After 4 hours of agonist treatment, colocalization of the receptor with EGFP-Rab5 (Figure 1F) and EGFP-Rab11a (Figure 2F) was remarkably reduced. Instead, a significant increase was exhibited in the colocalization of the receptor with EGFP-Rab7 (Figure 3F). These data suggest that internalized CXCR2 was transported to Rab7-positive late endosomes during this time.
Agonist-stimulated colocalization of the internalized CXCR2 with EGFP-Rab5 in HEK293 cells and RBL-2H3 cells.
HEK293 cells (A-D) and RBL-2H3 cells (E-F) stably expressing CXCR2 were transiently transfected with plasmids encoding EGFP-Rab5. Cells were treated with 200 nM CXCL8 at 37°C for different times as indicated, then fixed in methanol. Cells were incubated with a mouse monoclonal anti-CXCR2 antibody at room temperature for 30 minutes, followed by incubation with a rhodamine-conjugated antimouse antibody at room temperature for 30 minutes. Representative laser-scanning confocal micrographs from 3 independent experiments demonstrating the distribution of CXCR2 (red), EGFP-Rab5 (green), and colocalization of CXCR2 with EGFP-Rab5 (yellow) are shown. Images were processed using Photoshop software (Adobe, San Jose, CA). Bars, 10 μm.
Agonist-stimulated colocalization of the internalized CXCR2 with EGFP-Rab5 in HEK293 cells and RBL-2H3 cells.
HEK293 cells (A-D) and RBL-2H3 cells (E-F) stably expressing CXCR2 were transiently transfected with plasmids encoding EGFP-Rab5. Cells were treated with 200 nM CXCL8 at 37°C for different times as indicated, then fixed in methanol. Cells were incubated with a mouse monoclonal anti-CXCR2 antibody at room temperature for 30 minutes, followed by incubation with a rhodamine-conjugated antimouse antibody at room temperature for 30 minutes. Representative laser-scanning confocal micrographs from 3 independent experiments demonstrating the distribution of CXCR2 (red), EGFP-Rab5 (green), and colocalization of CXCR2 with EGFP-Rab5 (yellow) are shown. Images were processed using Photoshop software (Adobe, San Jose, CA). Bars, 10 μm.
Agonist-stimulated colocalization of internalized CXCR2 with EGFP-Rab11a in HEK293 cells and RBL-2H3 cells.
HEK293 cells (A-D) and RBL-2H3 cells (E-F) stably expressing CXCR2 were transiently transfected with plasmids encoding EGFP-Rab11a. Cells were treated with 200 nM CXCL8 at 37°C for different times as indicated, then fixed in methanol. Cells were incubated with a mouse monoclonal anti-CXCR2 antibody for 30 minutes, followed by incubation with a rhodamine-conjugated antimouse antibody for 30 minutes. Representative laser-scanning confocal micrographs from 3 independent experiments demonstrating the distribution of CXCR2 (red), EGFP-Rab11a (green), and colocalization of CXCR2 with EGFP-Rab11a (yellow) are shown. Images were processed using Photoshop software. Bars, 10 μm.
Agonist-stimulated colocalization of internalized CXCR2 with EGFP-Rab11a in HEK293 cells and RBL-2H3 cells.
HEK293 cells (A-D) and RBL-2H3 cells (E-F) stably expressing CXCR2 were transiently transfected with plasmids encoding EGFP-Rab11a. Cells were treated with 200 nM CXCL8 at 37°C for different times as indicated, then fixed in methanol. Cells were incubated with a mouse monoclonal anti-CXCR2 antibody for 30 minutes, followed by incubation with a rhodamine-conjugated antimouse antibody for 30 minutes. Representative laser-scanning confocal micrographs from 3 independent experiments demonstrating the distribution of CXCR2 (red), EGFP-Rab11a (green), and colocalization of CXCR2 with EGFP-Rab11a (yellow) are shown. Images were processed using Photoshop software. Bars, 10 μm.
Agonist-stimulated colocalization of CXCR2 with EGFP-Rab7 in HEK293 cells and RBL-2H3 cells.
HEK293 cells (A-D) and RBL-2H3 cells (E-F) stably expressing CXCR2 were transiently transfected with plasmids encoding EGFP-Rab7. Cells were treated with 200 nM CXCL8 at 37°C for different times as indicated, then fixed in methanol. Cells were incubated with a mouse monoclonal anti-CXCR2 antibody for 30 minutes, followed by incubation with a rhodamine-conjugated antimouse antibody for 30 minutes. Representative laser-scanning confocal micrographs from 3 independent experiments demonstrating the distribution of CXCR2 (red), EGFP-Rab7 (green), and colocalization of CXCR2 with EGFP-Rab7 (yellow) are shown. Images were processed using Photoshop software. Bars, 10 μm.
Agonist-stimulated colocalization of CXCR2 with EGFP-Rab7 in HEK293 cells and RBL-2H3 cells.
HEK293 cells (A-D) and RBL-2H3 cells (E-F) stably expressing CXCR2 were transiently transfected with plasmids encoding EGFP-Rab7. Cells were treated with 200 nM CXCL8 at 37°C for different times as indicated, then fixed in methanol. Cells were incubated with a mouse monoclonal anti-CXCR2 antibody for 30 minutes, followed by incubation with a rhodamine-conjugated antimouse antibody for 30 minutes. Representative laser-scanning confocal micrographs from 3 independent experiments demonstrating the distribution of CXCR2 (red), EGFP-Rab7 (green), and colocalization of CXCR2 with EGFP-Rab7 (yellow) are shown. Images were processed using Photoshop software. Bars, 10 μm.
