The proteasome inhibitor PS-341 inhibits nuclear factor-κB (NF-κB) activation, induces apoptosis in cancer cells, including multiple myeloma (MM) cells, and has marked clinical activity as a monotherapy for MM. In this study, we found that subtoxic concentrations of PS-341 potently sensitized MM cell lines and patient cells to DNA-damaging chemotherapeutic agents such as doxorubicin and melphalan, including cells resistant to these drugs and those isolated from a patient who had relapsed after PS-341 monotherapy. Moreover, PS-341 abolished cell adhesion–mediated drug resistance. Using gene expression profiling and proteomic analysis, we demonstrate that PS-341, among its other proapoptotic effects, down-regulates the expression of several effectors involved in the cellular response to genotoxic stress. These data suggest that, in addition to down-regulating the expression of apoptosis inhibitors, PS-341 inhibits genotoxic stress response pathways and thereby restores sensitivity to DNA-damaging chemotherapeutic agents. These studies, therefore, provide the framework for clinical use of this agent in combination with conventional chemotherapy.
Introduction
PS-341, a boronic acid dipeptide proteasome inhibitor, inhibits the activation of the transcription factor NF-κB (nuclear factor-κB),1,2 down-regulates the expression of several apoptosis inhibitors,3 induces caspase-dependent apoptosis of drug resistant multiple myeloma (MM) cell lines and patient cells,3,4 inhibits MM cell binding to bone marrow stromal cells (BMSCs), and inhibits production of MM growth and survival factors in the BM milieu.5 In a murine plasmacytoma model, PS-341 inhibits tumor growth in a dose-dependent fashion and prolongs host survival.6 In a phase 2 clinical trial of PS-341 in patients with relapsed, refractory MM, objective responses, including some complete responses, were observed.7
In this study, we characterized the effect of PS-341 combined with doxorubicin and melphalan on MM cells. We found that PS-341 lowered the apoptotic threshold to these chemotherapeutic agents and even reversed drug resistance. Gene expression profiling using oligonucleotide microarrays, as well as proteomic analysis, detected down-regulation of several effectors mediating the response to genotoxic stress. These studies, therefore, provide the framework for the future use of PS-341 combined with conventional chemotherapy.
Study design
Tissue culture
Human MM cell lines included MM.1S,8RPMI-8226/S, and its doxorubicin (Dox40)- and melphalan (LR5)-resistant sublines,8 ARP-1,8 S6B45,8NCI-H929 (American Type Culture Collection, Manassas, VA), and INA6 (a gift from Renate Burger, University of Erlangen-Nuernberg, Germany). Tumor cells were freshly isolated from the bone marrow of MM patients as previously described.8
Case report
A 58-year-old woman, with relapsed refractory immunoglobulin G (IgG) lambda MM, had received prior therapy with melphalan and prednisone; vincristine, doxorubicin, and dexamethasone (VAD) plus cyclophosphamide; high-dose melphalan and autologous stem cell transplantation; α-interferon; and thalidomide. After informed consent, she received cyclic PS-341, 1.3 mg/m2 intravenously twice a week for 2 weeks with 1 week off, per approved protocol of the institutional review board (IRB). Although serum paraprotein decreased from 5.1 g/dL to 3.3 g/dL after 3 cycles of PS-341, she developed fatigue and exacerbation of a preexisting peripheral neuropathy. PS-341 dose was, therefore, reduced to 1 mg/m2, and she completed 4 cycles of therapy. Because of progressive disease evidenced by increasing paraprotein (5.0 g/dL) and circulating plasma cells, dexamethasone (40 mg twice a week for 2 weeks each cycle) was added. Although her M-component slightly decreased (to 4.7 g/dL) and circulating plasma cells transiently cleared, she developed rapidly progressive disease, with 48% circulating plasma cells after the sixth cycle; PS-341 protocol treatment was discontinued. Subsequent therapy with intravenous cyclophosphamide, thalidomide, thalidomide alone, dexamethasone, and biaxin, as well as Doxil with thalidomide and dexamethasone, was ineffective. At this time, MM cells were isolated by BM aspiration and purified as previously described.8
Materials
PS-341 was provided by Millennium Pharmaceuticals (Cambridge, MA). Dexamethasone, doxorubicin, melphalan, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma Chemical (St Louis, MO). Ku 70 and 80 antibodies were acquired from Lab Vision (Fremont, CA).
