Lymphoblastic lymphoma (LBL) is a rare, clinically aggressive neoplasm of the young that frequently involves the bone marrow (BM) and/or central nervous system. Because LBL is similar to acute lymphoblastic leukemia, some centers prefer allogeneic hematopoietic stem cell (SC) transplantation to autologous SC transplantation. We retrospectively analyzed outcomes for patients who underwent autologous (auto, n = 128) or HLA-identical sibling (allo, n = 76) SC transplantations from 1989 to 1998 and were reported to International Bone Marrow Transplant Registry (IBMTR) or Autologous Blood and Marrow Transplant Registry (ABMTR). Allo stem cell transplant (SCT) recipients had higher treatment-related mortality (TRM) at 6 months (18% versus 3%, P = .002), and this disadvantage persisted at 1 and 5 years. Early relapse rates after alloSC transplantation and autoSC transplantation were similar, but significantly lower relapse rates were observed in alloSCT recipients at 1 and 5 years (32% versus 46%, P = .05; and 34% versus 56%,P = .004, respectively). No differences were noted in lymphoma-free survival rates between alloSC transplantations and autoSC transplantations (5-year rates 36% versus 39%,P = .82). AutoSCT recipients had higher overall survival at 6 months (75% versus 59%, P = .01), but survival did not significantly differ between the 2 groups at 1 and 5 years (60% versus 49%, P = .09; 44% versus 39%,P = .47, respectively). Multivariate analyses to account for confounding factors confirmed these results. Independent of SCT type, BM involvement at the time of transplantation and disease status more advanced than first complete remission were associated with inferior outcomes. In summary, alloSC transplantation for LBL is associated with fewer relapses than with autoSC transplantation, but higher TRM offsets any potential survival benefit.
Introduction
The lymphoblastic lymphomas (LBLs) are rare, accounting for approximately 2% of non-Hodgkin lymphomas (NHLs).1 These high-grade lymphomas usually are composed of precursor T cells, have a predilection for men and boys, and occur most commonly in children, adolescents, and young adults.2The disease is clinically aggressive, frequently involves the bone marrow and/or central nervous system, and in many respects is indistinguishable from acute lymphoblastic leukemia (ALL).3,4 Although patients with limited disease may fare well, the outcome for those with poor-risk features (bone marrow or central nervous system involvement or lactate dehydrogenase [LDH] > 300 IU/L) or recurrent disease is generally less favorable.5-7 Children have better outcomes than adults with poor-risk disease,6-9 but recurrent disease is infrequently cured regardless of age.10,11 Because of the high relapse rates and low cure rates for recurrent disease, both autologous and allogeneic hematopoietic stem cell transplantations are used in an attempt to improve survival. Long-term overall survival rates of 56% to 80% are reported with each approach in patients who received transplants in first complete remission.12-15 Prolonged remissions and long-term survival of patients with LBL are reported after both autologous and allogeneic transplantations for recurrent or refractory disease. Because these studies include small numbers of patients, it is not known which type of transplantation, if any, represents the better therapy for patients with LBL.16-19 Some evidence supports the existence of a graft-versus-lymphoma (GVL) effect,20-22 but the contribution of GVL toward survival remains unclear. We undertook a retrospective study of patients who underwent either autologous or allogeneic transplantation and analyzed the effect of several important factors, including stem cell source, on survival.
Patients and methods
Data sources
The International Bone Marrow Transplant Registry (IBMTR) is a voluntary working group of more than 350 transplantation centers worldwide that contribute detailed data on consecutive allogeneic hematopoietic stem cell transplantations to a Statistical Center at the Health Policy Institute of the Medical College of Wisconsin in Milwaukee. The Autologous Blood and Marrow Transplant Registry (ABMTR) is a voluntary organization of more than 250 transplantation centers primarily in North and South America that report data on consecutive autotransplantations to the same Statistical Center. On the basis of the data collected in the Centers for Disease Control Hospital Surveys23,24 and the US Government Accounting Office25 26 and worldwide surveys of transplantation activity, approximately 40% of allogeneic transplantations worldwide and more than 50% of autotransplantations in North and South America are registered with the IBMTR/ABMTR. Participating centers are required to report all transplantations consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally, with yearly follow-up. Computerized checks for errors, physician reviews of submitted data, and on-site audits of participating centers ensure the quality of data.
The IBMTR/ABMTR collects data at 2 levels: registration and research. Registration data include disease type, age, sex, pretransplantation disease stage and chemotherapy responsiveness, date of diagnosis, graft type (bone marrow and/or blood-derived stem cells), high-dose conditioning regimen, posttransplantation disease progression and survival, development of a new malignancy, and cause of death. Requests for data on progression or death for registered patients are at 6-month intervals. All IBMTR/ABMTR teams contribute registration data. Research data are collected on subsets of registered patients, including comprehensive pretransplantation and posttransplantation clinical information.