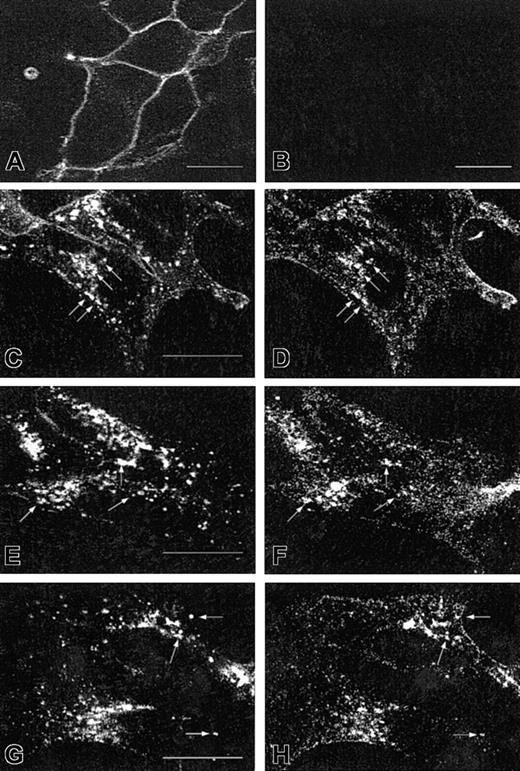
It may raise questions about whether the CXCR2 antibody used specifically recognizes the receptor and whether the overexpressed EGFP-Rab proteins exhibit the same pattern and behavior as the endogenous ones. The specificity of CXCR2 immunostaining was determined in HEK293 cells stably expressing or not expressing CXCR2. Only the cells expressing CXCR2 exhibited specific fluorescence on the cell surface (Figure 4A), whereas the cells without transfection of the receptor cDNA did not show any specific fluorescence (Figure 4B). To determine whether CXCR2 colocalizes with the native Rab proteins, HEK293 cells stably expressing CXCR2 were treated with CXCL8 for 1 hour or 4 hours, followed by immunostaining with an antibody mixture containing a monoclonal CXCR2 antibody and a rabbit antibody for Rab5, Rab11a, or Rab7. As shown in Figure 4, CXCR2 exhibited partial colocalization with Rab5 (Figure 4C-D) and Rab11a (Figure 4E-F) after 1 hour of agonist treatment. After 4 hours of agonist treatment, CXCR2 exhibited partial colocalization with native Rab7 (Figure 4G-H). These results, taken together with the data shown in Figures 1 to 3, clearly demonstrate that the overexpressed EGFP-Rab proteins exhibited the same behavior as the endogenous Rab proteins, consistent with the results obtained previously.35
Specific staining of CXCR2 and colocalization of CXCR2 with endogenous Rab5, Rab11a, and Rab7 in HEK293 cells.
For the specific staining of CXCR2, cells stably expressing (A) or not expressing (B) CXCR2 were fixed in methanol. Cells were incubated with a monoclonal CXCR2 antibody for 30 minutes, followed by incubation with an FITC-conjugated antimouse antibody for 30 minutes. Specific cell surface expression of CXCR2 was determined by confocal microscopy. For the colocalization of CXCR2 with the endogenous Rab proteins, cells stably expressing CXCR2 were treated with CXCL8 (200 nM) for 1 hour (C-F) or 4 hours (G-H), then fixed in methanol. Cells were incubated with a mixture of a monoclonal CXCR2 antibody and a rabbit antibody for Rab5 (C-D), Rab11a (E-F), or Rab7 (G-H), for 30 minutes. After washing with PBS, cells were incubated with a mixture of an FITC-conjugated antimouse antibody and a rhodamine-conjugated antirabbit antibody for 30 minutes. Representative confocal micrographs from 3 independent experiments demonstrating the intracellular localization of CXCR2 (C,E,G), Rab5 (D), Rab11a (F), and Rab7 (H) are shown. Arrows indicate the colocalization of CXCR2 with the individual Rab proteins. Bars, 10 μm.
Specific staining of CXCR2 and colocalization of CXCR2 with endogenous Rab5, Rab11a, and Rab7 in HEK293 cells.
For the specific staining of CXCR2, cells stably expressing (A) or not expressing (B) CXCR2 were fixed in methanol. Cells were incubated with a monoclonal CXCR2 antibody for 30 minutes, followed by incubation with an FITC-conjugated antimouse antibody for 30 minutes. Specific cell surface expression of CXCR2 was determined by confocal microscopy. For the colocalization of CXCR2 with the endogenous Rab proteins, cells stably expressing CXCR2 were treated with CXCL8 (200 nM) for 1 hour (C-F) or 4 hours (G-H), then fixed in methanol. Cells were incubated with a mixture of a monoclonal CXCR2 antibody and a rabbit antibody for Rab5 (C-D), Rab11a (E-F), or Rab7 (G-H), for 30 minutes. After washing with PBS, cells were incubated with a mixture of an FITC-conjugated antimouse antibody and a rhodamine-conjugated antirabbit antibody for 30 minutes. Representative confocal micrographs from 3 independent experiments demonstrating the intracellular localization of CXCR2 (C,E,G), Rab5 (D), Rab11a (F), and Rab7 (H) are shown. Arrows indicate the colocalization of CXCR2 with the individual Rab proteins. Bars, 10 μm.
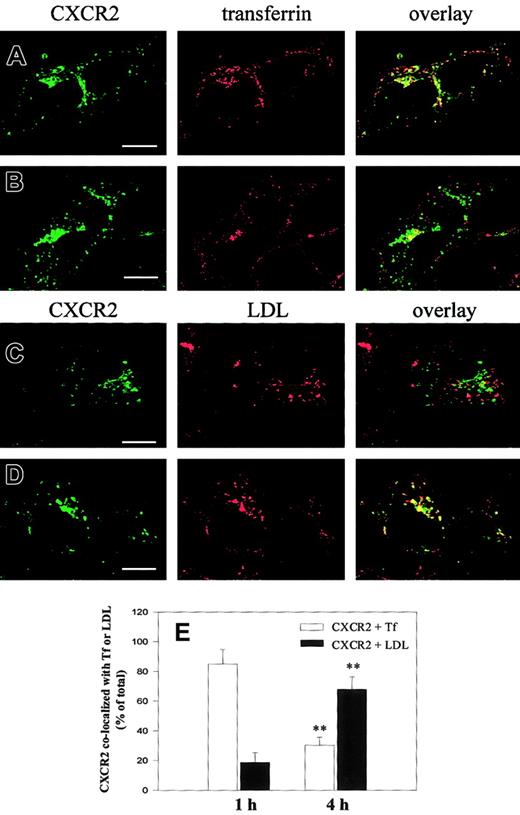
Time-dependent colocalization of CXCR2 with Rab5, Rab11a, and Rab7 strongly suggests that there are at least 2 trafficking pathways for the receptor. At the early stage of trafficking, the internalized receptors are delivered predominantly to the early and recycling endosomes. However, after prolonged agonist treatment, the receptors are transported to late endosomes/lysosomes. By analyzing the percentage of CXCR2 colocalized with transferrin, which is known to be transported quickly into early and recycling endosomes,38it was revealed that 84.9% ± 9.2% of internalized receptors were colocalized with transferrin in response to agonist treatment for 1 hour (Figure 5A,E). Colocalization was decreased to 30.2% ± 6.4% after agonist treatment for 4 hours (Figure 5B,E) (P < .01). Using LDL as a lysosomal probe39 to analyze the percentage of CXCR2 transported to lysosomes, it was revealed that 18.8% ± 6.4% of internalized receptors were colocalized with LDL after agonist treatment for 1 hour (Figure 5C,E), but colocalization was remarkably increased to 67.8% ± 8.5% after 4 hours of agonist exposure (Figure 5D-E) (P < .01).
