A modestly elevated total plasma homocysteine concentration (tHcy) is generally accepted as an independent and graded risk factor for various pathologies, including vascular diseases, neural tube defects, Alzheimer disease, and pregnancy complications. We analyzed 5 common functional polymorphisms in enzymes involved in homocysteine metabolism (ie, methylenetetrahydrofolate reductase [MTHFR] 677C>T and 1298A>C, methionine synthase [MTR] 2756A>G, cystathionine β-synthase [CBS] 844ins68, and methionine synthase reductase [MTRR] 66A>G) in 452 young adults, and quantified their independent and interactive effects on tHcy concentrations. Serum folate, red cell folate, vitamin B12, and tHcy concentrations were significantly influenced by MTHFR 677C>T genotypes. A particularly strong interaction was observed between theMTHFR 677TT genotype and serum folate, which led to a high tHcy phenotype that was more pronounced in males. The genetic contribution to the variance in tHcy was estimated to be approximately 9%, compared with approximately 35% that could be attributed to low folate and vitamin B12. Our study indicates that dietary factors are centrally important in the control of tHcy levels in young adults with additional, but somewhat weaker, genetic effects. These data underscore the potential benefits that may be gained by improving the dietary status of young adults, and provide support for the implementation of folate/B-vitamin food fortification programs.
Introduction
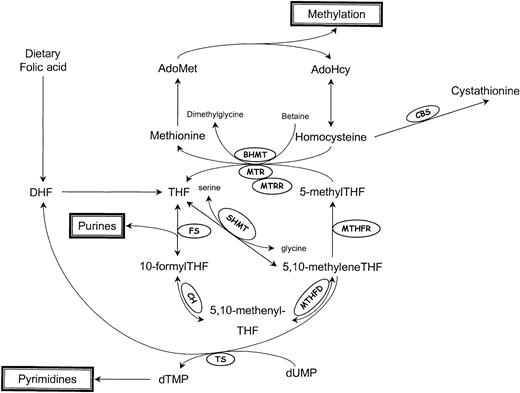
Homocysteine, a branch-point intermediate in the metabolism of the essential amino acid methionine, is a product of important transmethylation reactions that utilize S-adenosylmethionine (AdoMet) as a methyl donor (Figure 1). Once formed, homocysteine can be used to regenerate AdoMet, or can be catabolized to form the amino acid cysteine.
Schematic representation of homocysteine/folate metabolism.
AdoMet indicates S-adenosylmethionine; AdoHcy, S-adenosylhomocysteine; DHF, dihydrofolate; THF, tetrahydrofolate; BHMT, betaine-homocysteine methyltransferase; CBS, cystathionine β-synthase; CH, 5,10-methenylTHF cyclohydrolase; FS, 10-formylTHF synthase; MTHFD, 5,10-methylenetetrahydrofolate dehydrogenase; MTHFR, 5,10-methylenetetrahydrofolate reductase; MTR, methionine synthase; MTRR, methionine synthase reductase; SHMT, serine hydroxymethyltransferase; and TS, thymidylate synthase.
Schematic representation of homocysteine/folate metabolism.
AdoMet indicates S-adenosylmethionine; AdoHcy, S-adenosylhomocysteine; DHF, dihydrofolate; THF, tetrahydrofolate; BHMT, betaine-homocysteine methyltransferase; CBS, cystathionine β-synthase; CH, 5,10-methenylTHF cyclohydrolase; FS, 10-formylTHF synthase; MTHFD, 5,10-methylenetetrahydrofolate dehydrogenase; MTHFR, 5,10-methylenetetrahydrofolate reductase; MTR, methionine synthase; MTRR, methionine synthase reductase; SHMT, serine hydroxymethyltransferase; and TS, thymidylate synthase.