Patients
This study includes patients with LBL who underwent autologous or HLA-identical sibling transplantation from 1989 to 1998 and were reported to the IBMTR/ABMTR. Only patients who received single transplants were included. Excluded were patients who received T-cell–depleted grafts or nonmyeloablative conditioning regimens. A total of 76 (46%) of 165 registered HLA-identical sibling transplantations and 128 (41%) of 312 registered autologous transplantations had comprehensive data available for analysis and were included in the study. Comparison of 1-year probabilities of survival between patients with and without comprehensive data showed similar rates: autologous, 63% versus 57%, P = .46; HLA-identical sibling, 51% versus 56%, P = .81. Likewise, no differences in age distribution, sex, or histologic type were noted. Data analyzed were reported by 52 IBMTR teams from 24 countries and 64 ABMTR teams from 12 countries.
End points
Primary end points were treatment-related mortality (TRM), relapse, disease-free survival (DFS), and overall survival. TRM was defined as death during a continuous complete remission or death in the first 28 days after tranplantation. Relapse was defined as persistent or recurrent disease. Patients with persistent disease were classified as relapsed at day 28, even if they did not subsequently progress. Disease-free survival was defined as survival without evidence of disease. For analyses of DFS, relapses or deaths from any cause were considered as events. For analyses of overall survival, events were deaths from any cause.
Statistical analysis
Patient, disease, and treatment-related variables for the autologous and HLA-identical sibling transplantation cohorts were compared using the chi-square statistic for categorical variables and the Wilcoxon test for continuous variables. Univariate probabilities of DFS and survival were calculated using the Kaplan-Meier estimator; the log-rank test was used for univariate comparisons. Patients were censored at the time of last follow-up. Probabilities of TRM and relapse were calculated using cumulative incidence curves to accommodate competing risks.27 Relapse was used as competing risk for TRM and TRM as the competing risk for relapse. Ninety-five percent confidence intervals for all probabilities andP values of pairwise comparisons were derived from point-wise estimates and were calculated using standard techniques.28 TRM, relapse, DFS, and survival after autologous versus HLA-identical sibling transplantation were evaluated in multivariate analyses using Cox proportional hazards regression to adjust for other potentially confounding differences between the cohorts. Variables considered in multivariate analysis are as follows: type of transplant, age, sex, Karnofsky performance score at transplantation, disease stage at transplantation, chemosensitivity, bone marrow involvement at transplantation, central nervous system involvement, serum LDH, initial chemotherapy given, use of total body irradiation (TBI) and growth factors, graft source, and year of transplantation. We tested the proportional hazards assumption for each factor in the Cox model using time-dependent covariates. When this indicated differential effects over time (nonproportional hazards), models were constructed breaking the posttransplantation time course into 2 periods, using the maximized partial likelihood method to find the most appropriate breakpoint. Interactions between the main effect term and all covariates were tested prior to stepwise modeling. After modeling time-varying effects and interactions, the final multivariate model was built using a forward stepwise model selection approach. Each model contained the main effect (type of transplant, HLA-identical sibling versus autologous graft) to allow adjustments for other covariates. Factors significantly associated with the outcome variable at a 5% level were kept in the final model. First-order interactions were again examined between the type of transplant and all significant prognostic factors. Adjusted probabilities for DFS and overall survival were calculated with the multivariate Cox models described previously and weighted by the sample proportion value for each prognostic factor found significant in the regression models. To validate that results applied similarly to patients treated in remission and those with more advanced disease status, the multivariate analyses were repeated for patients in first complete remission alone and for all patients not in first complete remission. Examination for center effects used a random effects or frailty model.29 All P values are 2-sided.
Results
Patient, disease, and treatment-related characteristics
There were 76 HLA-identical sibling transplantations and 128 autologous transplantations available for analysis (Table1). Autologous transplant recipients were older, more likely to receive peripheral blood stem cells, more likely to receive transplants before 1994, and less likely to receive a TBI-containing conditioning regimen than allogeneic transplant recipients. The 2 groups did not differ significantly for other patient, disease, and treatment-related characteristics studied.