Agonist-induced colocalization of CXCR2 with transferrin and LDL in HEK293 cells.
Cells stably expressing CXCR2 were incubated with CXCL8 (200 nM) and transferrin–Texas Red (25 μg/mL) (A-B) or Dil-LDL (40 μg/mL) (C, D) at 37°C for 1 hour (A, C) or 4 hours (B, D). Cells were washed in cold PBS and fixed in methanol. Cells were incubated with a monoclonal CXCR2 antibody for 30 minutes at room temperature, followed by incubation with an FITC-conjugated antimouse antibody for 30 minutes at room temperature. Representative laser-scanning confocal micrographs from 3 independent experiments demonstrating the distribution of CXCR2 (green), transferrin–Texas Red (red), Dil-LDL (red), and colocalization of CXCR2 with transferrin–Texas Red (yellow; A-B) or CXCR2 with Dil-LDL (yellow; C-D) are shown. Bars, 10 μm. (E) Quantification of the percentage of CXCR2 colocalized with transferrin (■) or LDL (▪) was determined by counting the colocalized receptor fluorescence (yellow) from the total internalized receptor fluorescence (green and yellow). Data are means ± SEMs of 3 independent experiments (**P < .01). Tf indicates transferrin.
Agonist-induced colocalization of CXCR2 with transferrin and LDL in HEK293 cells.
Cells stably expressing CXCR2 were incubated with CXCL8 (200 nM) and transferrin–Texas Red (25 μg/mL) (A-B) or Dil-LDL (40 μg/mL) (C, D) at 37°C for 1 hour (A, C) or 4 hours (B, D). Cells were washed in cold PBS and fixed in methanol. Cells were incubated with a monoclonal CXCR2 antibody for 30 minutes at room temperature, followed by incubation with an FITC-conjugated antimouse antibody for 30 minutes at room temperature. Representative laser-scanning confocal micrographs from 3 independent experiments demonstrating the distribution of CXCR2 (green), transferrin–Texas Red (red), Dil-LDL (red), and colocalization of CXCR2 with transferrin–Texas Red (yellow; A-B) or CXCR2 with Dil-LDL (yellow; C-D) are shown. Bars, 10 μm. (E) Quantification of the percentage of CXCR2 colocalized with transferrin (■) or LDL (▪) was determined by counting the colocalized receptor fluorescence (yellow) from the total internalized receptor fluorescence (green and yellow). Data are means ± SEMs of 3 independent experiments (**P < .01). Tf indicates transferrin.
CXCR2 internalization is regulated by Rab5
To assess whether these Rab GTPases affect the receptor endocytosis, HEK293 cells stably expressing CXCR2 were transiently transfected with plasmids for EGFP-tagged Rab5, Rab11a, and Rab7, respectively, and ligand-bound receptor internalization was measured by a radioligand binding assay. Ligand-bound CXCR2 internalization was not affected by the overexpression of EGFP-tagged wild-type Rab5, Rab11a, or Rab7 (Figure 6A-C). Interestingly, overexpression of a dominant-negative mutant Rab5-S34N significantly attenuated the internalization of CXCR2 (Figure 6A), whereas the dominant-negative mutants of Rab11a and Rab7 exhibited little effect on receptor internalization (Figure 6B-C). The inhibitory effect of the dominant-negative Rab5 mutant on the internalization of CXCR2 was confirmed by confocal microscopy. As shown in Figure7, CXCR2 was internalized into cells expressing EGFP-Rab5-Q79L (constitutively active mutant) in response to agonist treatment for 30 minutes. The internalized receptor was colocalized with the EGFP-Rab5-Q79L (Figure 7A-B). However, the dominant-negative EGFP-Rab5-S34N mutant exhibited a diffuse cytosolic distribution, in contrast to the wild-type EGFP-Rab5 protein. Agonist-activated CXCR2 did not colocalize with the EGFP-Rab5-S34N mutant in this compartment (Figure 7C-D). Although some CXCR2 immunostaining was observed inside the cells expressing EGFP-Rab5-S34N, most CXCR2 staining was localized to vesicles that appeared to be associated with or to be retained close to the cell surface (Figure7C). These data suggest the regulation of CXCR2 internalization by Rab5.
Role of Rab GTPases in CXCR2 internalization in HEK293 cells.
Cells stably expressing CXCR2 were transiently transfected with plasmids for EGFP-Rab5 or EGFP-Rab5-S34N (A), plasmids for EGFP-Rab11a or EGFP-Rab11a-S25N (B), or plasmids for EGFP-Rab7 or EGFP-Rab5-T22N (C). Cells were incubated with 75 nCi/mL (2.775 KBq/mL)125I-CXCL8 at 4°C for 1 hour. Unbound125I-CXCL8 was removed by washing at 4°C. Cells were warmed to 37°C for the indicated time period. 125I-CXCL8 remaining at the cell surface was removed with acetic acid (0.2 M, pH. 2.5) containing 0.5 M NaCl, and the internalized125I-CXCL8 was quantified on a γ-counter. Values represent the means ± SEMs of 3 independent experiments performed in duplicate. Data were analyzed using Student paired t test (*P < .05).
Role of Rab GTPases in CXCR2 internalization in HEK293 cells.
Cells stably expressing CXCR2 were transiently transfected with plasmids for EGFP-Rab5 or EGFP-Rab5-S34N (A), plasmids for EGFP-Rab11a or EGFP-Rab11a-S25N (B), or plasmids for EGFP-Rab7 or EGFP-Rab5-T22N (C). Cells were incubated with 75 nCi/mL (2.775 KBq/mL)125I-CXCL8 at 4°C for 1 hour. Unbound125I-CXCL8 was removed by washing at 4°C. Cells were warmed to 37°C for the indicated time period. 125I-CXCL8 remaining at the cell surface was removed with acetic acid (0.2 M, pH. 2.5) containing 0.5 M NaCl, and the internalized125I-CXCL8 was quantified on a γ-counter. Values represent the means ± SEMs of 3 independent experiments performed in duplicate. Data were analyzed using Student paired t test (*P < .05).
