The WT1 tumor-suppressor gene is expressed by many forms of acute myeloid leukemia. Inhibition of this expression can lead to the differentiation and reduced growth of leukemia cells and cell lines, suggesting that WT1 participates in regulating the proliferation of leukemic cells. However, the role of WT1 in normal hematopoiesis is not well understood. To investigate this question, we have used murine cells in which the WT1 gene has been inactivated by homologous recombination. We have found that cells lacking WT1 show deficits in hematopoietic stem cell function. Embryonic stem cells lacking WT1, although contributing efficiently to other organ systems, make only a minimal contribution to the hematopoietic system in chimeras, indicating that hematopoietic stem cells lacking WT1 compete poorly with healthy stem cells. In addition, fetal liver cells lacking WT1 have an approximately 75% reduction in erythroid blast-forming unit (BFU-E), erythroid colony-forming unit (CFU-E), and colony-forming unit–granulocyte macrophage–erythroid–megakaryocyte (CFU-GEMM). However, transplantation of fetal liver hematopoietic cells lackingWT1 will repopulate the hematopoietic system of an irradiated adult recipient in the absence of competition. We conclude that the absence of WT1 in hematopoietic cells leads to functional defects in growth potential that may be of consequence to leukemic cells that have alterations in the expression of WT1.
Introduction
WT1 was isolated as a tumor-suppressor gene on the basis of its deletion in a subset of patients with Wilms tumor, a pediatric cancer of the kidney.1 In addition to its mutation in 5% to 10% of Wilms tumor patients, mutations ofWT1 have been implicated in a variety of other cancers including mesotheliomas, desmoplastic round cell tumors, and leukemias.1,WT1 encodes a protein of 52 to 54 kD, containing 4 zinc fingers of the Cys2 His2 class, that has been demonstrated to act as transcription factor.1 Potential transcriptional targets for WT1 include a variety of growth factor and growth factor receptor genes.1 An additional role for WT1 in RNA processing has also been proposed.1
Many leukemias, including up to 80% of acute myeloid leukemias (AMLs), have been demonstrated to express WT1.2-10 Mutations of WT1 have been found in approximately 5% to 10% of AMLs, similar to the level seen in Wilms tumors, and sporadically in a variety of other leukemias.11-18 Manipulation of WT1 expression in leukemia cells results in alterations in differentiation and growth potential.19-28 In the treatment of leukemia, expression of WT1 in the peripheral blood and bone marrow has been suggested to be an indicator of clinical relapse. Attempts have been made to correlate WT1 expression with progression of disease and prognosis in several types of hematologic malignancies.3,29-37 The clinical use of anti-WT1 cytotoxic T-lymphocytes (CTLs) for preferential destruction of WT1-expressing malignant cells in leukemia is under evaluation.38-41
A number of lines of evidence suggest that WT1 may play a role in regulating hematopoiesis. In the human hematopoietic system, WT1 appears to be normally expressed in bone marrow CD34+cells.2,20,42-47,WT1 mRNA levels are modulated during the in vitro differentiation of hematopoietic cells and leukemic cells lines.23,44,48,49 In vitro manipulation of WT1 expression has been shown to affect the growth potential and differentiation of hematopoietic cells, leukemia cells, and cell lines.19-28
Despite the use of WT1 as a therapeutic target and an indicator of prognosis in leukemia, the role of the WT1 gene in hematopoiesis is not clearly understood. We have used the targeted deletion of WT1 gene function in the mouse to directly investigate the role of WT1 in normal hematopoiesis. Because the absence of WT1 is lethal at embryonic day 13.5 (E13.5) to E15.5, we have used a combination of approaches—the generation of chimeric mice through the injection of normal C57BL6 blastocysts with embryonic stem (ES) cells lacking WT1, the reconstitution of normal mice with hematopoietic cells from embryos lacking WT1, and in vitro culturing of embryonic hematopoietic cells. We demonstrate that the absence of WT1 compromises the function of hematopoietic stem cells. In particular, stem cells lacking WT1 compete extremely poorly when contributed through ES cells to chimeric embryos. Despite this limitation, fetal liver cells lacking WT1 are competent to repopulate the hematopoietic lineages of lethally irradiated hosts. Although capable of in vivo reconstitution, these cells showed reduced in vitro colony-forming capacity in assays for erythroid colony-forming unit (CFU-E), granulocyte macrophage–colony-forming unit (CFU-GM), and colony-forming unit–granulocyte macrophage–erythroid–megakaryocyte (CFU-GEMM). These results suggest that the absence of WT1 compromises the viability, proliferation, or differentiation of hematopoietic stem and progenitor cells and that alteration in WT1 expression should be expected to have significant effects on these properties in leukemic cells.