Patient, disease, and transplant-related characteristics
| Variables . | HLA-identical sibling transplant . | Autologous transplant . | P . |
|---|---|---|---|
| N | 76 | 128 | |
| Age, y, median (range) | 27 (5-53) | 31 (2-67) | .01 |
| Men or boys (%) | 53 (70) | 91 (71) | .84 |
| Karnofsky performance score at transplantation (%) | .53 | ||
| 80% or lower | 47 (62) | 82 (64) | |
| 90%-100% | 27 (36) | 39 (30) | |
| Unknown | 2 (2) | 7 (6) | |
| Disease status at transplantation (%) | .86 | ||
| CR1 | 24 (32) | 47 (37) | |
| Second or subsequent CR | 16 (20) | 23 (18) | |
| Relapse | 18 (24) | 31 (24) | |
| Primary induction failure | 18 (24) | 27 (21) | |
| Bone marrow involvement at transplantation (%) | .09 | ||
| Absent | 51 (67) | 92 (72) | |
| Present | 10 (13) | 6 (5) | |
| Unknown | 15 (20) | 30 (23) | |
| CNS involvement at transplantation (%) | .20 | ||
| Absent | 61 (80) | 105 (83) | |
| Present | 12 (16) | 12 (9) | |
| Unknown | 3 (4) | 11 (8) | |
| Serum LDH at diagnosis (%) | .18 | ||
| Less than 1.5 upper normal limit | 63 (83) | 94 (74) | |
| 1.5 × upper normal limit or higher | 13 (17) | 31 (24) | |
| Unknown | — | 3 (2) | |
| Initial chemotherapy (%) | .55 | ||
| Adriamycin containing regimen | 42 (61) | 60 (47) | |
| Others, not adriamycin | 11 (16) | 13 (10) | |
| Others, not specified | 16 (23) | 55 (43) | |
| Chemosensitivity at transplantation | .09 | ||
| Sensitive | 57 (75) | 88 (69) | |
| Resistant | 11 (14) | 12 (9) | |
| Untreated/unknown | 8 (11) | 28 (22) | |
| Graft type | < .001 | ||
| Bone marrow | 56 (74) | 54 (42) | |
| Peripheral blood | 20 (26) | 61 (48) | |
| Both | — | 13 (10) | |
| Year of transplant | .04 | ||
| 1989-1993 | 12 (16) | 36 (28) | |
| 1994-1998 | 64 (84) | 92 (72) | |
| Use of TBI | 51 (67) | 50 (39) | < .001 |
| G-CSF given within 7 d after transplantation | .43 | ||
| No | 36 (47) | 68 (53) | |
| Yes | 40 (53) | 60 (47) | |
| Acute GVHD (%) | — | ||
| Grades 0-I | 53 (70) | — | |
| Grade II | 10 (13) | — | |
| Grade III | 9 (12) | — | |
| Grade IV | 4 (5) | — | |
| Chronic GVHD (%) | 13 (17)* | ||
| Median follow-up of survivors, mo (range) | 32 (3-122) | 44 (3-124) |
| Variables . | HLA-identical sibling transplant . | Autologous transplant . | P . |
|---|---|---|---|
| N | 76 | 128 | |
| Age, y, median (range) | 27 (5-53) | 31 (2-67) | .01 |
| Men or boys (%) | 53 (70) | 91 (71) | .84 |
| Karnofsky performance score at transplantation (%) | .53 | ||
| 80% or lower | 47 (62) | 82 (64) | |
| 90%-100% | 27 (36) | 39 (30) | |
| Unknown | 2 (2) | 7 (6) | |
| Disease status at transplantation (%) | .86 | ||
| CR1 | 24 (32) | 47 (37) | |
| Second or subsequent CR | 16 (20) | 23 (18) | |
| Relapse | 18 (24) | 31 (24) | |
| Primary induction failure | 18 (24) | 27 (21) | |
| Bone marrow involvement at transplantation (%) | .09 | ||
| Absent | 51 (67) | 92 (72) | |
| Present | 10 (13) | 6 (5) | |
| Unknown | 15 (20) | 30 (23) | |
| CNS involvement at transplantation (%) | .20 | ||
| Absent | 61 (80) | 105 (83) | |
| Present | 12 (16) | 12 (9) | |
| Unknown | 3 (4) | 11 (8) | |
| Serum LDH at diagnosis (%) | .18 | ||
| Less than 1.5 upper normal limit | 63 (83) | 94 (74) | |
| 1.5 × upper normal limit or higher | 13 (17) | 31 (24) | |
| Unknown | — | 3 (2) | |
| Initial chemotherapy (%) | .55 | ||
| Adriamycin containing regimen | 42 (61) | 60 (47) | |
| Others, not adriamycin | 11 (16) | 13 (10) | |
| Others, not specified | 16 (23) | 55 (43) | |
| Chemosensitivity at transplantation | .09 | ||
| Sensitive | 57 (75) | 88 (69) | |
| Resistant | 11 (14) | 12 (9) | |
| Untreated/unknown | 8 (11) | 28 (22) | |
| Graft type | < .001 | ||
| Bone marrow | 56 (74) | 54 (42) | |
| Peripheral blood | 20 (26) | 61 (48) | |
| Both | — | 13 (10) | |
| Year of transplant | .04 | ||
| 1989-1993 | 12 (16) | 36 (28) | |
| 1994-1998 | 64 (84) | 92 (72) | |
| Use of TBI | 51 (67) | 50 (39) | < .001 |
| G-CSF given within 7 d after transplantation | .43 | ||
| No | 36 (47) | 68 (53) | |
| Yes | 40 (53) | 60 (47) | |
| Acute GVHD (%) | — | ||
| Grades 0-I | 53 (70) | — | |
| Grade II | 10 (13) | — | |
| Grade III | 9 (12) | — | |
| Grade IV | 4 (5) | — | |
| Chronic GVHD (%) | 13 (17)* | ||
| Median follow-up of survivors, mo (range) | 32 (3-122) | 44 (3-124) |
CR indicates complete remission; CNS, central nervous system; LDH, lactate dehydrogenase; TBI, total body irradiation; GVHD, graft-versus-host disease; and —, not applicable.