Agonist-stimulated internalization of CXCR2 in HEK293 cells expressing EGFP-Rab5-Q79L or EGFP-Rab5-S34N.
HEK293 cells stably expressing CXCR2 were transiently transfected with plasmids for EGFP-Rab5-Q79L (A-B) or EGFP-Rab5-S34N (C-D). Cells were treated with 200 nM CXCL8 at 37°C for 30 minutes, then fixed in methanol. Cells were incubated with a mouse monoclonal anti-CXCR2 antibody for 30 minutes, followed by incubation with a rhodamine-conjugated antimouse antibody for 30 minutes. Representative confocal micrographs from 3 independent experiments demonstrating the distribution of CXCR2 (A, C) in cells expressing EGFP-Rab5-Q79L (B) or EGFP-Rab5-S34N (D) are shown. Small arrows indicate the examples of colocalization of CXCR2 with EGFP-Rab5-Q79L (A-B). Large arrows indicate the retardation of CXCR2 internalization in the EGFP-Rab5-S34N–expressing cells (C). Images were processed using Photoshop software. Bars, 10 μm.
Agonist-stimulated internalization of CXCR2 in HEK293 cells expressing EGFP-Rab5-Q79L or EGFP-Rab5-S34N.
HEK293 cells stably expressing CXCR2 were transiently transfected with plasmids for EGFP-Rab5-Q79L (A-B) or EGFP-Rab5-S34N (C-D). Cells were treated with 200 nM CXCL8 at 37°C for 30 minutes, then fixed in methanol. Cells were incubated with a mouse monoclonal anti-CXCR2 antibody for 30 minutes, followed by incubation with a rhodamine-conjugated antimouse antibody for 30 minutes. Representative confocal micrographs from 3 independent experiments demonstrating the distribution of CXCR2 (A, C) in cells expressing EGFP-Rab5-Q79L (B) or EGFP-Rab5-S34N (D) are shown. Small arrows indicate the examples of colocalization of CXCR2 with EGFP-Rab5-Q79L (A-B). Large arrows indicate the retardation of CXCR2 internalization in the EGFP-Rab5-S34N–expressing cells (C). Images were processed using Photoshop software. Bars, 10 μm.
CXCR2 recycling is regulated by Rab11a
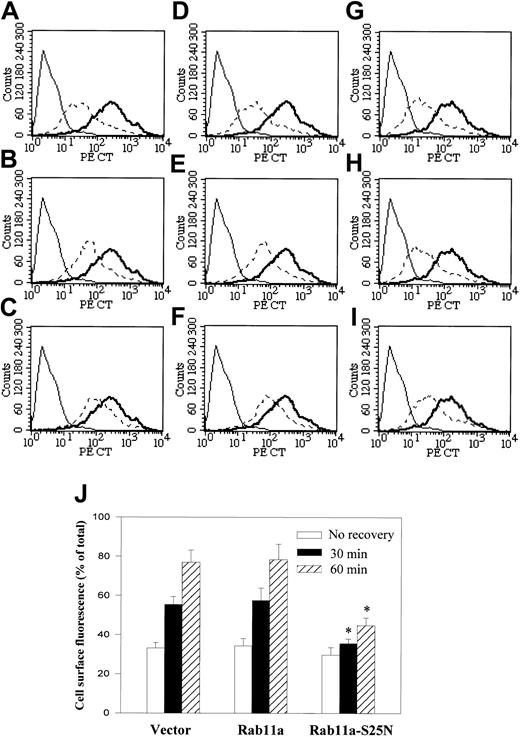
Previous studies on neutrophils have shown that internalized CXCR2 is recycled back to the plasma membrane on free-ligand (CXCL8) removal and recovery of the cells at 37°C.40 Rab11a, which is localized in the recycling endosomes, has been demonstrated to play a role in receptor recycling. Colocalization of the agonist-activated CXCR2 with EGFP-Rab11a (Figure 2) suggests that Rab11a regulates CXCR2 recycling. To test this hypothesis, we analyzed by fluorescence-activated cell sorter (FACS) the kinetics of recycling of CXCR2 on CXCL8 removal and recovery of the cells after CXCL8 exposure for 30 minutes at 37°C. Receptor re-expression levels following ligand removal were compared with the levels of their expression in control cells in which CXCL8-induced CXCR2 internalization was not elicited. As shown in Figure 8, without recovery, the internalized receptor levels are comparable among control cells (transfected with vector; Figure 8A,J), cells overexpressing Rab11a (Figure 8D,J), and cells overexpressing Rab11a-S25N (Figure8G,J). Following removal of the agonist and recovery of the cells at 37°C, CXCR2 was re-expressed on the cell membrane. On incubation at 37°C for 30 minutes, CXCR2 expression was restored to 55.4% ± 4.9% of the level in the control cells (Figure 8B,J). Recovery of receptor expression to 76.9% ± 6.3% was observed after 1 hour of incubation (Figure 8C,J). Overexpression of wild-type Rab11a did not affect the recovery of CXCR2 (57% ± 6.3% for 30 minutes of incubation, 78.4% ± 8.1% for 1 hour of incubation; Figure8E,F,J). In contrast, in cells overexpressing Rab11a-S25N, the recovery of CXCR2 levels was significantly reduced (35.5% ± 2.4% for 30 minutes of incubation, 44.8% ± 3.8% for 1 hour of incubation; Figure 8H-J).
Role of Rab11a in CXCR2 recycling.
HEK293 cells stably expressing CXCR2 were transiently transfected with plasmids for vector (A-C) Rab11a (D-F) or Rab11a-S25N (G-I). Cells were treated with CXCL8 at 37°C for 30 minutes, then the agonist was removed and the cells were recovered by incubation with agonist-free medium for 0 minutes (A,D,G), 30 minutes (B,E,H), or 1 hour (C,F,I) at 37°C. For the staining of the cell surface receptor, cells were incubated with a monoclonal CXCR2 antibody at 4°C for 1 hour, followed by incubation with FITC-conjugated antimouse IgG at 4°C for 30 minutes. Cells were washed and fixed in 2% formaldehyde in PBS and analyzed in FACScan. Thin solid line represents the staining of cells in the absence of primary CXCR2 antibody (background). Thick solid line represents the immunostaining of cell surface CXCR2 in the cells without CXCL8 treatment (total). Broken line indicates the immunostaining cell surface CXCR2 in the cells treated with CXCL8 and recovered for different intervals. (J) Relative cell surface fluorescence (percentage of total) of the cells recovered for different intervals was quantified. Data are means ± SEMs of 4 independent experiments (*P < .05).