Materials and methods
Mice
Mice containing a heterozygous deletion ofWT150 were maintained as heterozygotes on a mixed C57/B6 129SvJ background. Male and female C57/B6 mice for chimera host blastocysts were purchased from Taconic Farms (Germantown, NY). Female C57/B6 Ly5.2 mice were purchased from the National Cancer Institute (Frederick, MD).
Chimera production
ES cells homozygous for the absence of WT1 were generated by culturing delayed blastocysts from matings of heterozygous parents on a mixed C57/B6 129SvJ background. A second line was obtained by retargeting the remaining wild-type (WT) WT1 allele in the ES cells used to generated the WT1 null mouse using the original targeting vector with the hygromycin-resistance gene replacing the original neomycin-resistance gene. Chimeras were generated by microinjecting 10 to 15 cells into C57/B6 blastocysts.
GPI assays
Glucose-phosphate-isomerase (GPI) assays were performed as described.51 In brief, samples were lysed by 4 cycles of freezing and thawing, and GPI isoforms were analyzed by cellulose acetate electrophoresis of the samples followed by detection in an agarose gel containing fructose-6-phosphate, nicotinamide-adenine dinucleotide phosphate (NADP), methyl thiazolyl tetrazolium (MTT), phenazine methylsulfate (PMS), and glucose-6-phosphate dehydrogenase (all reagents were from Sigma [St Louis, MO]) at room temperature. This method permits the discrimination of the isoforms GPI-Iaa and GPI-Ibb present in 129Sv and C57BL strains, respectively.
Reconstitution of irradiated mice
Mating pairs of mice heterozygous for the absence ofWT1 were checked daily for the presence of a vaginal plug. The morning of detection was designated as E0.5. On E13.5, pregnant females were killed and the embryos were harvested. Each fetal liver was dissociated in sterile phosphate-buffered saline (PBS) and quantitated. Trypan blue–excluding nucleated cells (1 × 106) were injected in 200 μL PBS retro-orbitally into female C57/B6 Ly5.2 (8- to 10-week-old) recipients. These mice were lethally irradiated in 2 doses of 8 Gy and 4 Gy separated by 3 hours before receiving the donor cells.
Flow cytometry
For analysis of the contribution of ES cells to the peripheral blood and bone marrow, a 2-month-old female 90% (by coat color) chimera was anesthetized using tribromoethanol. One milliliter blood was obtained by heart puncture. Red cells were removed using 1% dextran T 500 followed by ammonium chloride lysis. Bone marrow cells were obtained by flushing both hind femurs and tibias into PBS. Peripheral blood and bone marrow cells were fractionated on a FACStar Plus (Becton Dickinson, San Jose, CA) using forward scatter and side scatter gates and then analyzed for ES cell contribution using the GPI assay. For analysis of the hematopoietic system of animals reconstituted with embryonic liver, blood samples were collected from the retro-orbital sinus. Red cells were removed using 1% dextran T 500 followed by ammonium chloride lysis. Cells were washed with PBS and then incubated with saturating quantities of fluorescein isothiocyanate (FITC)–conjugated anti-Ly5.2 (Pharmingen, San Diego, CA) and biotin-conjugated anti-Ly5.1 followed by phycoerythrin (PE)–conjugated streptavidin. Cells were washed and analyzed using FACStar Plus (Becton Dickinson) with forward scatter and side scatter gates. FITC emission was collected using a 530/30 dichroic filter (DF), and PE emission was collected using a 575/26 DF. A 560SP dichroic mirror (DM) was used to split the FITC and PE emissions.
In vitro progenitor cell assays
Mice heterozygous for the absence of WT1 were mated, and the morning of detection of a vaginal plug was designated as E0.5. On E14.5, pregnant females were killed and the embryos were harvested. Each fetal liver was dissociated in 1 mL Iscove/2% fetal bovine serum (FBS) and was cultured in Methocult GF M3434 (StemCell Technologies, Vancouver, BC, Canada) in triplicate at 1.5 × 104 cells/mL at 37°C and 5% CO2. CFU-Es were counted after 2 days of culture, CFU-GMs after 10 days, and CFU-GEMMs after 14 days. Significance was determined using analysis of variance (ANOVA) in the Statview statistical analysis program (SAS, Cary, NC).
Results
Embryonic stem cells lacking WT1 do not contribute to the hematopoietic system
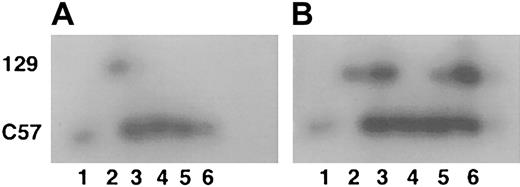
Mice lacking WT1 develop to between day E13.5 and E15.5 before they die in utero, apparently of hemorrhage into the pericardial cavity.50 52 At the time of death, the embryos appear to have a grossly normal hematopoietic system. To further investigate the hematopoietic functions of the WT1 gene, chimeric adult mice were generated from ES cells lacking WT1 and normal C57/Bl6 blastocysts to allow hematopoietic cells lacking WT1 the opportunity to develop past 15.5 days of embryogenesis. Tissues from mice with 90% to 100% agouti coat color were analyzed for ES cell contribution using the GPI assay. ES cells lacking WT1 contributed strongly to a number of organs, including the liver. However, the ES cell contribution to whole blood was at or below the detection threshold of the GPI assay system (approximately 5% ES cell or 2% 129 isoform because both forms of GPI were contained by the ES cells) (Figure 1). Peripheral blood and bone marrow from a 90% agouti coat color mouse were fractionated by size and side scatter into red cell, lymphocyte, and granulocyte populations. GPI assays on these cell fractions also did not detect any significant contribution by ES cells (data not shown). These results demonstrate that ES cells lacking WT1 failed to contribute to these populations significantly in adults.
GPI analysis of chimeric mice.
(A) Extracts of peripheral blood. (B) Extracts of liver. Lane 1 indicates C57/B6; lane 2, 129/SvJ; lanes 3 to 6, separate representative chimeras (total, 4). Upper band is the 129 GPI isoform; lower band is the C57 isoform.
GPI analysis of chimeric mice.
(A) Extracts of peripheral blood. (B) Extracts of liver. Lane 1 indicates C57/B6; lane 2, 129/SvJ; lanes 3 to 6, separate representative chimeras (total, 4). Upper band is the 129 GPI isoform; lower band is the C57 isoform.
Cells lacking WT1 can repopulate the mouse hematopoietic system
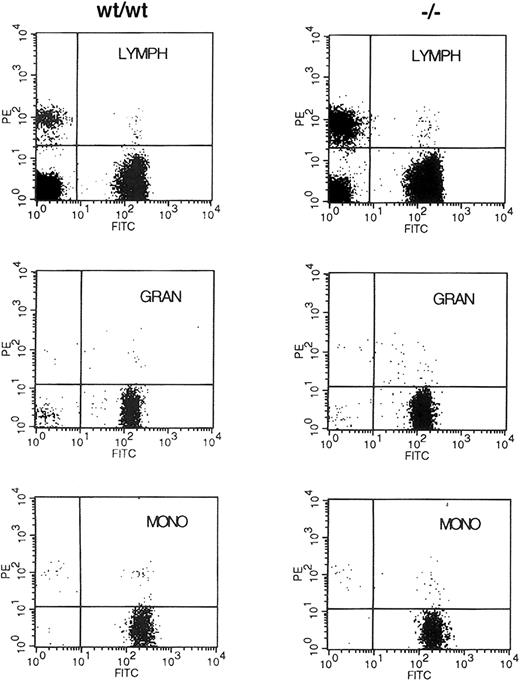
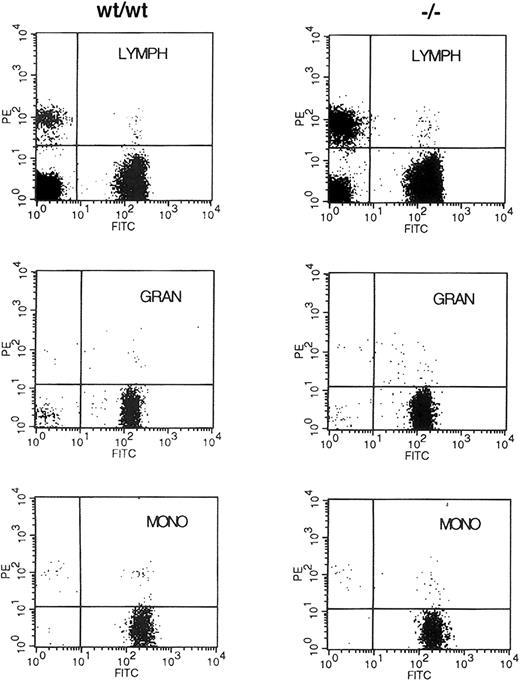
Because no abnormalities in early hematopoiesis had been reported in embryos lacking WT1, the results on the chimeric animals suggested it was possible that WT1 was necessary to effectively populate the hematopoietic system of the adult animal. The ability to distinguish donor from recipient cells by the polymorphism of the Ly5 antigen was used to assess the ability of early hematopoietic cells lacking WT1 to populate the adult hematopoietic lineages. NIH C57 female mice congenic for the Ly5.1 antigen were lethally irradiated and then reconstituted with 1 × 106 fetal liver cells from E13.5 WT1mutant, WT1 heterozygote, or wild-type embryos. Fourteen days after reconstitution, 4 of 4 of the buffer-injected control mice had died or been killed because of extreme morbidity, whereas 2 of 2 mice injected with cells from wild-type embryos, 7 of 7 injected withWT1 heterozygote cells, and 9 of 11 injected withWT1 mutant cells survived. At 4 weeks these mice all survived and had normal hematocrit levels of approximately 44%. At 2 months only one additional mouse reconstituted with cells lacking WT1 had died of unknown cause (Table 1). When peripheral blood was analyzed for its donor/host composition using staining with anti-Ly5.2 and Ly5.1, more than 90% of the staining cells were donor derived regardless of the genotype of the donor embryo. There was no alteration in this ratio when side and forward scatter were used to subdivide the cells into lymphocyte, granulocyte, and monocyte populations (Figure 2). These results demonstrate that stem cells lacking WT1 are capable of reconstituting and maintaining the adult hematopoietic system for at least 2 months after transplantation.
Fluorescence-activated cell-sorter (FACS) analysis of donor/host contribution to hematopoietic lineages of reconstituted mice.
Retro-orbital blood from animals reconstituted with E13.5 liver cells from wild-type (WT/WT) and WT1 mutant (−/−) embryos was incubated with antibodies to Ly5.2 and Ly5.1 specific for the donor and the lethally irradiated host, respectively. Cells were fractionated by size and side scatter into the lymphocyte (LYMPH), granulocyte (GRAN), and monocyte (MONO) populations and were analyzed for the expression of Ly5.1 (PE) and Ly5.2 (FITC) antigens.
Fluorescence-activated cell-sorter (FACS) analysis of donor/host contribution to hematopoietic lineages of reconstituted mice.
Retro-orbital blood from animals reconstituted with E13.5 liver cells from wild-type (WT/WT) and WT1 mutant (−/−) embryos was incubated with antibodies to Ly5.2 and Ly5.1 specific for the donor and the lethally irradiated host, respectively. Cells were fractionated by size and side scatter into the lymphocyte (LYMPH), granulocyte (GRAN), and monocyte (MONO) populations and were analyzed for the expression of Ly5.1 (PE) and Ly5.2 (FITC) antigens.
Cells lacking WT1 have reduced in vitro colony-forming potential
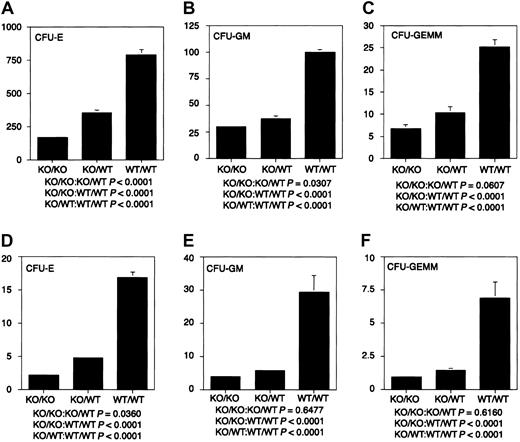
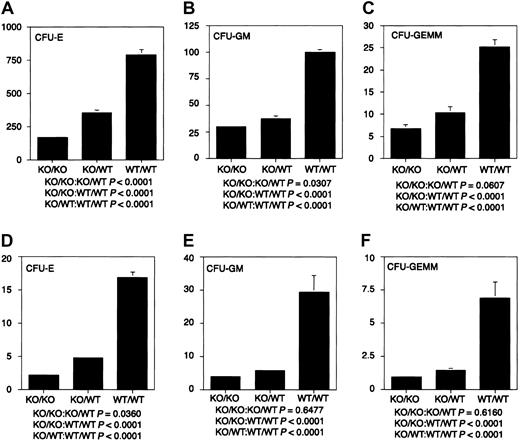
The ability of stem cells lacking WT1 to reconstitute the hematopoietic system of lethally irradiated adults, together with the failure of ES cells lacking WT1 to contribute effectively to the hematopoietic system of chimeric embryos in a competitive situation, suggested that WT1 might play a role in regulating the proliferation of differentiating hematopoietic cells. To characterize the growth and differentiation properties of hematopoietic cells lackingWT1 in vitro, fetal liver cells from E14.5 WT1 mutant, heterozygous, and wild-type embryos were placed in culture. Cultures from mutant embryos gave less than 30% the number of erythrocyte, granulocyte macrophage, and granulocyte macrophage—erythroid—megakaryocyte colonies as those derived from WT/WT embryos. Cultures of WT1 heterozygous cells gave less than 50% of the numbers of all classes of colonies as wild-type cultures (Figure 3). This suggests that the WT1 effect is quantitative and that upward or downward regulation of the level of WT1 expression can play a significant role in the number, proliferative potential, or survival of hematopoietic precursors. To determine whether the decrease in colony-forming potential was mirrored by hematologic deficiencies after birth, blood counts were taken on 31 wild-type and 21 heterozygous offspring. No statistically significant differences were found (data not shown).
Hematopoietic colony-forming potential of fetal liver cells.
Dissociated E14.5 fetal liver cells were cultured in Methocult GF M3434 (StemCell Technologies) in triplicate at 1.5 × 104cells/mL at 37°C and 5% CO2. CFU-Es were counted after 2 days of culture, CFU-GMs after 10 days of culture, and CFU-GEMMs after 14 days of culture. (A-C) CFU-E, CFU-GM, and CFU-GEMM per 1.5 × 104 fetal liver cells, respectively. (D-F) CFU-E × 10−4, CFU-GM × 10−4, and CFU-GEMM × 10−3 per fetal liver, respectively. Fifteen KO/KO embryos, 20 KO/WT embryos, and 16 WT/WT embryos were used in these analyses. KO indicates knockout.
Hematopoietic colony-forming potential of fetal liver cells.
Dissociated E14.5 fetal liver cells were cultured in Methocult GF M3434 (StemCell Technologies) in triplicate at 1.5 × 104cells/mL at 37°C and 5% CO2. CFU-Es were counted after 2 days of culture, CFU-GMs after 10 days of culture, and CFU-GEMMs after 14 days of culture. (A-C) CFU-E, CFU-GM, and CFU-GEMM per 1.5 × 104 fetal liver cells, respectively. (D-F) CFU-E × 10−4, CFU-GM × 10−4, and CFU-GEMM × 10−3 per fetal liver, respectively. Fifteen KO/KO embryos, 20 KO/WT embryos, and 16 WT/WT embryos were used in these analyses. KO indicates knockout.
Discussion
Hematopoietic cells lacking WT1 demonstrate functional deficits. When chimeric mice are formed between normal blastocysts and ES cells lacking WT1, contributions to the blood by the WT1-negative cells are negligible compared with those previously seen with normal 129 ES cells, suggesting a competitive disadvantage for hematopoietic stem cells lacking WT1.53 54 When WT1-negative fetal liver cells are tested by in vitro differentiation assay, colony numbers are significantly reduced, suggesting that the regulation of the formation or function of hematopoietic progenitors is also compromised. Despite these deficits, the observation that murine E13.5 fetal liver cells lacking WT1 can repopulate a lethally irradiated adult host demonstrates that WT1 function is not absolutely essential either for the initial development of the hematopoietic system or for the differentiation of its various lineages in the adult. These defects of the hematopoietic system suggest that, as a transcription factor expressed in early members of the hematopoietic lineage, WT1 may play a role in regulating the expression of one or more genes that, in turn, are significant regulators of function of hematopoietic stem cells, progenitors, or both.
WT1 has been shown to be capable of controlling the transcription of members of several classes of genes, which could account for this apparent regulation of hematopoiesis. These include a number of cell-signaling molecules, their receptors, and genes involved in key decision points in regulating the balance between cell cycle progression, differentiation, and apoptosis.1 The phenotypes of hematopoietic cells deficient for a number of such genes show significant resemblance to the phenotypes of WT1-deficient hematopoietic cells.
The phenotype of mice with targeted deletion of p21 cyclin–dependent kinase inhibitor may be of particular relevance to the phenotype of WT1-negative hematopoietic cells. The absence of p21 has a clear negative effect on the long-term proliferative capacity of hematopoietic stem cells because of an alteration in the balance between cell cycling and quiescence.55 p21-Deficient hematopoietic stem cells show greater 5-fluorouracil (5-FU) sensitivity than normal cells, presumably as a consequence of an increase in the proportion of actively cycling hematopoietic stem cells. In addition, p21-deficient cells are unable to undergo repeated serial bone marrow transplantation, suggesting that increased cycling leads to early depletion of these cells. WT1 has been shown to transcriptionally activate p21, and it may play a critical role in the regulation of p21 in hematopoietic stem cells.20 56 If so, WT1 absence could lead to a decrease in the competitive efficiency of stem cells through a failure to properly up-regulate p21 expression.
Mutations of the tyrosine kinase receptors c-kit and flk2/flt3 lead to hematopoietic phenotypes that show parallels to the phenotype of WT1 mutant mice. In each case, the hematopoietic system of animals with reduced or absent function for these tyrosine kinase receptors have decreased competitive efficiency of hematopoietic stem cells and negative effects on the differentiation of committed progenitors.57,58 Deficiencies in stem cells and committed progenitors resembling the WT1 defect have also been described for mice lacking the thrombopoietin (TPO) receptor c-mpl and for mice lacking the growth factors interleukin-6 (IL-6), kit ligand and Lif, and the HOX cofactor Pbx1.57 59-62
Hematopoietic defects of WT1-deficient mice could be the consequence of the failure of regulation by WT1 of one or more hematopoietic signaling pathways. If receptor expression levels are reduced by the absence of WT1, a hematopoietic phenotype resembling the WT1-negative hematopoietic cell would be expected. Alternatively, WT1 may function downstream and be involved in mediating the response to the activation of the pathway. Genes such as p21 and Pbx1may be direct WT1 targets or may participate in the same signaling pathways involved in hematopoiesis.
The transcriptional role of WT1 in hematopoiesis is likely to be complex. In a study of ectopic expression of WT1 in human cord blood progenitors and leukemic cells, WT1 expression appears to impact primitive hematopoietic precursors and lineage-specific progenitors.20 Our observations on WT1-deficient mice are consistent with this view. The targets of WT1 in committed lineage progenitors may differ from the targets of WT1 in primitive hematopoietic stem cells, adding levels to the understanding of the impact of WT1 on hematopoiesis.
These findings on WT1-deficient murine hematopoietic stem cells permit some suggestions to be made regarding the role of WT1 in leukemia. WT1 expression appears to activate genes that confer a competitive advantage to hematopoietic stem cells. WT1 expression in leukemic cells may play a significant role during the initial expansion of a leukemic clone or may be required for the effective preferential propagation of the leukemic clone. The occurrence of inactivating mutations to WT1 in some leukemias may reflect the impact of WT1 on the differentiation of later progenitors, permitting the leukemic clone to be maintained in a less differentiated state. Detailed understanding of the impact of WT1 expression in each of the contexts will be of great interest in establishing the usefulness of WT1 expression as an indicator of leukemic status and as a potential target for therapy in leukemia.
We thank Dr Rudolf Jaenisch for support during the early stages of this work. We also thank Glenn A. Paradis of the MIT Flow Cytometry Laboratory for assistance with flow cytometry.
Prepublished online as Blood First Edition Paper, December 5, 2002; DOI 10.1182/blood-2002-06-1656.
Supported by National Institutes of Health grant 2-PO1-CA42063-17, National Center for Human Genome Research (NCHGR) grant HG00061 (G.M.S.), National Cancer Institute Individual Research Service Award grant CA60464 (J.A.A.), and the Charles A. King Trust Medical Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David Housman, Center for Cancer Research, Massachusetts Institute of Technology, 77 Main St, Cambridge, MA 02139; e-mail: dhousman@mit.edu.