McCully postulated, 3 decades ago, that the clinical manifestation of vascular disease in 2 patients with different inborn errors of methionine metabolism was attributable to the effects of very high levels of total plasma homocysteine (tHcy), as severe hyperhomocysteinemia was the most prominent shared feature of the clinical phenotype.1 In recent years, many, though not all, prospective and retrospective studies have supported the association of mild hyperhomocysteinemia with an increased risk of cardiovascular diseases (CVDs).2 Meta-analysis of the available studies suggests that the risk is graded and independent of established CVD risk factors.3 Hyperhomocysteinemia has also been linked to an increased risk of neural tube defects,4 Alzheimer disease,5 pregnancy complications,6 and inflammatory bowel disease.7
The etiology of hyperhomocysteinemia is considered to be multifactorial, and includes genetic, nutritional, and lifestyle factors,2 and there is an ongoing debate regarding the relative contribution of each. The cDNAs of cystathionine β-synthase (CBS), methylenetetrahydrofolate reductase (MTHFR), methionine synthase (MTR), and methionine synthase reductase (MTRR) have all been cloned and analyzed for functional polymorphisms that affect homocysteine/folate metabolism. The most extensively studied variant is a 677C>T (Ala222Val) transition in MTHFR, that defines a mildly dysfunctional, “thermolabile” enzyme.8 TheMTHFR 677TT genotype is associated with elevated tHcy levels,8 especially in individuals with low folate status.9 Furthermore, it confers an increased risk of CVD in some,10 but not all,11 populations, and has also been associated with an increased risk of neural tube defects,12 recurrent early pregnancy loss,13and inflammatory bowel disease.7 Other common functional variants in the above enzymes include MTHFR 1298A>C (Glu429Ala),14MTR 2756A>G (Asp919Gly),15CBS 844ins68 (an insertion variant),16 and MTRR 66A>G (Ile22Met).17 The homocysteine-modifying impact of these polymorphisms, however, has been assessed in only a few studies. Nevertheless, these indicate that MTR 2756AA homozygotes have significantly higher tHcy compared with their 2756AG and 2756GG peers,18CBS 844ins68 carriers have lower tHcy levels than noncarriers, especially after methionine loading,16 and MTRR 66GG homozygotes have higher tHcy than 66AG heterozygotes and 66AA homozygotes.19 20
Most studies to date have assessed the effects of only a single polymorphism on tHcy levels, and have focused mainly on middle-aged or elderly individuals (ie, those who are old enough for diseases of aging to manifest). However, genetic effects are often more prominent in the young, as cumulative environmental factors have not had the time to substantially modify phenotype. We therefore undertook the current population-based study of young subjects aged 20 to 25 years, to investigate the genetic contribution to hyperhomocysteinemia in a relatively naive population. We analyzed the independent and interactive effects of various functional polymorphisms on tHcy levels and assessed potential interactions with the most relevant nutritional variables, serum and red cell folate (RCF), and vitamin B12status.
Patients, materials, and methods
Study subjects
This study was conducted as part of an ongoing longitudinal study, the Young Hearts (YH) Project, which initially examined the prevalence of coronary risk factors in a random sample of young people (N = 1015; aged 12 years and 15 years) in Northern Ireland. Sampling procedures, study design, and response rates of the first 2 screening phases (YH1 and YH2) are described in detail elsewhere.21 22 All subjects in the original cohort were invited to participate in the third screening phase (YH3; October 1997-October 1999), when aged between 20 and 25 years. Fasting blood samples were obtained if possible from all subjects. Ethical approval was gained from the Medical Research Ethical Committee of the Queen's University of Belfast, and written informed consent was obtained from all participating subjects prior to enrollment.
There were 250 males (49.7% of the male members of the cohort) and 239 females (46.7% of the original female members) who participated in YH3. As an indicator of socioeconomic position of the subjects, information about the occupation of the main breadwinner in the family was collected, and categorized using the Standard Occupational Classification of the Office of Population Consensus and Surveys Statistics (OPCS). The 6 categories (professional occupations; managerial and technical occupations; skilled nonmanual occupations; skilled manual occupations; partly skilled occupations; and unskilled occupations) were dichotomized into a nonmanual (upper 3 classes) and manual (lower 3 classes) social class.23 Response rates were higher in nonmanual social classes compared with manual social classes: of the subjects who attended YH3, 52.7% (n = 228) were from nonmanual social class defined at YH1, and 47.3% (n = 205) were from manual social classes (χ2 = 18.6, df = 1, P < .01). In the YH3 subset, nonattending males (but not nonattending females) were heavier and fatter, and had a higher saturated fat intake at YH1 than attending peers.