Role of Rab11a in CXCR2 recycling.
HEK293 cells stably expressing CXCR2 were transiently transfected with plasmids for vector (A-C) Rab11a (D-F) or Rab11a-S25N (G-I). Cells were treated with CXCL8 at 37°C for 30 minutes, then the agonist was removed and the cells were recovered by incubation with agonist-free medium for 0 minutes (A,D,G), 30 minutes (B,E,H), or 1 hour (C,F,I) at 37°C. For the staining of the cell surface receptor, cells were incubated with a monoclonal CXCR2 antibody at 4°C for 1 hour, followed by incubation with FITC-conjugated antimouse IgG at 4°C for 30 minutes. Cells were washed and fixed in 2% formaldehyde in PBS and analyzed in FACScan. Thin solid line represents the staining of cells in the absence of primary CXCR2 antibody (background). Thick solid line represents the immunostaining of cell surface CXCR2 in the cells without CXCL8 treatment (total). Broken line indicates the immunostaining cell surface CXCR2 in the cells treated with CXCL8 and recovered for different intervals. (J) Relative cell surface fluorescence (percentage of total) of the cells recovered for different intervals was quantified. Data are means ± SEMs of 4 independent experiments (*P < .05).
The effect of Rab11a on the recycling of CXCR2 was confirmed by confocal microscopy. HEK293 cells were cotransfected with plasmids for CXCR2, together with EGFP-Rab11a-S20V (constitutively active mutant) or EGFP-Rab11a-S25N (dominant-negative mutant). Figure9 shows the different pattern of distribution between EGFP-Rab11a-S20V and EGFP-Rab11a-S25N. EGFP-Rab11a-S20V was punctated in the cytoplasm as the expression pattern of wild-type Rab11a (Figure 9B,D). In contrast, EGFP-Rab11a-S25N exhibited diffuse cytosolic distribution (Figure9F,H). To observe the receptor recycling, we incubated the cells with CXCL8 for 30 minutes at 37°C and removed the agonist by washing the cells with agonist-free medium. Cells either were fixed immediately or were recovered by incubation in agonist-free medium for 1 hour at 37°C. As shown in Figure 9, without recovery, CXCR2 was localized in the cytoplasm in the cells expressing either EGFP-Rab11a-S20V (Figure 9A-B) or EGFP-Rab11a-S25N (Figure 9E,F). Following removal of the agonist and recovery of the cells at 37°C for 1 hour, a large proportion of CXCR2 was re-expressed on the cell surface in the cells overexpressing EGFP-Rab11a-S20V (Figure 9C-D), but not in the cells overexpressing EGFP-Rab11a-S25N (Figure 9G-H). In Figure 9C, the small arrows indicate that CXCR2 was recycled back to the cell surface in cells overexpressing EGFP-Rab11a-S20V. In Figure9G, the large arrow indicates that CXCR2 was retained in the cytoplasm in cells overexpressing EGFP-Rab11a-S25N. As an internal control in Figure 9G, in the cell without overexpression of EGFP-Rab11a-S25N (without arrow), the receptor was mostly recycled back to the cell surface. These data strongly suggest regulation of CXCR2 recycling by Rab11a.
Recycling of CXCR2 in HEK293 cells overexpressing EGFP-Rab11a-S20V or EGFP-Rab11a-S25N.
Cells coexpressing CXCR2 and Rab11a-S20V (A-D) or Rab11a-S25N (E-H) were treated with CXCL8 at 37°C for 30 minutes, then the agonist was removed. Cells either were fixed immediately (A-B,E-F) or were incubated with agonist-free medium for 1 hour at 37°C before being fixed (C-D,G-H). Cells were incubated with a mouse monoclonal anti-CXCR2 antibody for 30 minutes, followed by incubation with a rhodamine-conjugated antimouse antibody for 30 minutes. Representative confocal micrographs from 4 independent experiments demonstrating the intracellular distribution of CXCR2 (A,C,E,G) and EGFP-Rab11a-S20V (B,D) or EGFP-Rab11a-S25N (F,H) were shown. Small arrows indicate the recycled CXCR2 on the cell surface in the cells expressing EGFP-Rab11a-S20V (C). Large arrow indicates the nonrecycling of CXCR2 in the EGFP-Rab11a-S25N-expressing cell (G). Images were processed using Photoshop software. Bars, 10 μm.
Recycling of CXCR2 in HEK293 cells overexpressing EGFP-Rab11a-S20V or EGFP-Rab11a-S25N.
Cells coexpressing CXCR2 and Rab11a-S20V (A-D) or Rab11a-S25N (E-H) were treated with CXCL8 at 37°C for 30 minutes, then the agonist was removed. Cells either were fixed immediately (A-B,E-F) or were incubated with agonist-free medium for 1 hour at 37°C before being fixed (C-D,G-H). Cells were incubated with a mouse monoclonal anti-CXCR2 antibody for 30 minutes, followed by incubation with a rhodamine-conjugated antimouse antibody for 30 minutes. Representative confocal micrographs from 4 independent experiments demonstrating the intracellular distribution of CXCR2 (A,C,E,G) and EGFP-Rab11a-S20V (B,D) or EGFP-Rab11a-S25N (F,H) were shown. Small arrows indicate the recycled CXCR2 on the cell surface in the cells expressing EGFP-Rab11a-S20V (C). Large arrow indicates the nonrecycling of CXCR2 in the EGFP-Rab11a-S25N-expressing cell (G). Images were processed using Photoshop software. Bars, 10 μm.