A total of 75 patients were evaluable.
Treatment-related mortality
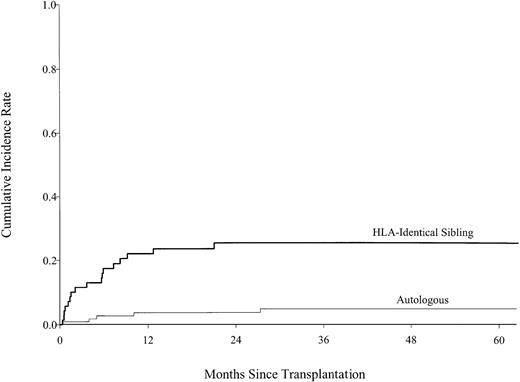
At every time point following transplantation (Table2), allogeneic transplant recipients were significantly more likely to die of treatment-related causes than autologous transplant recipients (Figure1). The 5-year cumulative incidences of TRM were 25% (95% confidence interval [CI], 16%-36%) after allogeneic transplantation and 5% (95% CI, 2%-10%) after autologous transplantation; pointwise P < 0.001. Multivariate analysis revealed that donor source was the only significant predictor of TRM. Patients who received an allogeneic transplant were 6.12 [95% CI, 2.25-16.56] times more likely to die of treatment-related causes than autologous transplant recipients. The findings remained the same when the analyses were done separately for patients in first complete remission and for patients with advanced stage disease. Graft-versus-host disease (GVHD) was reported as the cause of death in only 2 (7%) of the allogeneic transplant recipients; however, the cumulative incidence of GVHD in patients who died of TRM was 47% but only 15% in those who did not, suggesting that GVHD contributed to TRM, although it was not always the proximate cause of death. Infection, pneumonitis, and organ failure accounted for most TRM for both autologous and allogeneic transplant recipients.
Treatment-related mortality, relapse, disease-free survival, and death after transplantations for lymphoblastic lymphoma
| . | HLA-identical sibling transplant . | Autologous transplant . | P . |
|---|---|---|---|
| Treatment-related mortality* | |||
| 6 mo | 18 (10-27) | 3 (1-7) | .002 |
| 1 y | 22 (13-32) | 4 (1-8) | .0005 |
| 3 y | 25 (16-36) | 5 (2-10) | .0003 |
| 5 y | 25 (16-36) | 5 (2-10) | .0003 |
| Relapse* | |||
| 6 mo | 28 (18-38) | 30 (22-39) | .70 |
| 1 y | 32 (22-43) | 46 (37-55) | .05 |
| 3 y | 34 (23-45) | 52 (43-61) | .01 |
| 5 y | 34 (23-45) | 56 (45-65) | .004 |
| Disease-free survival† | |||
| 6 mo | 55 (43-65) | 66 (57-74) | .11 |
| 1 y | 46 (34-56) | 50 (40-58) | .61 |
| 3 y | 36 (24-48) | 42 (34-51) | .83 |
| 5 y | 36 (24-48) | 39 (30-48) | .82 |
| Survival† | |||
| 6 mo | 59 (48-68) | 75 (67-82) | .01 |
| 1 y | 49 (38-59) | 60 (52-68) | .09 |
| 3 y | 39 (29-49) | 46 (38-54) | .32 |
| 5 y | 39 (29-49) | 44 (36-52) | .47 |
| . | HLA-identical sibling transplant . | Autologous transplant . | P . |
|---|---|---|---|
| Treatment-related mortality* | |||
| 6 mo | 18 (10-27) | 3 (1-7) | .002 |
| 1 y | 22 (13-32) | 4 (1-8) | .0005 |
| 3 y | 25 (16-36) | 5 (2-10) | .0003 |
| 5 y | 25 (16-36) | 5 (2-10) | .0003 |
| Relapse* | |||
| 6 mo | 28 (18-38) | 30 (22-39) | .70 |
| 1 y | 32 (22-43) | 46 (37-55) | .05 |
| 3 y | 34 (23-45) | 52 (43-61) | .01 |
| 5 y | 34 (23-45) | 56 (45-65) | .004 |
| Disease-free survival† | |||
| 6 mo | 55 (43-65) | 66 (57-74) | .11 |
| 1 y | 46 (34-56) | 50 (40-58) | .61 |
| 3 y | 36 (24-48) | 42 (34-51) | .83 |
| 5 y | 36 (24-48) | 39 (30-48) | .82 |
| Survival† | |||
| 6 mo | 59 (48-68) | 75 (67-82) | .01 |
| 1 y | 49 (38-59) | 60 (52-68) | .09 |
| 3 y | 39 (29-49) | 46 (38-54) | .32 |
| 5 y | 39 (29-49) | 44 (36-52) | .47 |
Cumulative incidence.