Methods
RNA isolation, gene expression profiling, and data analysis were performed as previously described.3 High-throughput global proteomic analysis of the signaling state of PS-341–treated MM cells was performed by multiplex-immunoblotting arrays using the KPKS-1.0 and KPSS-1.0 platforms, as previously described.9,10Immunoblotting analysis and quantification of cell survival with the MTT assay were performed as previously described.11 All experiments were repeated at least 3 times, and each experimental condition was repeated at least in quadruplicate wells. Results from representative experiments are shown. LD50 values were calculated with the use of the SPSS-11.0 statistical package. Statistical significance was examined by a 2-way analysis of variance, followed by Duncan post hoc test. In all analyses,P < .05 was considered statistically significant.
Results and discussion
Synergistic anti-MM activity between PS-341 and conventional chemotherapeutics
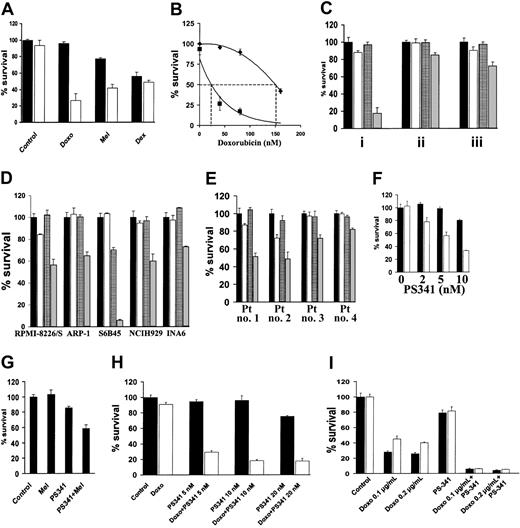
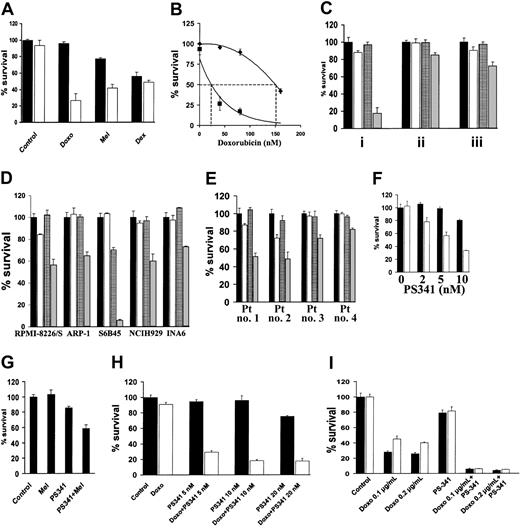
NF-κB confers resistance to DNA-damaging chemotherapy12; conversely, specific inhibition of NF-κB sensitizes MM cells to subtoxic concentrations of doxorubicin.13 We now investigated whether the proteasome inhibitor PS-341, which inhibits NF-κB activity,1sensitizes MM cells to conventional chemotherapy. As shown in Figure1A, PS-341, at a subtoxic concentration, markedly enhances sensitivity of MM.1S cells to subtoxic concentrations of doxorubicin and to melphalan (P < .001 in both cases). However, the subtoxic concentration of PS-341 did not increase the anti-MM effect of dexamethasone (P > .05), in agreement with our previous finding of only additive cytotoxicity between these 2 agents.5 Dose-response analysis demonstrated that the LD50 for doxorubicin in MM.1S cells was 150 nM in the absence and 26 nM in the presence of PS-341 (2 nM) (Figure 1B). The concentration of PS-341 is 10 to 30 nM in patients' serum, with peaks of 100 nM, which is sufficient to achieve this synergistic effect in vivo.