Rab7 is required for the transport of CXCR2 to lysosomes
We have demonstrated in Figure 5 that CXCR2 was colocalized with LDL in response to agonist treatment for 4 hours, suggesting that a proportion of CXCR2 was transported to lysosomes. To determine whether Rab7 is involved in lysosomal sorting of CXCR2, HEK293 cells stably coexpressing CXCR2 and the wild-type Rab7 or the dominant-negative mutant Rab7-T22N were treated with CXCL8 (200 nM) for 4 hours, and the colocalization of CXCR2 with Lamp-1 was determined by confocal microscopy. CXCR2 was colocalized with LAMP-1 in the control cells (Figure 10A), which is consistent with the colocalization of the receptor with LDL shown in Figure 5. Interestingly, the colocalization of CXCR2 with Lamp-1 appeared to be increased in the cells overexpressing wild-type Rab7 proteins (Figure10B). In contrast to wild-type Rab7, which did not change the perinuclear aggregation of lysosomes (Figure 10B), overexpressing a dominant-negative mutant Rab7-T22N significantly changed the morphology of Lamp-1 staining. Lamp-1 staining became smaller and was dispersed throughout the cytoplasm in cells overexpressing Rab7-T22N, as shown in Figure 10C, and the colocalization of CXCR2 with Lamp-1 was significantly decreased (Figure 10C). Rab7-T22N has been shown to block the lysosomal sorting of LDL for degradation,41 so that the colocalization of LDL with LAMP-1 in the Rab7- or the Rab7-T22N–expressing cells could be considered as a positive control for the effect of Rab7 on the transport of CXCR2 to lysosomes. Cells were loaded with Dil-LDL for 4 hours, and the colocalization of LDL with LAMP-1 was examined by confocal microscopy. As expected, LDL strongly colocalized with LAMP-1 in the cells overexpressing Rab7 (Figure 10D), but the colocalization was remarkably reduced in the cells overexpressing Rab7-T22N (Figure 10E). These data suggest that CXCR2 is targeted to degradation in lysosomes after prolonged agonist treatment and that Rab7 plays a role in the transport of the receptor to lysosomes.
Colocalization of CXCR2 or LDL with Lamp-1 in HEK293 cells overexpressing Rab7 or Rab7-T22N.
(A-C) Colocalization of CXCR2 with LAMP-1. HEK293 cells stably cotransfected with plasmids encoding CXCR2 and vector (A), CXCR2 and Rab7 (B), or CXCR2 and Rab7-T22N (C) were treated with CXCL8 at 37°C for 4 hours. Cells were fixed with methanol and incubated with a mixture of a rabbit CXCR2 antibody and a mouse monoclonal Lamp-1 antibody for 30 minutes, followed by a mixture of an FITC-conjugated antirabbit antibody and a rhodamine-conjugated antimouse antibody for 30 minutes. Representative laser-scanning confocal micrographs from independent experiments demonstrating the distribution of CXCR2 (green) and Lamp-1 (red) and the colocalization of CXCR2 with Lamp-1 (yellow) are shown. (D-E) Colocalization of LDL with LAMP-1. HEK293 cells stably expressing Rab7 (D) or Rab7-T22N (E) were incubated with Dil-LDL (40 μg/mL) at 37°C for 4 hours, then fixed in methanol. Cells were incubated with a monoclonal LAMP-1 antibody followed by incubation with an FITC-conjugated antimouse antibody. Representative confocal micrographs from 3 independent experiments demonstrating the distribution of LDL (red), Lamp-1 (green), and the colocalization of LDL with Lamp-1 (yellow) are shown. Images were processed using Photoshop software. Bars, 10 μm.
Colocalization of CXCR2 or LDL with Lamp-1 in HEK293 cells overexpressing Rab7 or Rab7-T22N.
(A-C) Colocalization of CXCR2 with LAMP-1. HEK293 cells stably cotransfected with plasmids encoding CXCR2 and vector (A), CXCR2 and Rab7 (B), or CXCR2 and Rab7-T22N (C) were treated with CXCL8 at 37°C for 4 hours. Cells were fixed with methanol and incubated with a mixture of a rabbit CXCR2 antibody and a mouse monoclonal Lamp-1 antibody for 30 minutes, followed by a mixture of an FITC-conjugated antirabbit antibody and a rhodamine-conjugated antimouse antibody for 30 minutes. Representative laser-scanning confocal micrographs from independent experiments demonstrating the distribution of CXCR2 (green) and Lamp-1 (red) and the colocalization of CXCR2 with Lamp-1 (yellow) are shown. (D-E) Colocalization of LDL with LAMP-1. HEK293 cells stably expressing Rab7 (D) or Rab7-T22N (E) were incubated with Dil-LDL (40 μg/mL) at 37°C for 4 hours, then fixed in methanol. Cells were incubated with a monoclonal LAMP-1 antibody followed by incubation with an FITC-conjugated antimouse antibody. Representative confocal micrographs from 3 independent experiments demonstrating the distribution of LDL (red), Lamp-1 (green), and the colocalization of LDL with Lamp-1 (yellow) are shown. Images were processed using Photoshop software. Bars, 10 μm.
The above data suggest that CXCR2 is transported from early endosomes to late endosomes/lysosomes. Thus, blocking lysosomal sorting by overexpressing the dominant-negative mutant Rab7-T22N may result in retention of the receptors in the early endosomes. In addition, because CXCR2 may traffic from early to recycling endosomes, blocking the lysosomal sorting may also result in the retention of some receptors in the recycling endosomes. To test this hypothesis, HEK293 cells stably coexpressing CXCR2 and Rab7 or Rab7-T22N were transiently transfected with cDNA for EGFP-Rab5 or EGFP-Rab11a. Cells were treated with CXCL8 for 4 hours, when most of the internalized receptors have left these 2 Rab protein-positive endosomes for late endosomes/lysosomes (Figures1D, 2D, 3D). As anticipated, CXCR2 exhibited little colocalization with EGFP-Rab5 and EGFP-Rab11 in the cells overexpressing wild-type Rab7 (Figure 11A, C) following CXCL8 treatment for 4 hours. However, the colocalization of CXCR2 and EGFP-Rab5 or EGFP-Rab11a was remarkably increased in the cells overexpressing Rab7-T22N (Figure 11B,D). These data suggest that blocking late endosomal/lysosomal sorting by overexpressing Rab7-T22N results in the retention of the receptor in early and recycling endosomes.
Colocalization of CXCR2 or LDL with EGFP-Rab5 or EGFP-Rab11a in HEK293 cells overexpressing Rab7 or Rab7-T22N.
(A-D) Colocalization of CXCR2 with EGFP-Rab5 or EGFP-Rab11a. HEK293 cells stably coexpressing CXCR2 and Rab7 (A, C) or CXCR2 and Rab7-T22N (B, D) were transiently transfected with plasmids encoding EGFP-Rab5 (A-B) or EGFP-Rab11a (C-D). Cells were treated with CXCL8 (200 ng/mL) at 37°C for 4 hours. Cells were fixed with methanol and incubated with a mouse monoclonal CXCR2 antibody for 30 minutes, followed by a Cy3-conjugated antimouse antibody for 30 minutes. Representative laser-scanning confocal micrographs from 3 independent experiments demonstrating the distribution of CXCR2 (red), EGFP-Rab5, or EGFP-Rab11a (green), and the colocalization (yellow) of CXCR2 with either EGFP-Rab5 (A-B) or EGFP-Rab11a (C-D) are shown. (E-F) Colocalization of LDL with EGFP-Rab5. HEK293 cells stably expressing Rab7 (E) or Rab7-T22N (F) were incubated with Dil-LDL (40 μg/mL) at 37°C for 4 hours, then fixed in methanol. Representative confocal micrographs demonstrating the distribution of LDL (red) and EGFP-Rab5 (green) and the colocalization of LDL with EGFP-Rab5 (yellow) are shown. Images were processed using Photoshop software. Bars, 10 μm.
Colocalization of CXCR2 or LDL with EGFP-Rab5 or EGFP-Rab11a in HEK293 cells overexpressing Rab7 or Rab7-T22N.
(A-D) Colocalization of CXCR2 with EGFP-Rab5 or EGFP-Rab11a. HEK293 cells stably coexpressing CXCR2 and Rab7 (A, C) or CXCR2 and Rab7-T22N (B, D) were transiently transfected with plasmids encoding EGFP-Rab5 (A-B) or EGFP-Rab11a (C-D). Cells were treated with CXCL8 (200 ng/mL) at 37°C for 4 hours. Cells were fixed with methanol and incubated with a mouse monoclonal CXCR2 antibody for 30 minutes, followed by a Cy3-conjugated antimouse antibody for 30 minutes. Representative laser-scanning confocal micrographs from 3 independent experiments demonstrating the distribution of CXCR2 (red), EGFP-Rab5, or EGFP-Rab11a (green), and the colocalization (yellow) of CXCR2 with either EGFP-Rab5 (A-B) or EGFP-Rab11a (C-D) are shown. (E-F) Colocalization of LDL with EGFP-Rab5. HEK293 cells stably expressing Rab7 (E) or Rab7-T22N (F) were incubated with Dil-LDL (40 μg/mL) at 37°C for 4 hours, then fixed in methanol. Representative confocal micrographs demonstrating the distribution of LDL (red) and EGFP-Rab5 (green) and the colocalization of LDL with EGFP-Rab5 (yellow) are shown. Images were processed using Photoshop software. Bars, 10 μm.
LDL is transported quickly from early endosomes to late endosomes/lysosomes after uptake.39 To examine whether the overexpression of Rab7-T22N results in the retention of LDL in early endosomes, HEK293 cells stably expressing Rab7 or Rab7-T22N were transfected with EGFP-Rab5. Cells were loaded with Dil-LDL (40 μg/mL) for 4 hours, and the colocalization of Dil-LDL with EGFP-Rab5 was determined by confocal microscopy. As anticipated, there was little colocalization between LDL and EGFP-Rab5 in the Rab7-expressing cells (Figure 11E). However, in the Rab7-T22N–expressing cells, LDL exhibited significantly increased colocalization with the EGFP-Rab5 (Figure 11F). These data, taken together with the effect of Rab7-T22N on the morphologic alteration of lysosomal compartments (Figure10C,E), suggest that Rab7-T22N causes the disorganization of lysosomes, thus blocking protein transport from early endosomes to lysosomes.
Discussion
To gain insight into the intracellular trafficking pathway of CXCR2 and to determine the fine-tuning of the receptor transport among endosomal compartments, we focused on the regulation of CXCR2 trafficking by various Rab GTPases, which have been implicated principally in the control of membrane budding, transport, vesicle docking, and membrane fusion.37 CXCR2 is expressed predominantly in neutrophils, but, because of the technical difficulties in transfection and confocal microscopy using the suspended neutrophils, we used a well-characterized model system (HEK293 cells) in which the receptor and the EGFP-tagged Rab GTPases are overexpressed. This system was shown to highly resemble neutrophils in ligand-binding affinity, receptor–G-protein coupling, homologous desensitization, internalization, and recycling of CXCR2.12,13 42-44 The present study demonstrated that multiple Rab GTPases are involved in the intracellular trafficking of CXCR2 in HEK293 cells. To provide evidence that these Rab proteins play the same role in the receptor trafficking in some other cell lines, we also determined the colocalization of CXCR2 with these Rab proteins in RBL-2H3 cells, which are widely used for the study of chemokine receptors.
Endocytosis is characterized by vesicular transport along numerous pathways. Common steps in each pathway include membrane budding to form vesicles, transport to a particular destination, and ultimately docking and fusion with the target membrane. Agonist-bound CXCR2 appears to internalize into vesicles through a dynamin-mediated formation of clathrin-coated pits,13 after which the receptors are rapidly transported to early and recycling endosomal compartments. This is evident by the colocalization of the receptor with Rab5,29 Rab11a,23 and transferrin.38 However, prolonged agonist exposure results in transport of the receptor to late endosomes and lysosomes, as can be seen by the colocalization of the receptor with Rab7, LDL, and LAMP-1.
CXCR2 internalization appears to be regulated by Rab5, based on the data that overexpression of a dominant-negative mutant Rab5-S34N attenuated the internalization of the ligand-bound CXCR2. Rab5 is important for sequestering receptors into clathrin-coated pits, subsequent fusion of vesicles with early endosomes, and homotypic fusion between early endosomes.45,46 Rab5-S34N likely prevents CXCR2 endocytosis before pinching off of the clathrin-coated vesicles from the plasma membrane. We also observed that internalized CXCR2 was colocalized with the constitutively active mutant Rab5-Q79L. In contrast to the D2 dopamine receptor,33 the overexpression of Rab5-Q79L did not significantly facilitate the internalization of CXCR2 as measured by radioligand-binding assay. These data are consistent with the report demonstrating that the overexpression of Rab5-Q79L did not enhance β2-AR internalization.31 Consequently, these data clearly demonstrated that Rab5 plays an important role in regulating the sequestration and endosomal membrane fusion of the endocytic vesicles that contain the internalized CXCR2.
CXCR2 has been demonstrated to colocalize with Rab11a on agonist exposure.47 An interesting observation in this study is that CXCR2 was colocalized with Rab11a in the same time-frame as the colocalization of the receptor with Rab5. Rab11a, which is concentrated in recycling endosomes, was initially demonstrated to be important for transferrin transport through recycling endosomes in nonpolarized cells.48,49 The small effect of the dominant-negative mutant of Rab11a on CXCR2 internalization excludes the possibility of a role for Rab11a in the early endosomal trafficking of CXCR2. Even though CXCR2 exhibited little recycling in the continued presence of agonist, it is recycled back to the cell membrane after removal of the agonist and recovery of the cells at 37°C. Receptor recycling was inhibited by overexpression of a dominant-negative mutant, Rab11a-S25N, suggesting the regulatory role of Rab11a in CXCR2 recycling. Another Rab protein that is localized in recycling endosomes is Rab4.23 It has been postulated that Rab4 plays a role in the recycling of β2-AR because a dominant-negative mutant of Rab4 blocked the recycling of β2-AR.31 However, we did not observe colocalization of CXCR2 with Rab4 on agonist stimulation (data not shown), implying that Rab4 does not associate with the Rab11a-positive endosomal compartments associated with CXCR2 trafficking. Our data suggest that various GPCRs are regulated differentially by the Rab GTPases.
Previous studies have demonstrated that prolonged agonist stimulation induces degradation of CXCR2.9,13,24 The inhibition of CXCR2 degradation by the lysosomal inhibitor chloroquine (data not shown) suggests that CXCR2 is degraded in lysosomes. We observed a dramatic increase in the colocalization of CXCR2 with Rab7 in response to prolonged agonist stimulation (4 hours). In the meantime, CXCR2 was found to be colocalized with Lamp-1 and LDL. These data suggest that most of the internalized CXCR2 is transported from early endosomes to late endosomes/lysosomes on prolonged ligand stimulation. In addition, studies on CXCR4 trafficking also demonstrated that CXCR4 colocalized with Lamp-1 after 3 hours of CXCL12 treatment.50 It is unclear why it takes longer for lysosomal sorting of chemokine receptors than of LDL.
Rab7 likely plays an important role in the transport of CXCR2 to lysosomes. The inhibition of lysosomal sorting of CXCR2 by the overexpression of the dominant-negative mutant Rab7-T22N could be explained in 2 different ways. One interpretation is that Rab7 plays a role in the transport of the internalized receptor from late endosomes to lysosomes, which is hypothesized by Meresse et al51based on their observation that a GTPase-defective Rab7 mutant (constitutively active) exhibits enhanced association with lysosomes. In contrast to the cells expressing the wild-type Rab7, which showed an aggregation of lysosomes in the perinuclear region, in the cells overexpressing the dominant-negative Rab7-T22N mutant, perinuclear lysosome aggregation disappeared and lysosomes identified by Lamp-1 became dispersed throughout the cytoplasm. Thus, Rab7 is considered to control the aggregation and fusion of late endocytic structures/lysosomes, which is essential for maintenance of the perinuclear lysosome compartment.52 The second interpretation is that Rab7 is essential for the transport from early to late endosomes.53 This hypothesis is supported by our observation that overexpression of the dominant-negative Rab7-T22N mutant, which blocks the transport of CXCR2 to lysosomes, resulted in the retention of CXCR2 in the Rab5-positive early endosomes. In addition, because there is a pathway from early to recycling endosomes, it is anticipated that overexpression of the dominant-negative Rab7-T22N mutant resulted in the localization of CXCR2 in recycling endosomes.
Taken together, this study clearly demonstrated that the chemokine receptor CXCR2 undergoes a recycling pathway and lysosomal sorting pathways under different conditions. CXCR2 is sequestered into early endosomes on agonist exposure. The receptors are delivered to recycling endosomes and are partially recycled back to the cell membrane after removal of the agonist, though the recycling rate is very slow.54 In the prolonged presence of a saturated concentration of agonist, CXCR2 is transported predominantly to lysosomes for degradation, but this process is relatively slow compared with the quick lysosomal sorting of LDL.39 These data are important for understanding the regulation of the receptor functions under physiological and pathologic conditions. It is well known that one of the most important functions of chemokine receptors is to mediate chemotaxis of neutrophils and lymphocytes to the inflammatory sites, and it is postulated that receptor internalization and recycling play an important role in this process.12,13 55Neutrophils and lymphocytes migrate to the inflammatory sites along the gradient concentrations of chemokines. When the cells are far away from the inflammatory sites where the chemokine concentrations are relatively low, some internalized chemokine receptors are able to undergo recycling and resensitization, so as to respond to the continued stimulation of the ligands for chemotaxis. When the cells reach the inflammatory sites, chemokine receptors are targeted for degradation in lysosomes without recycling because of the exposure of high concentrations of ligands. These result in termination of the chemotactic response and accumulation of neutrophils and lymphocytes in the inflammatory sites.
We thank Dr Angela Wandinger-Ness in the University of New Mexico Health Sciences Center for the generous gifts of the wild-type and mutant Rab7 plasmids, Dr Stephan S. G. Ferguson of the University of Western Ontario, London, ON, Canada, for the generous gifts of the wild-type and mutant Rab5 plasmids; Dr Richard Roberts of Vanderbilt University for the generous gifts of transferrin–Texas Red and Dil-LDL; and Dr Ricardo Richard of Meharry Medical College for offering us the RBL-2H3 cells. We also thank Dr Sam Wells of Vanderbilt University Ingram-Cancer Center for confocal analysis, and we thank Catherine Allen, Lynn Butler, and Melanie Smith of Nashville VA Medical Center for their assistance with FACS analysis. In addition, we thank Linda W. Horton and Yingchun Yu in our laboratory for technical help.
Prepublished online as Blood First Edition Paper, October 31, 2002; DOI 10.1182/blood-2002-07-1965.
Supported by a Department of Veterans Affairs career scientist grant (A.R.), National Cancer Institute grant CA 34590 (A.R.), Vanderbilt-Ingram Cancer Center support grant CA68485, and National Institutes of Health grants DK48370 (J.R.G.) and DK43405 (J.R.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ann Richmond, Department of Cancer Biology, Vanderbilt University School of Medicine, Nashville, TN 37232; e-mail:ann.richmond@vanderbilt.edu.