Biochemical parameters
Blood samples were obtained after overnight fast. Plasma for tHcy determination was separated with minimal delay, and stored at −20°C until analysis. tHcy levels were assayed using an established high-performance liquid chromatography (HPLC) method.24 Serum folate and vitamin B12concentrations were measured using time-resolved immunofluorescence on an AutoDelfia analyzer (Perkin Elmer Life Sciences, Cambridge, United Kingdom). RCF levels were determined by microbiologic assay as previously described.25
DNA extraction and genetic analyses
Genomic DNA was isolated from peripheral blood leukocytes using an established method.26 All polymorphic variants, except for the 844ins68 variant in the CBS gene, were analyzed using heteroduplex generator (HG) techniques. Briefly, this technology is an adaptation of single-stranded conformation polymorphism analysis, in which each DNA segment that contains the polymorphic nucleotide being tested is coamplified with a synthetic constructed HG. An HG is identical to the genomic DNA segment except for a microdeletion close to the polymorphic site. Denaturation of the DNA strands and subsequent reannealing lead to the formation of homoduplexes of both genomic and HG origin and heteroduplexes comprising mixed hybrids of genomic and HG DNA. The presence or absence of the polymorphic variant leads to the formation of heteroduplexes with distinct protruding loops that mandate different migration properties in polyacrylamide gel electrophoresis. We have developed a multiplex genotyping system, that allows simultaneous genotyping of theMTHFR 677C>T, MTHFR 1298A>C, MTR2756A>G, and CBS 844ins68 variants in a single tube.27 In addition, a similar HG assay was developed for analysis of the MTRR 66A>G transition.19 20The duplexes were separated in 12% polyacrylamide/5% glycerol gels at 150 V for 12 to 16 hours, and visualized by ethidium bromide staining and ultraviolet (UV) illumination.
Statistical analyses
The distributions of tHcy, vitamin B12, RCF, and serum folate concentrations were all skewed; therefore, data were logarithmically transformed prior to all statistical analyses. Differences in the above biochemical and nutritional variables among different genotype subgroups were assessed by one-way analysis of variance (ANOVA) followed by pair-wise t tests, corrected for multiple comparisons (Bonferroni). Differences in genotype frequencies among different tHcy strata, and deviations from Hardy-Weinberg equilibrium were assessed by χ2 analysis. Odds ratios (OR) and 95% confidence intervals (95% CIs) were calculated using logistic regression analysis. Bivariate correlations were estimated using the Pearson correlation test. Gene-gene, and gene-environment interactions were assessed using 2-way ANOVA, which allowed the assessment of any interaction effect over and above the main effects of the independent factors in the model. The relative contribution of the factors to the variability in tHcy levels was calculated from the adjusted R2 estimate in the model being tested. All statistical analyses were performed using SPSS for Windows version 9.0 (Statistical Product and Service Solutions, Chicago, IL), and statistical significance was accepted for a 2-tailedP < .05.
Results
Characteristics of the study group
The study group consists of 250 (51.1%) males and 239 (48.9%) females. The median (range) tHcy, serum folate, RCF, and vitamin B12 concentrations in males and females are presented in Table 1. Despite statistically significantly poorer RCF and vitamin B12 status (P < .001 for each variable), females had tHcy levels similar to those of males. Serum folate levels were also similar in both sexes. Geometric mean tHcy levels were similar in women who used hormonal contraception (ie, pill, minipill, and contraceptive injections) versus those who used other measures or no contraception (tHcy, 9.4 μM vs 8.8 μM; P = .23). Alcohol intake had no significant effect on tHcy levels (geometric mean, 9.5 μM in users vs 8.8 μM in nonusers; P = .11), nor on folate levels (geometric mean, 13.6 nM in both groups; P = .96).