Adjusted probability estimate, weighted on other significant factors in the multivariate model.
Cumulative incidence of treatment-related mortality by type of transplant, showing statistically lower treatment-related mortality rates in the autologous transplant group than those in the HLA-identical sibling transplant group.
Cumulative incidence of treatment-related mortality by type of transplant, showing statistically lower treatment-related mortality rates in the autologous transplant group than those in the HLA-identical sibling transplant group.
Relapse
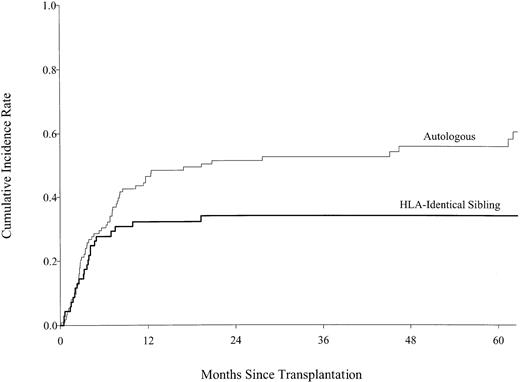
For the first 6 months following transplantation, there was no statistically significant difference in the likelihood of relapse or persistent LBL after autologous versus allogeneic transplantation (Table 2; Figure 2). Among patients who survived in remission for 6 months, subsequent relapse was more likely in autologous transplant recipients than in patients who received allogeneic transplants, leading to a statistically significant difference in the cumulative incidence of relapse by 1 year after transplantation. The 5-year cumulative incidences of relapse were 34% (95% CI, 23%-45%) after allogeneic transplantation and 56% (95% CI, 45%-65%) after autologous transplantation; pointwiseP = .004. Multivariate analysis revealed that donor source, lymphomatous involvement of the bone marrow at transplantation, and disease status at transplantation were independent predictors of persistent or recurrent lymphoma after transplantation (Table3). There was no statistically significant effect of acute or chronic GVHD on the risk of relapse for allogeneic transplant recipients, but low statistical power could miss a small effect.
Cumulative incidence of relapse by type of transplant, showing statistically lower relapse rates in the HLA-identical sibling transplant group than those in the autologous transplant group after 6 months.
Cumulative incidence of relapse by type of transplant, showing statistically lower relapse rates in the HLA-identical sibling transplant group than those in the autologous transplant group after 6 months.
Multivariate analysis of relapse (persistent or recurrent lymphoma)
| Variables . | Relative risk of relapse (95% confidence interval) . | P . |
|---|---|---|
| HLA-identical sibling transplant versus autologous transplant | ||
| First 6 mo after transplantation | 0.93 (0.52-1.64) | .80 |
| Beyond 6 mo after transplantation | 0.25 (0.09-0.73) | .01 |
| Other significant variables | ||
| Bone marrow involvement at transplantation | ||
| Absent | 1.00 | |
| Present | 2.08 (1.00-4.31) | .05 |
| Unknown | 1.80 (1.08-2.98) | .02 |
| Disease status at transplantation3-150 | ||
| CR1 | 1.00 | |
| Second or subsequent CR | 2.24 (1.14-4.42) | .02 |
| Relapse | 4.00 (2.16-7.42) | < .0001 |
| Primary induction failure | 2.92 (1.49-5.69) | .002 |
| Variables . | Relative risk of relapse (95% confidence interval) . | P . |
|---|---|---|
| HLA-identical sibling transplant versus autologous transplant | ||
| First 6 mo after transplantation | 0.93 (0.52-1.64) | .80 |
| Beyond 6 mo after transplantation | 0.25 (0.09-0.73) | .01 |
| Other significant variables | ||
| Bone marrow involvement at transplantation | ||
| Absent | 1.00 | |
| Present | 2.08 (1.00-4.31) | .05 |
| Unknown | 1.80 (1.08-2.98) | .02 |
| Disease status at transplantation3-150 | ||
| CR1 | 1.00 | |
| Second or subsequent CR | 2.24 (1.14-4.42) | .02 |
| Relapse | 4.00 (2.16-7.42) | < .0001 |
| Primary induction failure | 2.92 (1.49-5.69) | .002 |
CR indicates complete remission; and RR, relative risk.
≥ Relapse 1 versus ≥ CR2, RR, 1.78 (95% CI, 0.98-3.26),P = .06; primary induction failure versus ≥ CR2, RR, 1.30 (95% CI, 0.67-2.51), = .43; primary induction failure versus ≥ relapse 1, RR, 0.73 (95% CI, 0.41-1.30),P = .28.