PS-341 sensitizes MM cells to DNA-damaging chemotherapy.
(A) MM.1S cells were pretreated with doxorubicin (Doxo; 40 nM), melphalan (Mel; 1 μM), or dexamethasone (Dex; 0.5 μM) for 24 hours, and then PS-341 (2 nM) was added for additional 24 hours (black bars, without PS-341; white bars, with PS-341). PS-341 sensitizes MM.1S cells to DNA-damaging chemotherapy. (B) Dose-response analysis for the effect of doxorubicin on MM.1S cells in the presence (▪) or absence (♦) of PS-341 (2 nM) reveals that PS-341 decreases the LD50 of doxorubicin from 150 to 26 nM. (C) MM.1S cells were pretreated with doxorubicin (50 ng/mL) for 24 hours, and then PS-341 (2 nM) was added for an additional 24 hours (i), or pretreated with PS-341 for 24 hours and then doxorubicin for an additional 24 hours (ii), or treated with PS-341 and doxorubicin together for 24 hours (iii). In all cases a synergistic effect is found, but the strongest synergy is observed when the cells are pretreated with doxorubicin followed by PS-341 treatment (black bars, control; white bars, doxorubicin alone; grid bars, PS-341; gray bars, doxorubicin plus PS-341). (D) RPMI-8226/S, ARP-1, S6B45, NCH-H929, and INA6 cells were pretreated with doxorubicin (50 ng/mL) for 24 hours and then with PS-341 (2 nM) for an additional 24 hours (black bars, control; white bars, doxorubicin alone; grid bars, PS-341; gray bars, doxorubicin plus PS-341). (E) Primary MM cells from 4 PS-341–naive patients were pretreated with doxorubicin (50 ng/mL) for 24 hours and then with PS-341 (2 nM) for an additional 24 hours. PS-341 sensitizes all MM cells to doxorubicin (black bars, control; white bars, doxorubicin alone; grid bars, PS-341; gray bars, doxorubicin plus PS-341). (F) Doxorubicin-resistant RPMI-Dox40 cells were pretreated with (white bars) or without (black bars) doxorubicin (800 ng/mL) for 24 hours, and then PS-341 (2-10 nM) was added for an additional 24 hours (black bars, without doxorubicin; white bars, with doxorubicin). PS-341 sensitizes RPMI-Dox40 cells to doxorubicin. (G) Melphalan-resistant LR5 cells were pretreated with or without melphalan (5 μM) for 24 hours, and then PS-341 (2 nM) was added for an additional 24 hours. PS-341 sensitizes LR5 cells to melphalan. (H) MM cells isolated from a patient who had relapsed following treatment with PS-341 were pretreated with (white bars) or without (black bars) doxorubicin (100 ng/mL) for 24 hours, and then PS-341 (5-20 nM) was added for an additional 24 hours. Pretreatment with doxorubicin overcomes resistance to PS-341. (I) MM.1S cells were treated for 24 hours with doxorubicin (100-200 ng/mL) in wells coated with (white bars) or without (black bars) fibronectin (FN). PS-341 (10 nM) was added for additional 24 hours. In all cases, percentage of cell survival (mean ± SD) is quantified by MTT. All experiments were repeated at least 3 times, and each experimental condition was repeated at least in quadruplicate wells in each experiment. Results from representative experiments are shown.
PS-341 sensitizes MM cells to DNA-damaging chemotherapy.
(A) MM.1S cells were pretreated with doxorubicin (Doxo; 40 nM), melphalan (Mel; 1 μM), or dexamethasone (Dex; 0.5 μM) for 24 hours, and then PS-341 (2 nM) was added for additional 24 hours (black bars, without PS-341; white bars, with PS-341). PS-341 sensitizes MM.1S cells to DNA-damaging chemotherapy. (B) Dose-response analysis for the effect of doxorubicin on MM.1S cells in the presence (▪) or absence (♦) of PS-341 (2 nM) reveals that PS-341 decreases the LD50 of doxorubicin from 150 to 26 nM. (C) MM.1S cells were pretreated with doxorubicin (50 ng/mL) for 24 hours, and then PS-341 (2 nM) was added for an additional 24 hours (i), or pretreated with PS-341 for 24 hours and then doxorubicin for an additional 24 hours (ii), or treated with PS-341 and doxorubicin together for 24 hours (iii). In all cases a synergistic effect is found, but the strongest synergy is observed when the cells are pretreated with doxorubicin followed by PS-341 treatment (black bars, control; white bars, doxorubicin alone; grid bars, PS-341; gray bars, doxorubicin plus PS-341). (D) RPMI-8226/S, ARP-1, S6B45, NCH-H929, and INA6 cells were pretreated with doxorubicin (50 ng/mL) for 24 hours and then with PS-341 (2 nM) for an additional 24 hours (black bars, control; white bars, doxorubicin alone; grid bars, PS-341; gray bars, doxorubicin plus PS-341). (E) Primary MM cells from 4 PS-341–naive patients were pretreated with doxorubicin (50 ng/mL) for 24 hours and then with PS-341 (2 nM) for an additional 24 hours. PS-341 sensitizes all MM cells to doxorubicin (black bars, control; white bars, doxorubicin alone; grid bars, PS-341; gray bars, doxorubicin plus PS-341). (F) Doxorubicin-resistant RPMI-Dox40 cells were pretreated with (white bars) or without (black bars) doxorubicin (800 ng/mL) for 24 hours, and then PS-341 (2-10 nM) was added for an additional 24 hours (black bars, without doxorubicin; white bars, with doxorubicin). PS-341 sensitizes RPMI-Dox40 cells to doxorubicin. (G) Melphalan-resistant LR5 cells were pretreated with or without melphalan (5 μM) for 24 hours, and then PS-341 (2 nM) was added for an additional 24 hours. PS-341 sensitizes LR5 cells to melphalan. (H) MM cells isolated from a patient who had relapsed following treatment with PS-341 were pretreated with (white bars) or without (black bars) doxorubicin (100 ng/mL) for 24 hours, and then PS-341 (5-20 nM) was added for an additional 24 hours. Pretreatment with doxorubicin overcomes resistance to PS-341. (I) MM.1S cells were treated for 24 hours with doxorubicin (100-200 ng/mL) in wells coated with (white bars) or without (black bars) fibronectin (FN). PS-341 (10 nM) was added for additional 24 hours. In all cases, percentage of cell survival (mean ± SD) is quantified by MTT. All experiments were repeated at least 3 times, and each experimental condition was repeated at least in quadruplicate wells in each experiment. Results from representative experiments are shown.
We next investigated whether the sequence of administration of doxorubicin and PS-341 affects their synergistic anti-MM effect. MM.1S cells were therefore (1) pretreated with doxorubicin for 24 hours and then treated with PS-341 for an additional 24 hours, (2) pretreated with PS-341 for 24 hours and then treated with doxorubicin for an additional 24 hours, or (3) treated concomitantly with PS-341 and doxorubicin for 24 hours. Although the combination of PS-341 and doxorubicin was more potent than either drug alone under any of these conditions (P < .05 in all cases), the most pronounced synergy was observed when MM cells are pretreated with doxorubicin followed by PS-341 (Figure 1C).
We extended our studies to additional MM cell lines (RPMI-8226/S, ARP-1, S6B45, NCI-H929, and INA6, Figure 1D) and primary patient MM cells (Figure 1E) and confirmed that PS-341 sensitizes MM cells to doxorubicin. Importantly, the same sensitizing effect is observed in cells that have been selected for resistance to doxorubicin (RPMI-Dox40 cells, Figure 1F) or melphalan (LR5 cells, Figure 1G), indicating that PS-341 increases chemosensitivity in both drug-sensitive and -resistant MM cells.
We next assessed the effect of PS-341 on the chemosensitivity of primary MM cells isolated from a patient who had relapsed following conventional and high-dose chemotherapy, interferon-α, thalidomide alone and combined with cytotoxic drugs or steroids, liposomal doxorubicin, as well as PS-341 alone and in combination with dexamethasone. These patient MM cells have low sensitivity to either PS-341 (IC50 > 50 nM compared with IC50 < 5 nM in PS-341-sensitive patient MM cells2) or doxorubicin monotherapy in vitro. Nonetheless, the combination of these 2 agents results in significant MM cell death (Figure 1H). Therefore, synergy between PS-341 and chemotherapy can reverse resistance to either agent alone.
PS-341 abolishes cell adhesion–mediated drug resistance (CAM-DR)
Previous studies have shown that sensitivity of MM cells to doxorubicin is decreased on tumor cell binding to extracellular matrix components, in particular fibronectin.14-17 This CAM-DR is associated with increased availability of caspase inhibitor FLICE-inhibitory protein (FLIP) for binding to the death receptor Fas and decreased activation of caspase-8.18 Because we have demonstrated that PS-341 lowers FLIP expression and facilitates Fas-dependent caspase-8 activation,3 we hypothesized that PS-341 could inhibit CAM-DR. As shown in Figure 1I, MM.1S cells are less sensitive to doxorubicin in the presence than in the absence of fibronectin, but PS-341 completely overcomes this antiapoptotic effect (P < .05).
Mechanism of chemosensitization by PS-341
We next investigated the mechanism of the chemosensitizing activity of PS-341. We have previously demonstrated that PS-341 decreases the expression of Bcl-2, A1, cIAP-2, X-linked inhibition of apoptosis (XIAP), and FLIP.3 These effects may be due, at least in part, to the inhibition of NF-κB activation by PS-341,1,2 because specific inhibition of NF-κB down-regulates these apoptosis inhibitors and sensitizes MM cells to doxorubicin.13 Our findings are consistent with the ability of NF-κB to promote resistance to genotoxic agents in other models12 and suggest a possible mechanism for the chemosensitizing activity of PS-341.
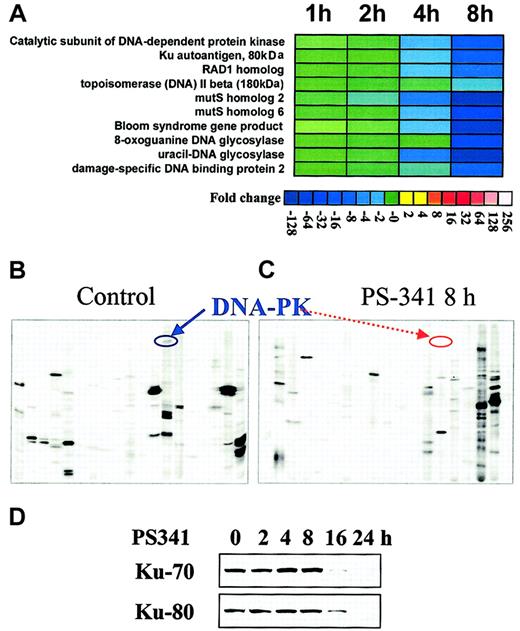
To further characterize the effect of the proteasome inhibitor PS-341 on the transcriptional profile of MM cells, we performed oligonucleotide gene microarray and proteomic analysis of MM.1S cells treated with PS-341 versus control cells. As we have previously reported,3 PS-341 induced changes in transcripts involved in the regulation of apoptosis, cell growth, proteasome function, and heat shock response. In this study, we specifically report the effects of PS-341 on transcripts modulating response to chemotherapy (Figure2A). PS-341 down-regulated the transcripts for several effectors of the protective cellular response to genotoxic stress: topoisomerase II beta, that relaxes DNA torsion on replication, transcription, and cell division and is inhibited by mitoxantrone, doxorubicin, and etoposide19; the Bloom syndrome gene product, involved in maintenance of genome integrity and stability through its cooperation with p5320; 8-oxoguanine DNA glycosylase and uracil-DNA glycosylase, involved in base-excision repair and protection from oxidative DNA damage21; the mutS homologs 2 and 6, that are involved in mismatch repair22; the catalytic subunit of DNA-dependent protein kinase and Ku autoantigen, which function in the repair of DNA double-strand breaks caused by physiologic oxidation reactions, V(D)J recombination, ionizing radiation, and chemotherapeutic drugs23; the damage-specific DNA binding protein 2; and the RAD1 homolog, which is involved in nucleotide excision repair and recombination repair. Selected changes were further confirmed at the protein level. For example, our proteomic-based analysis confirms the down-regulation of DNA-dependent protein kinase (Figure 2B-C); moreover, conventional immunoblotting confirms time-dependent down-regulation of the Ku subunits (80 and 70 kD) triggered by PS-341(Figure 2D).
PS-341 down-regulates the expression of several proteins involved in DNA repair.
(A) Transcriptional profile detected by oligonucleotide-microarray analysis in MM-1S cells treated with PS-341. Transcriptional changes induced by PS-341 (100 nM, 1-8 hours) included down-regulation of a functional cluster of molecules implicated in the response to genotoxic stress. Color saturation is proportional to magnitude of the difference from the respective control. (B-C) Proteomic analysis of the signaling state of PS-341–treated MM-1S cells. Proteomic analysis detects down-regulation of DNA-PK following 8-hour incubation with PS-341 (as depicted by respective arrows). (D) Immunoblotting confirms that PS-341 decreases protein expression of Ku 80 and Ku 70.
PS-341 down-regulates the expression of several proteins involved in DNA repair.
(A) Transcriptional profile detected by oligonucleotide-microarray analysis in MM-1S cells treated with PS-341. Transcriptional changes induced by PS-341 (100 nM, 1-8 hours) included down-regulation of a functional cluster of molecules implicated in the response to genotoxic stress. Color saturation is proportional to magnitude of the difference from the respective control. (B-C) Proteomic analysis of the signaling state of PS-341–treated MM-1S cells. Proteomic analysis detects down-regulation of DNA-PK following 8-hour incubation with PS-341 (as depicted by respective arrows). (D) Immunoblotting confirms that PS-341 decreases protein expression of Ku 80 and Ku 70.
In a phase 2 multicenter clinical trial of PS-341 treatment of patients with relapsed, refractory MM, remarkable antitumor activity has been demonstrated, including some complete responses.7 Our prior in vitro preclinical studies combining PS-341 with other anti-MM agents have revealed an additive antitumor effect with dexamethasone5 and a synergistic effect with the immunomodulatory derivatives (IMiDs) of thalidomide.24However, the magnitude of the synergy between PS-341 and conventional chemotherapy shown in the present study far exceeds these previous findings. Significant sensitization to anticancer therapies by proteasome inhibitors without increased toxicity has also been demonstrated in other animal models, independent of functional p53 status.25 Therefore, the use of PS-341 as an adjuvant to conventional chemotherapy has significant potential utility to overcome resistance, even in patients with advanced disease.
In conclusion, we report that PS-341 sensitizes MM cells to chemotherapy and overcomes CAM-DR. We propose a dual mechanism for this phenomenon, related both to attenuation of the protective cellular response to genotoxic stress and to down-regulation of antiapoptotic protein expression. Our study suggests that the combination of PS-341 with conventional chemotherapy will augment clinical effectiveness and overcome resistance in patients with relapsed refractory MM.
Prepublished online as Blood First Edition Paper, November 7, 2002; DOI 10.1182/blood-2002-06-1768.
Supported by the Multiple Myeloma Research Foundation (N.M., C.S.M.), Laurie Strauss Leukemia Foundation (N.M., C.S.M), National Institutes of Health Grants RO-1 50947 and PO-1 78378, National Institutes of Health Grant R24 DK58739 (T.A.L.), the Leukemia and Lymphoma Society Scholar in Translational Research Award and VA Merit Award (N.C.M.), the Myeloma Research Fund (K.C.A.), and the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.).
N.M. and C.S.M. have equally contributed to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth C. Anderson, Jerome Lipper Multiple Myeloma Center, Dana Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail:kenneth_anderson@dfci.harvard.edu.