Plasma creatinine levels were only weakly, but positively, associated with tHcy levels (r = 0.134, P < .01) in the overall study group, an association that was sex dependent (in females,r = 0.155, P = .04; in males,r = 0.084, P = .21).
Genotyping
Genotypes were obtained from 452 study subjects. The frequencies of the MTHFR 677TT, MTHFR 1298CC, MTR2756GG, CBS 844ins68 WI, and MTRR 66GG genotypes were, respectively, 13.5%, 10.6%, 2.0%, 17.7%, and 29.6%, comparable with those reported in the literature for each of these genotypes in white populations, including the Northern Ireland population.9 18-20 All genotype distributions were similar in males and females, and were in accordance with Hardy-Weinberg predictions (data not shown).
We combined the MTHFR 677C>T and 1298A>C genotypes to generate composite MTHFR genotypes, which established that 1298C rarely occurs in cis with 677T. In our study group of 452 individuals, only 3 (0.7%) recombinant genotypes were observed: 2 individuals had the 677TT/1298AC genotype, and one had the 677CT/1298CC genotype. Assuming that there are no double recombinants among those with the 677CT/1298AC genotypes, the frequencies for the 677C/1298C, 677C/1298A, 677T/1298A, and recombinant 677T/1298C alleles were 31.2%, 33.2%, 35.3% and 0.3%, respectively; these allele frequencies mandate expected composite genotype frequencies that are similar to those observed (χ2 = 2.025; df = 7;P = .96; data not shown).
Associations between genotypes and biochemical variables
We assessed the associations between the MTHFR, MTR, MTRR, and CBS genotypes and tHcy, RCF, serum folate, and vitamin B12 (Table2). These associations were similar in males and females, and both groups were therefore combined. TheMTHFR 677C>T genotypes significantly influence tHcy (P < .0005, ANOVA); pair-wise Bonferroni ttests showed that individuals with the MTHFR 677TT genotype have significantly higher tHcy than those with the 677CT and 677CC genotypes (P < .0005 for either comparison). Serum folate levels were also significantly associated with the MTHFR677C>T genotypes (P = .010, ANOVA); 677TT homozygotes had significantly lower serum folate levels compared with 677CC homozygotes (P < .02), with the levels in 677CT heterozygotes being intermediate. A similar association between MTHFR 677C>T genotypes and RCF was observed: RCF levels were lowest in 677TT homozygotes, highest in 677CC homozygotes, and intermediate in 677CT heterozygotes (P < .03 for all intergenotype comparisons). Furthermore, vitamin B12 levels were significantly lower in those with the MTHFR 677TT genotype than in those with the MTHFR 677CT genotype (P = .02).
The relative risk of being in the top 5%, 10%, 20%, and 50% of the tHcy distribution for individuals with the MTHFR 677TT genotype versus those with the MTHFR 677CC genotype was calculated separately for males and females (Table3). The MTHFR 677TT genotype confers a much higher risk of hyperhomocysteinemia in males than in females at each of the different, sex-specific tHcy rank cutoff values. For males, there is a highly significant 4.2-fold risk of being in the top 50% of the tHcy distribution (ie, tHcy > 9.2 μM) for 677TT homozygotes relative to 677CC homozygotes (P < .005), a risk that increases to more than 40-fold for being in the top 5% of the distribution (ie, tHcy > 20.8 μM). In females, the corresponding risk estimates increase from 2.4 (top 50%; tHcy > 8.6 μM) to 7.1 (top 5%; tHcy > 17.9 μM). The risk that theMTHFR 677TT genotype will lead to a potentially pathogenic Hcy phenotype is therefore much more extreme in males than in females.
We also assessed the effect of the composite MTHFR genotypes on tHcy; individuals with the 677TT/1298AA genotype have the highest tHcy concentrations, significantly higher than those of all others with nonrecombinant genotypes. In contrast to an earlier report,28 tHcy levels in MTHFR 677CT/1298AC compound heterozygotes (median [range], 8.7 μM [4.4−29.7 μM]; n = 86) were similar to those in subjects who are singly heterozygous for the 677C>T polymorphism, that is, those with theMTHFR 677CT/1298AA genotype (8.6 μM [5.1 − 34.0 μM]; n = 89). RCF was significantly influenced by compositeMTHFR genotype (P = .001; ANOVA); individuals with the MTHFR 677TT/1298AA genotype had lower RCF than those with the MTHFR 677CC/1298AA (P = .06), 677CC/1298AC (P = .001), and 677CC/1298CC (P = .03) genotypes (Bonferroni-corrected ttests). The effects of the composite MTHFR genotypes on serum folate and serum vitamin B12 levels were not statistically significant (ANOVA, P = .15 andP = .17, respectively). An association was also observed between the MTRR 66A>G genotypes and RCF (ANOVA,P = .03); however, none of the subsequent Bonferroni-corrected t tests showed significant relationships. None of the genotypes defined by the other polymorphisms showed significant associations with levels of tHcy, serum folate, RCF, or vitamin B12 (Table 2), although an apparent trend was observed between tHcy concentrations and the MTR 2756A>G genotypes, consistent with an earlier observation.18
Interaction analysis
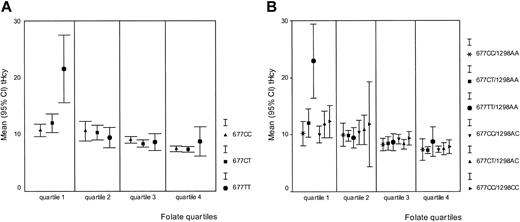
Serum folate and vitamin B12 concentrations were both inversely related to tHcy in this study population (r = −0.481 and r = −0.369, respectively;P < .01 for both correlations); in addition, their levels were dependent on MTHFR 677C>T genotypes. We therefore assessed the potential interaction between serum folate and vitamin B12 status and the MTHFR genotypes. In each quartile of the folate distribution, we plotted the mean tHcy concentration per MTHFR 677C>T genotype. Figure2A shows that the influence of theMTHFR 677TT genotype on tHcy levels is confined to the lowest folate quartile; in contrast to the top 3 quartiles of the folate distribution, MTHFR 677TT homozygotes in the lowest quartile (n = 17) have significantly higher tHcy (median, 17.3 μM) than their 677CT (n = 42) and 677CC (n = 30) peers (median tHcy, 10.7 and 10.9 μM, respectively; P < .01). Subdivision by sex of the 17 MTHFR 677TT individuals in the lowest folate quartile confirmed a divergent impact of this genotype on tHcy concentration in males and females; that is, 6 of 7 MTHFR677TT males, but only 2 of 10 MTHFR 677TT females (both using hormonal contraception) in the lowest folate quartile have a tHcy of 18.7 μM or higher (95th percentile of entire study group; χ2 = 7.1, df = 1, P < .01). Creatinine levels were within the normal range in these males and females. An analysis of variance with MTHFR 677TT, folate, sex, and a sex–MTHFR 677TT interaction term showed a significant contribution of the sex-genotype interaction (P < .02) on tHcy variance. Figure 2B shows the association between the compositeMTHFR genotypes and tHcy in each of the folate quartiles. The potentiation of a high tHcy phenotype is restricted to those with the 677TT/1298AA composite genotype (n = 15; median tHcy, 19.1 μM) who are in the lowest folate quartile. Interaction analyses between vitamin B12 and the MTR 2756A>G andMTRR 66A>G genotypes showed that none of the MTRor MTRR genotypes modify tHcy levels in a vitamin B12–dependent manner (data not shown).
The relationships between serum folate, tHcy, and
MTHFR genotypes. The mean tHcy (μM) was plotted separately for each genotype within the 4 quartiles of the folate distribution. (A) Relationship between MTHFR 677C>T genotypes, folate, and tHcy. Folate concentrations increase from quartile 1 to quartile 4. Quartile 1: 9.50 nM or less; quartile 2: 9.51 to 12.95 nM; quartile 3: 12.96 to 18.83 nM; and quartile 4: 18.84 nM or more. (B) Relationship between composite MTHFR 677C>T and 1298A>C genotypes, folate, and tHcy. Folate quartiles are identical to those described in panel A.
The relationships between serum folate, tHcy, and
MTHFR genotypes. The mean tHcy (μM) was plotted separately for each genotype within the 4 quartiles of the folate distribution. (A) Relationship between MTHFR 677C>T genotypes, folate, and tHcy. Folate concentrations increase from quartile 1 to quartile 4. Quartile 1: 9.50 nM or less; quartile 2: 9.51 to 12.95 nM; quartile 3: 12.96 to 18.83 nM; and quartile 4: 18.84 nM or more. (B) Relationship between composite MTHFR 677C>T and 1298A>C genotypes, folate, and tHcy. Folate quartiles are identical to those described in panel A.
The effects of the single genotypes and genotype combinations on the variation in plasma tHcy levels were assessed using 2-way ANOVA, in which the interactive effects can be estimated, over and above the main effects in the model being tested. The overall variance in tHcy levels explained by the genetic factors under consideration was 9%. After inclusion of folate and vitamin B12 concentrations in the model, almost 42% of the variation in tHcy levels could be explained. The latter estimate changed to 45% after inclusion of creatinine in the model.
Discussion
In the current study, we have determined the genotypes for 5 common functional variants of enzymes involved in homocysteine metabolism (ie, MTHFR, MTR, MTRR, and CBS) and tHcy levels in subjects aged 20 to 25 years. The contribution of these genetic factors and important environmental factors (ie, folate, vitamin B12, and creatinine) to the variability in tHcy concentrations has been estimated. In this young adult population, the only genetic polymorphism that significantly influenced tHcy, serum folate, RCF, and vitamin B12 levels was MTHFR 677C>T. TheMTHFR 677TT genotype strongly interacted with low folate levels to produce a high tHcy phenotype, an effect that was more pronounced in males than in females.
In previous studies of a male population aged 30 to 49 years from the same geographical region, we found that MTR 2756AA andMTRR 66GG homozygotes had significantly elevated tHcy levels compared with their MTR 2756GG and MTRR 66AA peers, respectively.18-20 In the younger population studied here, however, tHcy concentrations did not differ according toMTRR genotype, and the difference in tHcy levels between theMTR 2756AA, MTR 2756AG, and MTR 2756GG genotypes did not reach statistical significance, although an apparent trend toward higher tHcy levels in MTR 2756AA homozygotes was observed. These results suggest that there may be additional environmental, nutritional, or genetic factors that act cumulatively to potentiate, via the tHcy-raising MTR and MTRRgenotypes, a phenotypic effect that becomes more prominent (and significant) over time. There is precedent for such an environmental factor: in middle-aged Australian men, Wang et al demonstrated that smoking interacts with the MTR 2756GG genotype to increase the risk of coronary artery disease to a level greater than that observed in smokers with the other MTRgenotypes29; however, as tHcy concentrations were not reported by these investigators, the precise nature of the interaction with respect to biochemical aspects of Hcy metabolism is not clear.
In a recent study of subjects ranging in age from 21 to 82 years (mean age, 48.9 years) recruited from the upper midwestern region of the US, Tsai et al30 estimated that only 1.49% of the variability in fasting tHcy was attributable to genetic factors. This is much lower than the estimates that we have calculated for the Northern Ireland population; in men aged 30 to 49, we have calculated that genetic factors account for approximately 7% of the variability in tHcy,18,19 and in the younger population reported here it is somewhat higher at approximately 9%. Taken together, these data suggest that the genetic contribution to a high tHcy phenotype is generally more prominent in early life and that cumulative environmental factors may become more important in modifying phenotype as individuals reach middle age. The results shown in Table 3 also support a more pronounced genetic effect on tHcy levels in young subjects, especially males, as the risk estimates of having a tHcy in the top 5%, 10%, and 20%, conferred by the MTHFR 677TT genotype relative to the MTHFR 677CC genotype (ie, 40.8-, 11.4-, and 6.8-fold, respectively), are all much higher than those observed in the published study of 30 to 49 year old males (ie, 9.7-, 5.7-, and 2.6-fold, respectively).9 Furthermore, in the younger population, we observed interactive effects between theMTHFR 677TT genotype, the MTR 2756AA genotype, and the MTRR 66GG genotype that contribute significantly to the variance in tHcy. In contrast with an earlier observation,16 carriers of the CBS 844ins68 insertion variant had similar tHcy levels as noncarriers.
Although the comparison was based on a limited number of individuals, we observed a striking difference in the risk of being hyperhomocysteinemic between MTHFR 677TT males and females. This difference was not dependent on kidney function and may be explained by sex-specific differential interactions betweenMTHFR 677C>T genotypes and folate. The primary phenotypic effect of being a MTHFR 677TT female is the reduced level of circulating 5-methylTHF, without accompanying strong effects on tHcy levels. In contrast, tHcy concentrations are strongly influenced by theMTHFR 677TT genotype in combination with low folate in males. A similar disparity between the sexes was recently reported in a French study,31 in which tHcy levels differed according toMTHFR 677C>T genotype in men, but not in women. This suggests that there may be fundamental differences in the interactions between nutritional and genetic variables in males and females with respect to elicited biochemical phenotype.
In the present study, approximately 35% of the variability in tHcy could be explained by both folate and vitamin B12, confirming our a priori expectations that, as in older subjects, dietary intake of these micronutrients by young adults is centrally important in the control of tHcy levels. According to the definition of hyperhomocysteinemia used in a recent European study32(ie, a fasting tHcy concentration more than 12 μM), 16.1% (n = 68) of our 20- to 25-year-old subjects are hyperhomocysteinemic. This indicates that 1 in every 6 of those entering the third decade of life already has a tHcy concentration that, in subjects of more advanced age, is strongly associated with a range of pathologies, including CVD, inflammatory bowel disease, and Alzheimer disease.3,5 7
Although conclusive evidence supporting a clinically beneficial effect of folic acid supplementation on CVD incidence is still lacking, recent data from clinical trials on intermediate end points showed that homocysteine-lowering intervention by improving folate and B vitamin status led to a reduction in the frequency of abnormal exercise electrocardiography tests33 and restenosis rate after angioplasty.34 Our data may therefore have implications for governments worldwide that are currently considering new legislation to introduce mandatory fortification of food with folic acid, primarily aimed at the prevention of neural tube defects but recognizing the potential benefits on the incidence of CVD via homocysteine lowering. In the US such a fortification policy, introduced in 1998, has proved to be highly effective in reducing the prevalence of low folate status (< 3 ng/mL ≅ 7 nM) from 22.0% to 1.7%, and of mild hyperhomocysteinemia (a fasting tHcy > 13 μM) from 18.9% to 9.8%.35 Of direct clinical importance, the occurrence of neural tube defects has declined by almost 20% since the introduction of mandatory folic acid fortification.36 The data reported here, showing the much greater importance of dietary factors compared with genetic effects in determining tHcy concentration, support the introduction of fortification elsewhere.
In conclusion, the data presented here are consistent with the genetic factors that influence tHcy levels being more prominent in young subjects than in those of more advanced age. Nevertheless, the proportion of the variance in tHcy levels that is attributable to genetic factors is relatively modest. As is the case in the older population, the genetic effect is considerably smaller than that attributable to dietary factors, including folate and vitamin B12. If the outcomes of ongoing intervention trials support a clinically beneficial effect of homocysteine-lowering regimens, our data would suggest that long-term disease prevention benefits may be gained by improving folate and vitamin B12 status in the young, regardless of genetic factors, via the implementation of government mandated food fortification programs.
Supported by National Institutes of Health (NIH) grant HD 39081; European Union Biomed Demonstration Project BMH 4983549, Abbott, Germany; and the British Heart Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alexander S. Whitehead, Department of Pharmacology, University of Pennsylvania School of Medicine, 153 Johnson Pavilion, 3620 Hamilton Walk, Philadelphia, PA 19104-6084; e-mail: aswhitehead@pharm.med.upenn.edu.