Disease-free and overall survival
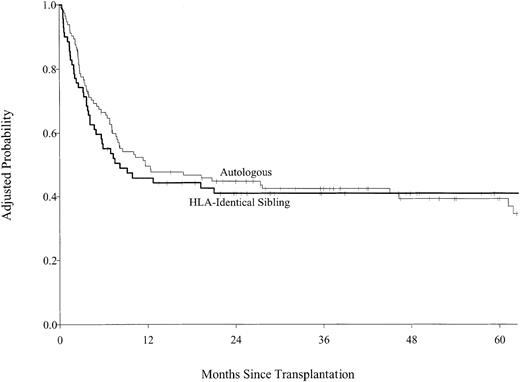
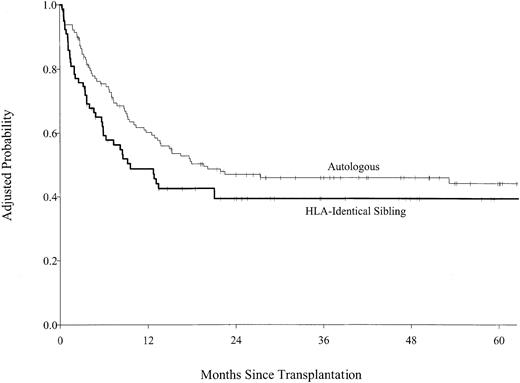
Multivariate analysis of DFS found that allogeneic transplant recipients had inferior DFS for the first 6 months following transplantation and superior disease-free survival after 6 months, but these differences did not reach statistical significance (Figure3). The 5-year adjusted probabilities of DFS were 36% (95% CI, 24%-48%) after allogeneic transplantations and 39% (95% CI, 30%-48%) after autologous transplantation; pointwise P = .82. Disease status at transplantation was more important than source of stem cells. Patients who received transplants in first complete remission had higher DFS than transplant recipients who had more advanced disease (Table4). No other variable predicted DFS. When subgroup analyses were performed for patients in first complete remission and for patients in first complete remission without prior bone marrow involvement, the results were the same. Allogeneic transplant recipients were almost twice as likely to die during the first 6 months after transplantation compared with autologous transplant recipients. Among patients who survived the first 6 months, subsequent survival was similar for autologous and allogeneic transplant recipients (Figure 4; Table5), but treatment failure patterns differed. Patients who received autologous grafts were more likely to die of persistent or recurrent lymphoma in the early and late posttransplantation periods than were allograft recipients. No single cause accounted for a majority of deaths after allografts; deaths from lymphoma, pneumonitis, organ failure, and GVHD were equally common. The 5-year adjusted probabilities of survival were 39% (95% CI, 29%-49%) after allogeneic transplantation and 44% (95% CI, 36%-52%) after autologous transplantation; pointwise P = .47. Advanced disease status and poor performance status at transplantation were the only other factors predictive of higher mortality (Table 5).
Probability of disease-free survival by type of transplant adjusted for disease status at transplantation.
Probability of disease-free survival by type of transplant adjusted for disease status at transplantation.
Multivariate analysis of disease-free survival
| Variables . | Relative risk of treatment failure (95% confidence interval) . | P . |
|---|---|---|
| HLA-identical sibling transplant versus autologous transplant | ||
| First 6 mo after transplantation | 1.49 (0.92-2.40) | .10 |
| Beyond 6 mo after transplantation | 0.58 (0.27-1.22) | .15 |
| Other significant variables | ||
| Disease status at transplantation4-150 | ||
| CR1 | 1.00 | |
| CR2 or more | 1.82 (1.03-3.22) | .04 |
| Relapse 1 or more | 3.17 (1.69-5.32) | < .0001 |
| Primary induction failure | 2.65 (1.55-4.53) | .0004 |
| Variables . | Relative risk of treatment failure (95% confidence interval) . | P . |
|---|---|---|
| HLA-identical sibling transplant versus autologous transplant | ||
| First 6 mo after transplantation | 1.49 (0.92-2.40) | .10 |
| Beyond 6 mo after transplantation | 0.58 (0.27-1.22) | .15 |
| Other significant variables | ||
| Disease status at transplantation4-150 | ||
| CR1 | 1.00 | |
| CR2 or more | 1.82 (1.03-3.22) | .04 |
| Relapse 1 or more | 3.17 (1.69-5.32) | < .0001 |
| Primary induction failure | 2.65 (1.55-4.53) | .0004 |
CR indicates complete remission; and RR, relative risk.
≥ Relapse 1 versus ≥ CR2, RR, 1.74 (95% CI, 1.00-3.03),P = .05; primary induction failure versus ≥ CR2, RR, 1.46 (95% CI, 0.82-2.58), P = .20; primary induction failure versus ≥ relapse 1, RR, 0.84 (95% CI, 0.50-1.40), P = 0.49.
Probability of survival by type of transplant adjusted for Karnofsky performance score and disease status at transplantation.
Probability of survival by type of transplant adjusted for Karnofsky performance score and disease status at transplantation.
Multivariate analysis of death
| Variables . | Relative risk of death (95% confidence interval) . | P . |
|---|---|---|
| HLA-identical sibling transplant versus autologous transplant | ||
| First 6 mo after transplantation | 1.93 (1.17-3.20) | .01 |
| Beyond 6 mo after transplantation | 0.89 (0.47-1.68) | .72 |
| Other significant variables | ||
| Karnofsky performance score at transplantation | ||
| 80% or less | 1.00 | |
| 90%-100% | 0.62 (0.42-0.92) | .02 |
| Disease status at transplantation5-150 | ||
| CR1 | 1.00 | |
| Second subsequent CR | 1.66 (0.90-3.07) | .10 |
| Relapse | 3.45 (2.06-5.78) | < .0001 |
| Primary induction failure | 2.77 (1.62-4.74) | .0002 |
| Variables . | Relative risk of death (95% confidence interval) . | P . |
|---|---|---|
| HLA-identical sibling transplant versus autologous transplant | ||
| First 6 mo after transplantation | 1.93 (1.17-3.20) | .01 |
| Beyond 6 mo after transplantation | 0.89 (0.47-1.68) | .72 |
| Other significant variables | ||
| Karnofsky performance score at transplantation | ||
| 80% or less | 1.00 | |
| 90%-100% | 0.62 (0.42-0.92) | .02 |
| Disease status at transplantation5-150 | ||
| CR1 | 1.00 | |
| Second subsequent CR | 1.66 (0.90-3.07) | .10 |
| Relapse | 3.45 (2.06-5.78) | < .0001 |
| Primary induction failure | 2.77 (1.62-4.74) | .0002 |
CR indicates complete remission; and RR, relative risk.
≥ Relapse 1 versus ≥ CR2, RR, 2.07 (95% CI, 1.17-3.67),P = .01; primary induction failure versus ≥ CR2, RR, 1.66 (95% CI, 0.92-3.02), P = .09; primary induction failure versus ≥ relapse 1, RR, 0.80 (95% CI, 0.49-1.30), P = .38.
Discussion
Hematopoietic stem cell transplantation is an option for some patients with LBL, particularly those with recurrent or refractory disease. Some investigators suggested that transplantation has a role for LBL in first complete remission,13,15 and in a prospective randomized trial recipients of an autologous transplant had superior relapse-free survival compared with patients who received conventional consolidative and maintenance chemotherapy.12In this study, approximately one third of the patients received their transplant in first complete remission. Whether stem cell transplantation will continue to have a role in the management of LBL in first complete remission is not known. Newer approaches, such as the application of pediatric ALL therapy to adult LBL,7 may result in low relapse rates, although that goal is not yet achieved in patients with very high risk features. Not surprisingly, the present study revealed that transplant recipients who had less advanced disease and good performance status were more likely to become long-term survivors. Overall survival was similar for both autologous and allogeneic transplant recipients, regardless of disease stage, bone marrow involvement, time from diagnosis to transplantation, or other potentially confounding factors. However, the pattern of treatment failure was different for the 2 groups.
Treatment-related mortality was higher with allogeneic transplantation
In the early months after transplantation, TRM was substantially higher in allogeneic than autologous transplant recipients, leading to a survival advantage with autologous transplantation through the first 6 months. A single explanation for higher TRM is not apparent. Reported causes of death included pneumonitis, infection, and organ failure. Although grade II-IV GVHD developed in 28% of allograft recipients, 47% of the patients with TRM were diagnosed with acute GVHD at some point prior to death. It is likely that GVHD contributed to mortality even though the proximate cause of death was reported as infection, organ failure, or pneumonitis. However, GVHD does not necessarily account for all the higher TRM between allograft or autograft recipients. Allograft recipients were more likely to receive radiation-containing conditioning regimens and use bone marrow as the source of stem cells. It is possible that these factors contributed to increased mortality through greater organ toxicity and/or slower engraftment, but proof to support this contention is lacking. Higher TRM but fewer relapses after allogeneic compared with autologous transplantation is observed in other comparative studies of transplantation for hematologic malignancies.30-33
Relapses were less likely with allogeneic transplantation
As expected, bone marrow involvement and advanced disease stage at transplantation were associated with relapse independent of transplant type. We found that an allogeneic source of stem cells was associated with a reduced risk of relapse, even though survival did not improve because of higher TRM. The observation of fewer relapses in allogeneic transplantation held true in multivariate analysis, after adjustment for the possibly confounding effects of age, sex, Karnofsky performance score at transplantation, disease stage at transplantation, chemosensitivity, bone marrow involvement at transplantation, central nervous system involvement, serum LDH level, initial chemotherapy given, use of TBI and growth factors, graft source, time from diagnosis to transplantation, and year of transplantation.
Three possible explanations for this observation are suggested. First, a graft-versus-lymphoma effect provided protection against relapse. Although the existence of a GVL effect is supported by clinical observations,20-22 there are limited data for its activity in LBL. One prior report found relapse was less likely for patients with more advanced disease who received an allogeneic compared with autologous transplant. As in the present study, the antilymphoma benefit of an allogeneic transplantation was offset by higher TRM.14 In our study, we found that there were far fewer relapses after allogeneic than autologous transplantation, regardless of disease stage. We did not find a statistically significant protective effect of GVHD against relapse. However, it is also well documented that clinically evident GVHD is not a mandatory prerequisite for GVL.34 A second explanation is that reinfusion of autologous stem cells contaminated with tumor cells contributed to the higher relapse rate in the autologous transplant group. More than 90% of the stem cell collections were not purged, and, in any event, the effectiveness of purging to prevent reintroduction of lymphoma is not established. Finally, it is possible that the difference in the relapse rates may be that TRM in the allogeneic transplant group occurred preferentially in those destined to relapse.
This current study suggests that, as practiced in the previous decade, the choice between allogeneic versus autologous stem cells made little difference in overall transplantation outcome, regardless of disease status or other important factors. However, the pattern of treatment failure differed and gives important clues as to future strategies. Because most patients lack an available allogeneic donor, new strategies to improve the outcome of autologous transplantation are needed. The lack of an obvious plateau in relapses after autologous transplantation indicates that late disease control is a major problem. Incorporation of current or future monoclonal antibody therapies, perhaps as posttransplantation consolidation, may be of value for these patients.
Because TRM was the most important cause of treatment failure for the allogeneic patients, strategies to reduce TRM without compromising the GVL effect may be useful. Results from animal studies suggest that drug strategies that prevent GVHD while preserving GVL may be possible.35,36 Alternatively, modest reductions in conditioning intensity may decrease TRM without increasing the risk of relapse.37,38 However, the full measure of the toxicity associated with reduced intensity transplantation, such as GVHD, is not yet known, and reduction in intensity may compromise tumor control. Some centers are exploring high-dose therapy with autologous transplantation followed by reduced intensity allogeneic transplantation with promising results in certain settings, including high-risk lymphoma.39 40 A carefully designed clinical trial to test reduced intensity allogeneic transplantation for patients with lymphoblastic lymphoma may also be worth considering.
The Lymphoma Study Writing Committee of the International Bone Marrow Transplant Registry and Autologous Blood and Marrow Transplant Registry includes Asad Bashey, Brian J. Bolwell, Linda J. Burns, Mitchell S. Cairo, Richard E. Champlin, Cesar O. Freytes, John Gibson, Steve C. Goldstein, Mary J. Laughlin, John Lister, David I. Marks, Richard T. Maziarz, Alan M. Miller, Gustavo A. Milone, Santiago Pavlovsky, Andrew L. Pecora, J. Douglas Rizzo, Gary Schiller, Harry C. Schouten, and Mei Jie Zhang.
Prepublished online as Blood First Edition Paper, November 27, 2002; DOI 10.1182/blood-2002-05-1483.
Supported by Public Health Service Grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung, and Blood Institute; Contract No. CP-21161 from the National Cancer Institute of the US Department of Health and Human Services; Grant No. DAMD17-95-I-5002 from the Department of the US Army Medical Research and Development Command; and grants from Abgenix, AmCell Corporation, American Cancer Society, American Society of Clinical Oncology, Amgen, Anonymous, Aventis Pharmaceuticals, Berlex Laboratories, Blue Cross and Blue Shield Association, Lynde and Harry Bradley Foundation, Bristol-Myers Squibb Oncology, Center for Advanced Studies in Leukemia, Cerus, Chimeric Therapies, Chiron Therapeutics, Eleanor Naylor Dana Charitable Trust, Deborah J. Dearholt Memorial Fund, Empire Blue Cross Blue Shield, Fujisawa Healthcare, Gambro BCT, Genentech, GlaxoSmithKline, Human Genome Sciences, ICN Pharmaceuticals, IDEC Pharmaceuticals, Immunex, IntraBiotics Pharmaceuticals, Kettering Family Foundation, Kirin Brewery, Robert J. Kleberg Jr and Helen C. Kleberg Foundation, LifeTrac/Allianz, The Liposome Company, Nada and Herbert P. Mahler Charities, Market Certitude LLC, Mayer Ventures, MedImmune, Merck & Co, Milliman & Robertson, Milstein Family Foundation, The Greater Milwaukee Foundation/Elsa Schoeneich Research Fund, NeoRx, Nexell Therapeutics, Novartis Pharmaceuticals, Orphan Medical, Ortho Biotech, John Oster Family Foundation, Pfizer US Pharmaceuticals, Pharmacia, Principal Life Insurance Company, Response Oncology, RGK Foundation, Roche Laboratories, SangStat, Schering AG, Schering Oncology/Biotech, Stackner Family Foundation, The Starr Foundation, SuperGen, TheraTechnologies, Unicare Life & Health Insurance, and Wyeth/Genetics Institute.
A complete list of the members of the Lymphoma Study Writing Committee of the International Bone Marrow Transplant Registry and Autologous Blood and Marrow Transplant Registry appears in the “.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mary M. Horowitz, IBMTR/ABMTR Statistical Center, Medical College of Wisconsin, 8701 Watertown Plank Rd, PO Box 26509, Milwaukee, WI 53226; e-mail: marymh@mcw.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal