Bcl-XL is essential for the survival and normal maturation of erythroid cells, especially at the late stage of erythroid differentiation. It remains unclear whether Bcl-XL serves only as a survival factor for erythroid cells or if it can induce a signal for differentiation. We have previously shown that dimethyl sulfoxide (DMSO) induction of erythroid differentiation in murine erythroleukemia (MEL) cells correlates with delay of apoptosis and specific induction of Bcl-XL. In this study, we investigate the contribution of Bcl-2 and Bcl-XL to survival and erythroid differentiation by generating stable MEL transfectants expressing these antiapoptotic regulators. Overexpression of Bcl-2 completely prevented apoptosis of MEL cells before and after DMSO induction, whereas overexpression of Bcl-XL only delayed it. Overexpression of Bcl-2 or Bcl-XL neither induced spontaneous erythroid differentiation nor accelerated DMSO-induced differentiation. Inhibition of Bcl-XL by antisense transcripts accelerated apoptosis in DMSO-treated MEL cells and blocked the synthesis of hemoglobin without altering the growth arrest associated with terminal erythroid differentiation. An antisense oligonucleotide to Bcl-XL did not induce apoptosis in MEL cells overexpressing Bcl-2 but greatly decreased their hemoglobin synthesis when treated with DMSO, suggesting that Bcl-XL is necessary for erythroid differentiation independently of its antiapoptotic function. Importantly, Bcl-XL antisense transcripts prevented heme synthesis but not globin mRNA induction in DMSO-treated MEL cells. Furthermore, inhibition of hemoglobin synthesis by Bcl-XLantisense was reversed by addition of exogenous hemin. Finally, Bcl-XL localized to mitochondria during MEL erythroid differentiation, suggesting that it may mediate a critical mitochondrial transport function related to heme biosynthesis.
Introduction
The Bcl-2 family of proteins has been reported to participate in the regulation of apoptosis and, more recently, in cell growth and differentiation. Some members of the Bcl-2 family, such as Bcl-2, Bcl-XL, and Mcl-1, function as antiapoptotic proteins, whereas Bax, Bad, Bak, and Bid work as cell death promoters.1 Among these Bcl-2 family members, Bcl-XL appears to be especially important for erythropoiesis. Several studies have revealed that Bcl-XL, but not Bcl-2, is required for the survival and normal maturation of erythroid cells. The important role of Bcl-XL in the control of erythropoiesis was first suggested by studies showing that Bcl-X−/− mouse embryos exhibit apoptosis of fetal liver hematopoiesis and pallor2 and its up-regulation during terminal erythroid differentiation.3Further studies in vivo have shown that Bcl-X−/− embryonic stem (ES) cells in chimeric mice do not contribute to circulating adult definitive erythrocytes, indicating that Bcl-XL is central for full erythroid maturation.4
Erythropoietin (EPO) allows erythroid precursors to proliferate and mature while protecting them from apoptosis. Several studies reported that EPO induces Bcl-XL in different EPO-dependent erythroid cell lines.5,6 The regulation of Bcl-XL function is likely to play a major role in the antiapoptotic effect of EPO. Recent reports have demonstrated that EPO-dependent activation of the factor STAT5 is probably involved in the induction of Bcl-XL expression.7,8 The transcriptional factor GATA-1 is also crucial for the maturation and survival of erythroid precursors and erythroblasts.9GATA-1 strongly induces the expression of Bcl-XL in erythroid cells and cooperates with EPO signaling in this regard.10 So, the EPO and GATA-1 signaling pathways converge to promote erythroid cell survival through activation of Bcl-XL expression.
Findings using in vitro hematopoietic differentiation of mouse bcl-X−/− ES cells clearly indicate that Bcl-XL is essential at a late stage of erythroid maturation.4,10 Finally, Wagner et al have recently described the phenotypic analysis of adult mice that are conditionally deficient in the Bcl-X gene.11 The animals suffered from severe hemolytic anemia and profound splenomegaly. Moreover, according to earlier findings in vitro, they demonstrated that Bcl-XL is only required for the survival of erythroid cells at the end of maturation, which includes enucleated reticulocytes in circulation.
Taken together, the data point to an essential role for Bcl-XL in maintaining the survival of differentiated erythroid cells. However, it remains unclear whether suppression of apoptosis is the sole function of Bcl-XL in terminal erythroid differentiation. To investigate this, we have employed the Friend murine erythroleukemia (MEL) cell model which provides a useful system for studying the mechanism of erythroid differentiation. MEL cells are erythroid precursors that are blocked at the proerythroblast stage. When exposed to dimethyl sulfoxide (DMSO) or other inducers, MEL cells undergo phenotypic changes that resemble the final stages of normal erythropoiesis, including hemoglobin synthesis.12,13 We have previously reported that DMSO induction of terminal erythroid differentiation in MEL cells correlates with a delay of apoptosis and a specific induction of the Bcl-XL protein.14 Here, we have created MEL cell lines which overexpressed Bcl-2 or Bcl-XL and a variant cell line transfected with a Bcl-XL antisense construct. Our results demonstrate that Bcl-2 prevents apoptosis in differentiated MEL cells, whereas Bcl-XL only delays it. Bcl-XL antisense transcripts accelerated apoptosis in DMSO-induced MEL cells and blocked their production of hemoglobin. Furthermore, we show that an antisense oligonucleotide to Bcl-XL may block hemoglobin synthesis in MEL cells in the absence of apoptosis. Finally, heme synthesis, but not globin mRNA expression, was prevented when Bcl-XL was inhibited in MEL cells.
Materials and methods
Chemicals and antibodies
DMSO was from BDH (Poole, United Kingdom) and bovine hemin was from Sigma Aldrich (St Quentin Fallavier, France). The primary antibodies used in Western blot analysis were mouse monoclonal anti–Bcl-2 from DAKO (Trappes, France), rabbit polyclonal anti–Bcl-X from BD Transduction Laboratories (Le Pont de Claix, France), rabbit polyclonal anti-actin from Sigma Aldrich, and mouse monoclonal anti–cytochrome oxidase subunit IV from Molecular Probes (Eugene, OR).
Plasmid constructs
The Bcl-2 expression vector pSFFVbcl-2neo described by S. J. Korsmeyer15 was kindly provided by Dr J. Bréard (INSERM Unit 461, Chatenay-Malabry, France). Human Bcl-XLcDNA was excised from its plasmid vector (gift from Dr Craig B. Thompson, Chicago, IL)16 as a 0.8-kb EcoR1 fragment and ligated into EcoR1-digested pSFFVneo with the sense and antisense orientation. The resulting expression vectors contain the Bcl-XL sense (pSFFVbcl-XL-S) or antisense (pSFFVbcl-XL-AS) downstream of the SFFV-LTR and the neomycin gene as a selective marker.
Cell lines and transfection
MEL cells, strain 745A, and derived transfectants were grown in RPMI-1640 medium (Gibco-BRL Life Technologies, Cergy Pontoise, France) containing 10% fetal calf serum (FCS; Eurobio, Les Ulis, France). 745A cells were transfected by electroporation as previously described17 with either the pSFFVneo plasmid (745A/Neo) or plasmid constructs. Individual cell clones were selected with 300 μg/mL geneticin (G418; Gibco-BRL). Clones expressing high levels of Bcl-2 or Bcl-XL were further subcloned by limiting dilution. Representative subclones each of 745A/Neo, 745A/Bcl-2, and 745A/Bcl-XL cells were used for the studies described below. Similarly, clones transfected with pSFFVbcl-XL-AS vector that did not express Bcl-XL were subcloned and a representative subclone was selected for molecular and biologic analysis.
Benzidine staining and heme quantification
Cells were seeded at 1 × 105 cells/mL and DMSO was added at a final concentration of 2% (vol/vol). The degree of erythroid differentiation was scored on day 4 or day 5 by counting the percentage of benzidine-positive cells as previously described.18 In some experiments hemin was added to the medium at a final concentration of 100 μM. Heme concentration in untreated and DMSO-treated cells was determined by the oxalic acid–fluorometric procedure of Sassa.19
Spectrophotometric assay for hemoglobin levels
Intracellular hemoglobin levels were determined by a previously described method.20 Briefly, cells were washed twice with phosphate-buffered saline (PBS), resuspended in 0.5 mL distilled water, and lysed by 4 cycles of freezing and thawing in a water bath at 37°C for 3 minutes. The cell lysate was centrifuged at 14 000 rpm in a microfuge for 15 minutes at 40°C. A portion of the supernatant (300 μL) was transferred to a new tube. The assay mixture was prepared in duplicate by adding the reagents in the following order: 100 μL supernatant, 900 μL distilled water, 100 μL freshly prepared benzidine-HCl (10 mg/mL in 0.5% acetic acid) and finally, 40 μL of 30% H2O2. The contents were mixed well and after exactly 90 seconds the absorbance at 604 nm was measured in a spectrophotometer. Hemoglobin concentration was determined by comparing this absorbance value to a standard curve prepared by similar treatments of pure human hemoglobin solutions.
Treatment with antisense oligonucleotide to Bcl-XL
Cells were incubated for 5 days in the presence of 30 μM bcl-XL antisense (AS) phosphorothioate derivatised oligodeoxynucleotide (purchased from Eurobio). Fresh oligonucleotide was added every day. Antisense DNA oligonucleotide with a phosphorothioate backbone was synthesized and purified by high-performance liquid chromatography (HPLC) (Eurobio). The antisense oligonucleotide used in this study encoded a sequence complementary to the coding region 5′ to the translation initiation codon site for the bcl-XL gene and extending 3′ downstream for a total of 18 bases (100% sequence identity between mouse and human). The oligonucleotide sequence used was 5′-CCGGTTGCTCTGAGACAT-3′ as previously described by Pollman et al.21
Semiquantitative RT-PCR for globin mRNA analysis
Transcripts of α- and β-globin were analyzed by using reverse transcriptase–polymerase chain reaction (RT-PCR) as previously described.22 Total RNA was extracted from each sample using Trizol reagent (Gibco-BRL) and cDNA was synthesized from 1 μg total RNA using the Advantage RT-for-PCR Kit (Clontech, Palo Alto, CA) according to the protocol of the manufacturer. The PCR mixture was amplified by using 1 μL RT reaction sample with the following primer sequences: α-globin (forward 5′-CTCTCTGGGAAGACAAAAGCAAC-3′, reverse 5′-GGTGGCTAGCCAAGGTCACCAG-CA-3′), β-globin (forward 5′-CTGACAGATGCTCTCTTGGG-3′, reverse 5′-CACAACCCCAGAAAACAGACA-3′), glyceraldehyde phosphate dehydrogenase (GAPDH) (forward 5′-GGTGAAGGTCGGAGTCAACGGA-3′, reverse 5′-GAGGGATCTCGCTCCTGGAAGA-3′). The sequence of the primers used for the analysis of α- and β-globin expression was reported by Vannucchi et al.23GAPDH-specific oligonucleotides were used as the internal control. PCR conditions were as follows: 94°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute. PCR was performed at different cycle numbers to determine the linear amplification and 25 PCR cycles was the cycle number chosen to allow a linear RT-RNA dose response for the 3 genes. PCR products were electrophoresed in agarose gels and visualized by ethidium-bromide staining. Band intensities for α- and β-globin PCR products were quantitated using a densitometer (Image Quant; Molecular Dynamics) and normalized to the intensity of the GAPDH signal.
Western blot analysis
Protein lysates were separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), blotted onto PolyScreen membranes (NEN, Paris, France) and processed for Western blot analysis as previously described.14
Preparation of mitochondria and S-100 fraction
The isolation of mitochondria was adapted from a previously described method.24 Briefly, cells were centrifuged and washed twice with ice PBS. The cell pellet was then resuspended in an ice-cold hypotonic buffer containing 10 mM NaCl, 1.5 mM MgCl2, and 10 mM Tris (tris(hydroxymethyl)aminomethane)–HCl, pH 7.5. After 20 minutes incubation in ice, cells were ruptured with several strokes in a Dounce homogenizer. Immediately after homogenization, the homogenate was mixed with an iso-osmotique MS buffer (final concentration 210 mM mannitol, 70 mM sucrose, 5 mM Tris-HCl, pH 7.5, and 1 mM EDTA [ethylenediaminetetraacetic acid], pH 7.5) and centrifuged at 750g for 10 minutes at 4°C to pellet nuclei and unbroken cells. The supernatant was then centrifuged at 17 000g for 15 minutes at 4°C. After washing once with MS buffer, the pellet containing mitochondria was resuspended in MS buffer and stored at −80°C. The supernatant of the 17 000g spin was further centrifuged at 100 000g for 1 hour at 4°C and the resulting supernatant (S-100) containing the cytosolic fraction was frozen at −80°C. The protein concentrations of mitochondria and S-100 were determined and 25 μg protein was used for Western blot analysis of Bcl-XL.
Analysis of cell viability and apoptosis
Cells were treated by adequate stimulus and after the appropriate time, viable cells were counted using the trypan blue dye exclusion assay. The number of total and viable cells was counted every 24 hours. The percent cell viability was determined by dividing the number of viable cells by the number of total cells. Apoptosis was analyzed by staining with a mixture of ethidium bromide (EB) and acridine orange (AO). Cell suspension (25 μL) was mixed with 25 μL of a solution containing 500 μg/mL EB and 150 μg/mL AO. Cell morphology was studied using a fluorescence microscope. Live cells fluoresce green and dead cells orange. In advanced apoptosis, nuclei take the form of bright spherical beads which are distinguishable from the spheric form in necrotic cells.
Analysis of DNA fragmentation
DNA fragmentation was analyzed as previously described.14 Briefly, 2 × 107 cells were washed in PBS and resuspended in 50 μL of 50 mM Tris-HCl, pH 8.0, 10 mM EDTA, and 0.5 mg/mL proteinase K. After incubation at 50°C for 1 hour, 1 μL of 10 mg/mL RNaseA was added for an additional 1 hour at 37°C. The samples were mixed with 25 μL of 10 mM EDTA, pH 8.0, containing 1% (wt/vol) low melting point agarose, 0.25% bromophenol blue, and 40% sucrose at 70°C. DNA was separated in 2% agarose gels and visualized by ultraviolet illumination after ethidium-bromide staining.
TUNEL assays
Apoptotic DNA fragments were end-labeled in situ with fluorescein-12-dUTP by terminal deoxynucleotide transferase (TdT) using the Apoptosis Detection System from Roche Diagnostics (Meylan, France). Cells were analyzed on FACScan flow cytometer (Becton Dickinson, Le Pont de Claix, France) and a total of 4000 events were collected.
Results
Establishment of stable MEL transfectants expressing Bcl-2 and Bcl-XL transcript
We have previously shown that Bcl-XL, but not Bcl-2, is expressed in Friend MEL cells. Moreover, DMSO-induced erythroid differentiation of MEL cells is associated with an enhancement of Bcl-XL expression correlated with a delay of apoptosis.14 To investigate the potential role of these 2 antiapoptotic proteins in regulating erythroid differentiation, we introduced Bcl-2 or Bcl-XL sense expression vectors into 745A MEL cells. Cells were transfected by electroporation with linearized plasmid DNA from one of the expression vectors. 745A cells were also transfected with the control neo vector. Cells stably expressing constructs were selected by growth in G418 and 10 to 20 individual clones for each group were obtained by limiting dilution. All cellular clones were examined for their protein expression in absence of DMSO by Western blotting. A single clone that expresses high levels of Bcl-2 (745A/Bcl-2) or Bcl-XL(745A/Bcl-XLS) was used in the studies presented here (Figure 1A). The cell population–doubling time of 745A/Bcl-2 and 745A/Bcl-XLS cells was not significantly different from that of parental 745A or 745A/Neo cells (data not shown).
Bcl-2, but not Bcl-XL, prevents apoptosis before and after DMSO-induced erythroid differentiation in MEL cells.
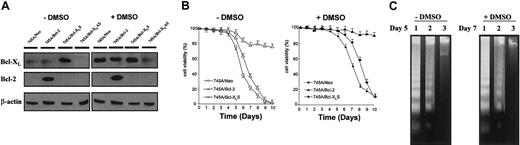
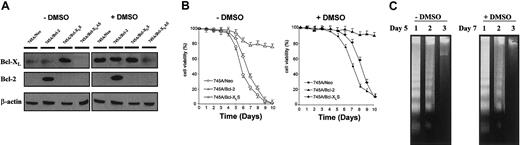
(A) Expression of Bcl-2 and Bcl-XL in MEL cells and derived clones. Cells were grown in the absence or in the presence of DMSO for 5 days. Total protein was isolated from 745A/Neo cells (745A clone stably transfected with neo gene), 745A/Bcl-2 cells (745A clone stably transfected with Bcl-2 sense transcripts), 745A/Bcl-XLS cells (745A clone stably transfected with Bcl-XL sense transcripts), and 745A/Bcl-XLAS cells (745A clone stably transfected with Bcl-XL antisense transcripts), and analyzed by Western blotting with anti–Bcl-2 and anti–Bcl-XL antibodies. Evaluation of β-actin expression was used to control for equal loading. (B) Cell viability of MEL cells. Cells were initiated at 105 cells/mL and cultured with or without 2% DMSO. At the indicated times (days), cell viability was measured by trypan blue dye exclusion. Data represent the mean of triplicate cultures ± standard deviation. (C) DNA fragmentation analyzed by gel electrophoresis followed by staining with ethidium bromide in MEL cells cultured without DMSO (day 5) or with DMSO (day 7). Lane 1: 745A/Neo cells; lane 2: 745A/Bcl-XLS cells; lane 3: 745A/Bcl-2 cells.
Bcl-2, but not Bcl-XL, prevents apoptosis before and after DMSO-induced erythroid differentiation in MEL cells.
(A) Expression of Bcl-2 and Bcl-XL in MEL cells and derived clones. Cells were grown in the absence or in the presence of DMSO for 5 days. Total protein was isolated from 745A/Neo cells (745A clone stably transfected with neo gene), 745A/Bcl-2 cells (745A clone stably transfected with Bcl-2 sense transcripts), 745A/Bcl-XLS cells (745A clone stably transfected with Bcl-XL sense transcripts), and 745A/Bcl-XLAS cells (745A clone stably transfected with Bcl-XL antisense transcripts), and analyzed by Western blotting with anti–Bcl-2 and anti–Bcl-XL antibodies. Evaluation of β-actin expression was used to control for equal loading. (B) Cell viability of MEL cells. Cells were initiated at 105 cells/mL and cultured with or without 2% DMSO. At the indicated times (days), cell viability was measured by trypan blue dye exclusion. Data represent the mean of triplicate cultures ± standard deviation. (C) DNA fragmentation analyzed by gel electrophoresis followed by staining with ethidium bromide in MEL cells cultured without DMSO (day 5) or with DMSO (day 7). Lane 1: 745A/Neo cells; lane 2: 745A/Bcl-XLS cells; lane 3: 745A/Bcl-2 cells.
Bcl-2, but not Bcl-XL, prevents apoptosis in MEL cells before and after DMSO treatment
Cell viability was studied in both untreated 745A/Bcl-2 and 745A/Bcl-XLS cells and compared with that of 745A/Neo cells (Figure 1B). We noted that 745/Bcl-2 cells had a greater life span compared with that of 745A/Neo cells. All 745A/Neo cells were dead after 9 days of culture, whereas 745A/Bcl-2 cells showed 80% viability at this time. In contrast, 745A/Bcl-XLS cells only exhibited delayed spontaneous cell death compared with 745A/Neo cells and were totally dead after 9 days of culture. As seen in Figure1B, DMSO-induced 745A/Neo cells began to die after 6 days of treatment and more than 90% were dead 10 days after DMSO treatment. The behavior of 745A/Bcl-2 cells in the presence of DMSO was clearly different from that observed in 745A/Bcl-XLS cells. DMSO-treated 745A/Bcl-XLS cells died later than DMSO-treated 745A/Neo cells but they did not survive after 10 days of treatment. In contrast, DMSO-treated 745A/Bcl-2 cells were totally resistant to death after 10 days of treatment and even after 15 days (data not shown).
These results showed that overexpression of Bcl-XL delayed but did not prevent spontaneous cell death in untreated and DMSO-treated MEL cells. In contrast, cell death was totally inhibited by overexpression of Bcl-2 in MEL cells before and after DMSO treatment. To further study cell death in MEL cells, DNA fragmentation reflecting the endonuclease activity characteristic of apoptosis was analyzed. As seen in Figure 1C, DNA fragmentation was not evident in untreated 745A/Bcl-2 cells after 5 days of culture, whereas at this time DNA genomic from 745A/Neo and 745A/Bcl-XLS cells was degraded into oligonucleosomal fragments. Similarly, after 7 days of culture with DMSO, DNA fragmentation was observed in 745A/Neo and 745A/Bcl-XLS cells but not in 745A/Bcl-2 cells. Therefore, Bcl-2 provided better protection than Bcl-XL against apoptosis in MEL cells induced to differentiate and prevented them from apoptosis after differentiation.
Transfection with antisense Bcl-XL induces premature apoptosis in DMSO-induced MEL cells
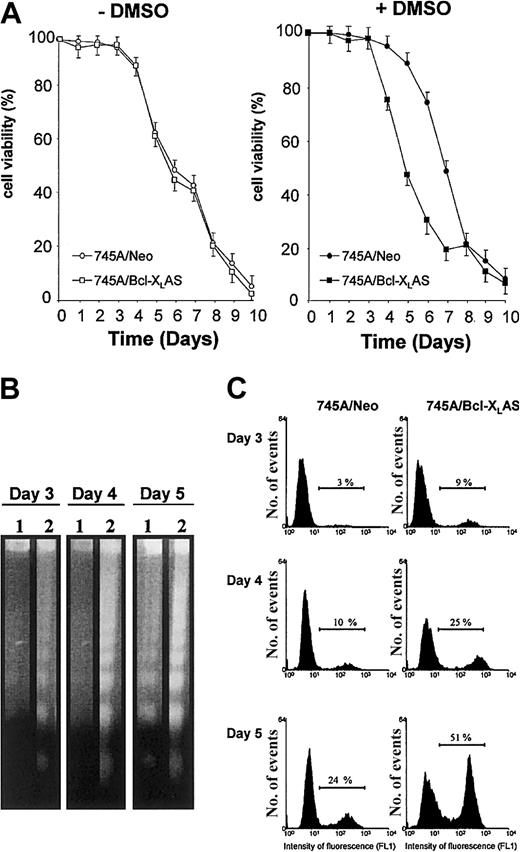
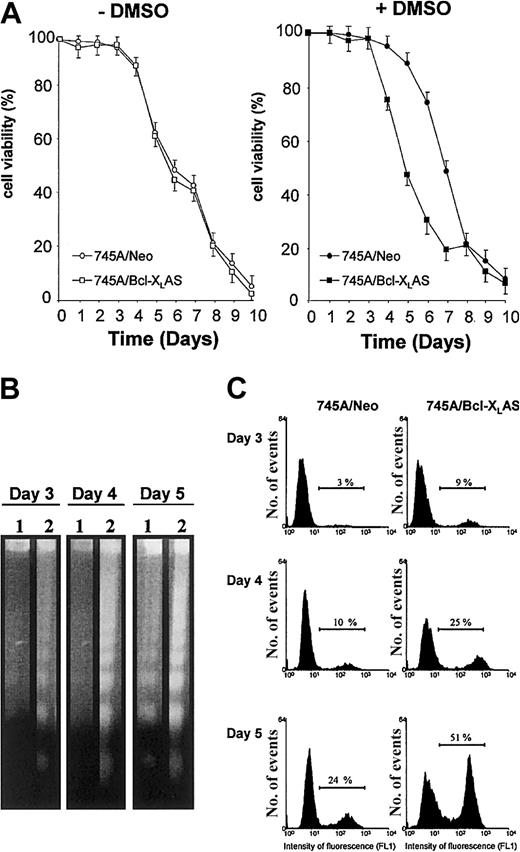
We previously reported that DMSO treatment induced a marked increase of Bcl-XL expression correlated with a transient resistance to spontaneous apoptosis of 745A cells.14 To understand whether Bcl-XL may play a role in protecting MEL cells from apoptosis during DMSO-induced differentiation, we generated 745A clones stably transfected with Bcl-XL antisense expression vector. We selected a 745A/Bcl-XLAS clone that did not show basal expression of Bcl-XL protein and displayed a marked inhibition of Bcl-XL protein levels following DMSO induction as compared with 745A/Neo cells (Figure 1A). Viability of untreated 745A/Bcl-XLAS cells was very similar to that of untreated 745A/Neo cells (Figure2A), suggesting that Bcl-XLdoes not play an antiapoptotic role in undifferentiated MEL cells. In contrast, in the presence of DMSO inducer, 745A/Bcl-XLAS cells presented an acceleration of cell death as compared with 745A/Neo cells (Figure 2A). At day 4 of DMSO treatment, 25% of 745A/Bcl-XLAS cells were dead, whereas at the same time DMSO-treated 745A/Neo cells were all alive. After 5 days of DMSO treatment, the viability of 745A/Bcl-XLAS cells was only 45%, compared with 90% in 745A/Neo cells. Viability of both 745A/Neo and 745A/Bcl-XLAS cells then declined rapidly after 6 days of DMSO treatment, reaching less than 20% by day 8. These results indicate that the inhibition of Bcl-XL expression in DMSO-induced MEL cells resulted in premature death at the time of maximum hemoglobin synthesis (on day 4 to day 5 of culture). The loss of viability in 745A/Bcl-XLAS cells was due to the activation of an apoptotic process as shown by the degradation of DNA (Figure 2B) and by the TdT-mediated dUTP nick end labeling (TUNEL) assay (Figure 2C) as early as 3 days after DMSO treatment.
Bcl-XL antisense induces premature apoptosis in DMSO-induced MEL cells.
(A) Cell viability of MEL cells in the absence or in the presence of DMSO. 745A/Neo and 745A/Bcl-XLAS cells were seeded at 105 cells/mL with or without 2% DMSO. At the times indicated (days) after growth initiation, cell viability was measured by trypan blue dye exclusion. Data represent the mean of triplicate cultures ± standard deviation. (B) DNA fragmentation analyzed by gel electrophoresis followed by staining with ethidium bromide in MEL cells treated with DMSO for indicated times (days). Lane 1: 745A/Neo cells; lane 2: 745A/Bcl-XLAS cells. (C) DNA breaks determined by TUNEL assay in MEL cells treated with DMSO for indicated days. Numbers on the horizontal bar indicate apoptotic cells as the percent of total cell number.
Bcl-XL antisense induces premature apoptosis in DMSO-induced MEL cells.
(A) Cell viability of MEL cells in the absence or in the presence of DMSO. 745A/Neo and 745A/Bcl-XLAS cells were seeded at 105 cells/mL with or without 2% DMSO. At the times indicated (days) after growth initiation, cell viability was measured by trypan blue dye exclusion. Data represent the mean of triplicate cultures ± standard deviation. (B) DNA fragmentation analyzed by gel electrophoresis followed by staining with ethidium bromide in MEL cells treated with DMSO for indicated times (days). Lane 1: 745A/Neo cells; lane 2: 745A/Bcl-XLAS cells. (C) DNA breaks determined by TUNEL assay in MEL cells treated with DMSO for indicated days. Numbers on the horizontal bar indicate apoptotic cells as the percent of total cell number.
Antisense Bcl-XL blocks erythroid differentiation in DMSO-induced MEL cells
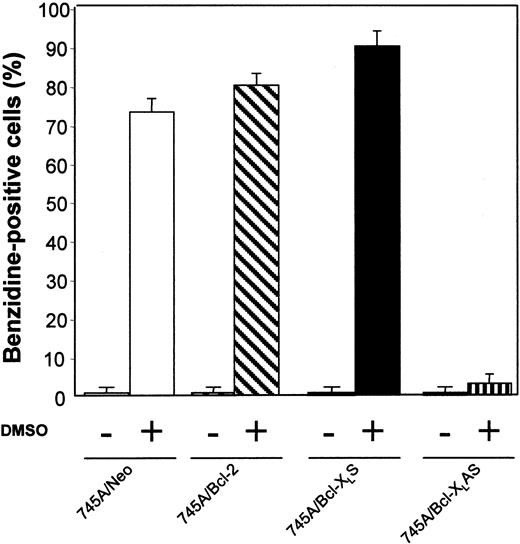
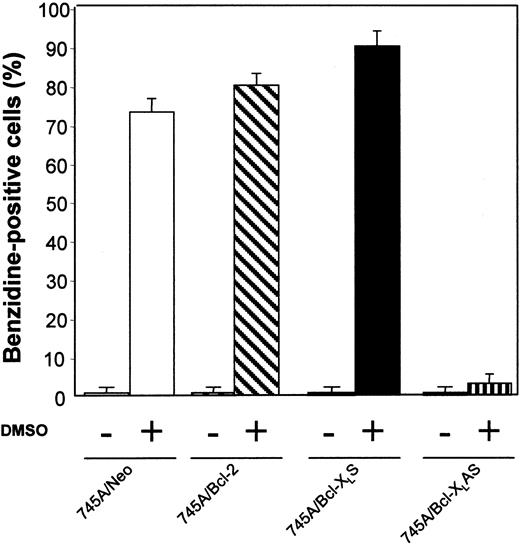
We have examined whether prolonged survival induced by Bcl-2 or Bcl-XL overexpression increased erythroid differentiation in 745A cells. Clones were stained with benzidine to detect hemoglobin synthesis. In the absence of DMSO inducer, 745A/Bcl-2 and 745A/Bcl-XLS cells did not exhibit spontaneous erythroid differentiation (≤ 1% of benzidine-positive cells) like 745A/Neo cells (Figure 3). In the presence of DMSO, both 745A clones overexpressing Bcl-2 or Bcl-XLexhibited high levels of hemoglobin synthesis on day 4 (≥ 80% of benzidine-positive cells) similar to 745A/Neo cells (Figure 3). In contrast, the DMSO-treated 745A/Bcl-XLAS cells showed a very low (≤ 3%) percentage of benzidine-positive cells. Therefore, the inhibition of Bcl-XL expression by antisense transcripts in MEL cells blocks the hemoglobin synthesis induced by DMSO treatment.
Bcl-XL antisense inhibits erythroid differentiation in DMSO-induced MEL cells.
MEL cells were initiated at a cell density of 105 cells/mL and cultured with or without 2% DMSO. After 4 days of culture, the proportion of differentiated cells was measured by benzidine staining. All data represent the mean of triplicate cultures ± standard deviation.
Bcl-XL antisense inhibits erythroid differentiation in DMSO-induced MEL cells.
MEL cells were initiated at a cell density of 105 cells/mL and cultured with or without 2% DMSO. After 4 days of culture, the proportion of differentiated cells was measured by benzidine staining. All data represent the mean of triplicate cultures ± standard deviation.
Antisense Bcl-XL does not prevent MEL cells from cell growth arrest after DMSO induction
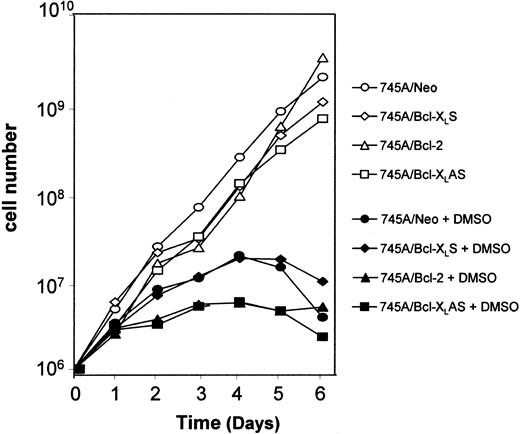
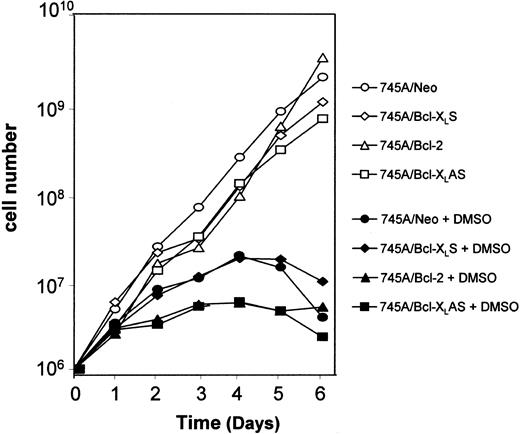
Incubation of MEL cells with DMSO inducer leads to the stimulation of 2 programs: one responsible for hemoglobin synthesis and the other for inhibition of cell growth. Several studies have indicated that the coordination of both programs is not obligatory.25 That is why we examined the effect of Bcl-2 and Bcl-XL on loss of proliferative potential cell in DMSO-treated MEL cells. To study the loss of proliferative potential after DMSO induction, cells were cultured in suspension and diluted every 2 days. When MEL cells were cultured without DMSO, they grew logarithmically after each transfer. A similar growth was observed in 745A/Neo, and both 745A/Bcl-2 and 745A/Bcl-XLS cells (Figure4). When 745A/Neo cells were cultured in the presence of DMSO, they progressively lost their capacity to grow in culture. A loss of growth arrest was also observed in 745A/Bcl-2 and 745A/Bcl-XLS cells after DMSO treatment. Similarly, DMSO induced cell growth arrest in 745A/Bcl-XLAS cells, indicating that the program responsible for cessation of proliferation was not blocked by antisense Bcl-XL during terminal MEL erythroid differentiation.
Overexpression or inhibition of Bcl-XL does not prevent growth arrest of DMSO-treated MEL cells.
MEL cells were seeded at 105 cells/mL with or without 2% DMSO. Every 2 days, an aliquot of each culture was removed and transferred to fresh medium without or with inducer at 105cells/mL. The results are expressed as the cumulative cell number versus time.
Overexpression or inhibition of Bcl-XL does not prevent growth arrest of DMSO-treated MEL cells.
MEL cells were seeded at 105 cells/mL with or without 2% DMSO. Every 2 days, an aliquot of each culture was removed and transferred to fresh medium without or with inducer at 105cells/mL. The results are expressed as the cumulative cell number versus time.
Bcl-XL antisense oligonucleotide inhibits hemoglobin synthesis without inducing apoptosis in DMSO-treated 745A/Bcl-2 cells
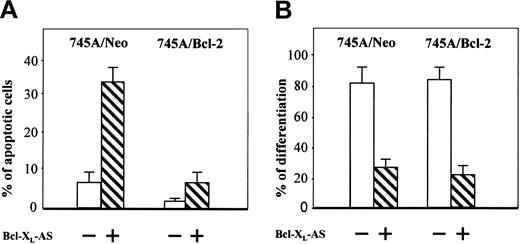
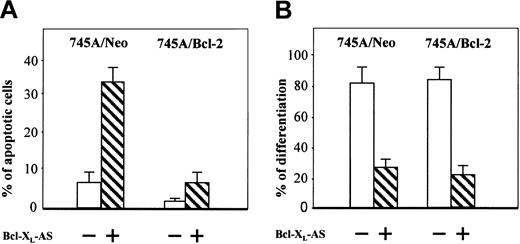
Recent results suggest that Bcl-XL is necessary to maintain erythroid cell viability during normal terminal erythroid maturation.4,10 11 Our results showing premature apoptosis in the DMSO-treated 745A/Bcl-XLAS cells might explain the inhibition of hemoglobin synthesis observed in these cells. To determine whether Bcl-XL is required for erythroid differentiation in MEL cells by its antiapoptotic function, we decided to study the effect of Bcl-XL antisense oligonucleotide in 745A/Bcl-2 cells. The efficacy of this antisense oligonucleotide was first studied in 745A/Neo cells (Figure5A). Incubation for 5 days with Bcl-XL antisense oligonucleotide increased the number of apoptotic cells in DMSO-treated 745A cells, according to the above findings that the inhibition of Bcl-XL decreased viability in 745A/Bcl-XLAS cells. In contrast, incubation for 5 days with Bcl-XL antisense oligonucleotide in DMSO-treated 745A/Bcl-2 cells slightly affected their cell survival. These results indicate that the overexpression of Bcl-2 in 745A cells overcame the apoptotic effect of the Bcl-XL antisense oligonucleotide. When we examined hemoglobin synthesis, Bcl-XL antisense oligonucleotide greatly decreased the percentage of benzidine-positive cells in DMSO-treated 745A/Neo cells as well as in DMSO-treated 745A/Bcl-2 cells (Figure 5B). These results show that inhibition of Bcl-XL in MEL cells may block hemoglobin synthesis in the absence of apoptosis, suggesting that Bcl-XL may be necessary for erythroid differentiation independently of its antiapoptotic function.
Bcl-XL antisense oligonucleotide does not induce apoptosis but inhibits hemoglobin synthesis in MEL cells overexpressing Bcl-2.
745A/Neo and 745A/Bcl-2 cells were seeded at 105 cells/mL and cultured in the presence of 2% DMSO with or without Bcl-XL antisense oligonucleotide (Bcl-XL-AS). At 5 days after DMSO treatment, the following parameters were determined: (A) percentage of apoptotic cells measured by staining cells with a mixture of ethidium bromide (EB) and acridine orange (AO) (data represent the mean of quadruplicate cultures ± standard deviation); (B) proportion of differentiated cells measured by benzidine staining (data represent the mean of quadruplicate cultures ± standard deviation).
Bcl-XL antisense oligonucleotide does not induce apoptosis but inhibits hemoglobin synthesis in MEL cells overexpressing Bcl-2.
745A/Neo and 745A/Bcl-2 cells were seeded at 105 cells/mL and cultured in the presence of 2% DMSO with or without Bcl-XL antisense oligonucleotide (Bcl-XL-AS). At 5 days after DMSO treatment, the following parameters were determined: (A) percentage of apoptotic cells measured by staining cells with a mixture of ethidium bromide (EB) and acridine orange (AO) (data represent the mean of quadruplicate cultures ± standard deviation); (B) proportion of differentiated cells measured by benzidine staining (data represent the mean of quadruplicate cultures ± standard deviation).
Antisense Bcl-XL inhibits heme synthesis but not globin mRNA expression in DMSO-induced MEL cells
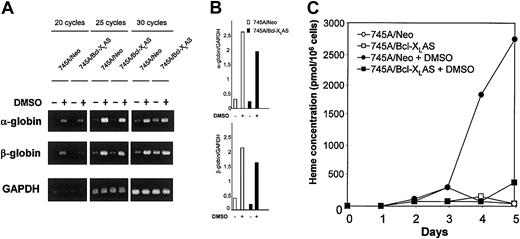
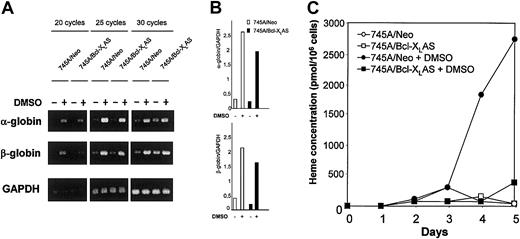
Treatment of MEL cells with agents such as DMSO induces accumulation of α- and β-globin mRNA and heme biosynthesis leading to hemoglobin production.13,19 26 We have examined the effect of antisense Bcl-XL on both globin RNA expression and heme synthesis. Globin mRNA expression was tested by semiquantitative RT-PCR (Figure 6A-B). Treatment with DMSO induced α- and β-globin mRNA after 4 days of culture in both 745A/Neo and 745A/Bcl-XLAS cells, indicating that inhibition of Bcl-XL in MEL cells did not prevent expression of globin. Heme concentration was determined by fluorometry. The total heme concentration in untreated MEL cells remained very low (Figure 6C). In 745A/Neo cells grown in the presence of DMSO, a significant increase of heme concentration became apparent after 4 days of culture. Approximately 2.7 nmol heme/106 cells were found by the fifth day of DMSO treatment, that is 50 times more heme per cell than in untreated cells. In contrast, the heme content was not increased in 745A/Bcl-XLAS cells after 4 or 5 days of DMSO treatment. These results indicate that antisense Bcl-XLprevents heme synthesis but not globin mRNA induction in DMSO-treated MEL cells.
Bcl-XL antisense inhibits heme synthesis without altering α- and β-globin mRNA expression in DMSO-induced MEL cells.
(A) Expression of α- and β-globin transcripts in 745A/Neo and 745A/Bcl-XLAS cells. Cells were seeded at 105cells/mL with or without 2% DMSO. After 4 days of culture, total RNA was isolated and subjected to RT-PCR as described in “Materials and methods.” α-globin, β-globin, and GAPDH were amplified for 20, 25, and 30 cycles. Aliquots of the PCR reactions were separated on 1.2% agarose gels and visualized with ethidium bromide. (B) Semiquantitative RT-PCR analysis of α- and β-globin in 745A/Neo (■) and 745A/Bcl-XLAS (▪) cells cultured for 4 days in the absence or in the presence of DMSO. Values are taken from densitometric analysis of bands obtained with 25 cycle amplifications and are normalized to GAPDH values from the same sample. (C) Heme production in 745A/Neo and 745A/Bcl-XLAS cells. Cells were seeded at 105 cells/mL with or without 2% DMSO and harvested at the times indicated (days) after growth initiation. Heme concentration was determined by a fluorometric procedure.
Bcl-XL antisense inhibits heme synthesis without altering α- and β-globin mRNA expression in DMSO-induced MEL cells.
(A) Expression of α- and β-globin transcripts in 745A/Neo and 745A/Bcl-XLAS cells. Cells were seeded at 105cells/mL with or without 2% DMSO. After 4 days of culture, total RNA was isolated and subjected to RT-PCR as described in “Materials and methods.” α-globin, β-globin, and GAPDH were amplified for 20, 25, and 30 cycles. Aliquots of the PCR reactions were separated on 1.2% agarose gels and visualized with ethidium bromide. (B) Semiquantitative RT-PCR analysis of α- and β-globin in 745A/Neo (■) and 745A/Bcl-XLAS (▪) cells cultured for 4 days in the absence or in the presence of DMSO. Values are taken from densitometric analysis of bands obtained with 25 cycle amplifications and are normalized to GAPDH values from the same sample. (C) Heme production in 745A/Neo and 745A/Bcl-XLAS cells. Cells were seeded at 105 cells/mL with or without 2% DMSO and harvested at the times indicated (days) after growth initiation. Heme concentration was determined by a fluorometric procedure.
Inhibition of hemoglobin synthesis by antisense Bcl-XL is reversed by addition of exogenous hemin
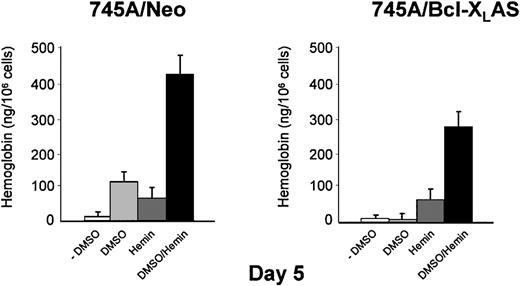
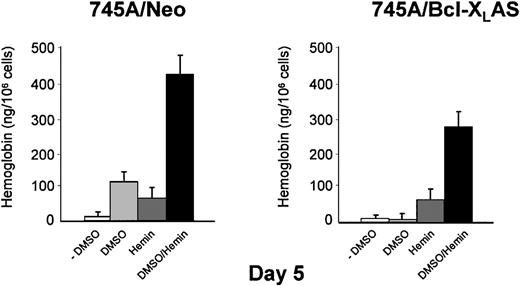
Because α- and β-globin mRNA was induced by DMSO in 745A/Bcl-XLAS cells, we investigated whether the addition of exogenous hemin can reverse the inhibition of hemoglobin synthesis. Treatment of 745A/Neo and 745A/Bcl-XLAS cells with hemin alone did not result in erythroid differentiation as measured by benzidine staining (Table 1). However, hemin induced detectable quantities of intracellular hemoglobin in both cell lines (50 ng/106 cells) when hemoglobin was measured by spectrophotometry (Figure 7). DMSO also induced hemoglobin production in 745A/Neo cells (110 ng/106 cells) but not in 745A/Bcl-XLAS cells (Figure 7) in agreement with results observed by benzidine staining (Table 1 and Figure 3). When hemin was added in DMSO-treated 745A/Neo cells, the percentage of benzidine-positive cells was optimal (97%) as compared with DMSO alone (80%). After exposure to DMSO and hemin for 5 days, 45% of 745A/Bcl-XLAS cells were benzidine positive (Table 1). Moreover, following hemin addition, DMSO-treated 745A/Bcl-XLAS cells were able to accumulate high levels of intracellular hemoglobin (280 ng/106 cells). These results indicated that treatment with exogenous hemin overcame the block of hemoglobin synthesis induced by Bcl-XL antisense in DMSO-treated MEL cells. These results further argued that the inhibition of Bcl-XL caused a defect in the induction of heme synthesis.
Addition of exogenous hemin reversed inhibition of hemoglobin synthesis induced by Bcl-XL antisense in DMSO-treated MEL cells.
745A/Neo and 745A/Bcl-XLAS cells were treated with DMSO (2%), hemin (100 μM), or the combined addition of DMSO (2%) and hemin (100 μM) for 5 days. Cellular hemoglobin production was determined by a spectrophotometric assay. Data represent the mean of triplicate cultures ± standard deviation.
Addition of exogenous hemin reversed inhibition of hemoglobin synthesis induced by Bcl-XL antisense in DMSO-treated MEL cells.
745A/Neo and 745A/Bcl-XLAS cells were treated with DMSO (2%), hemin (100 μM), or the combined addition of DMSO (2%) and hemin (100 μM) for 5 days. Cellular hemoglobin production was determined by a spectrophotometric assay. Data represent the mean of triplicate cultures ± standard deviation.
Mitochondrial localization of Bcl-XL in MEL cells after DMSO induction
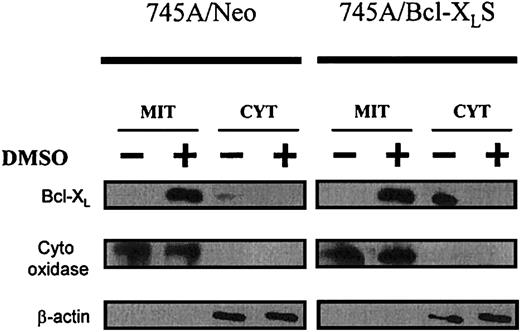
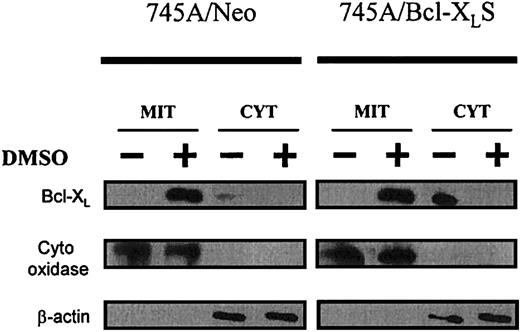
To better understand the function of Bcl-XL in erythroid differentiation of MEL cells, we studied its localization before and after DMSO in parental 745A cells and in derived clones. The subcellular distribution of Bcl-XL protein was analyzed in both purified mitochondria and cytosol (Figure8). Before DMSO treatment, low levels of Bcl-XL protein were found in the cytosolic fraction of 745A/Neo cells. After DMSO induction in 745A/Neo cells, Bcl-XL was induced and seen only in the mitochondria. 745A/Bcl-XLS cells expressed high levels of Bcl-XL before and after DMSO treatment. However, the protein was detected in the cytosol of untreated 745A/Bcl-XLS cells whereas, in the presence of DMSO, the protein was predominantly present in the mitochondria. These results indicate that Bcl-XL was translocated from the cytosol to the mitochondria during DMSO-induced erythroid differentiation of MEL cells.
Bcl-XL localizes to mitochondria during MEL erythroid differentiation.
745A/Neo and 745A/Bcl-XLS cells were cultured with or without 2% DMSO. After 5 days of culture, cells were lysed and separated into mitochondrial (MIT) and cytosolic (CYT) fractions. Proteins from each cellular fraction were analyzed by Western blotting with anti–Bcl-XL, anti–cytochrome oxidase (subunit IV), and anti–β-actin antibodies.
Bcl-XL localizes to mitochondria during MEL erythroid differentiation.
745A/Neo and 745A/Bcl-XLS cells were cultured with or without 2% DMSO. After 5 days of culture, cells were lysed and separated into mitochondrial (MIT) and cytosolic (CYT) fractions. Proteins from each cellular fraction were analyzed by Western blotting with anti–Bcl-XL, anti–cytochrome oxidase (subunit IV), and anti–β-actin antibodies.
Discussion
The relationship between cell survival and cell maturation programs remains unclear during hematopoietic differentiation. Several studies have shown that Bcl-XL is necessary for normal erythroid differentiation by maintaining survival of erythroid cells at the end of their maturation. We report here that the block of Bcl-XL during terminal erythroid differentiation of MEL cells resulted not only in premature apoptosis but also in inhibition of hemoglobin synthesis, due to a block of heme production. It is notable that the inhibition of hemoglobin synthesis by Bcl-XL antisense occurred in MEL cells even when apoptosis was overcome by Bcl-2 overexpression. We suggest that Bcl-XL has 2 independent roles in erythroid maturation: cell survival and heme synthesis.
Both Bcl-2 and Bcl-XL have been implicated in survival of erythroid progenitors but only Bcl-XL is required at the end of erythroid maturation.4,10,11 A delay of apoptosis and an up-regulation of Bcl-XL are found during chemical induction of MEL erythroid differentiation.14 However, MEL cell differentiation terminates with apoptosis and high levels of Bcl-XL in MEL cells are not sufficient to protect them from apoptosis after maturation. In contrast, overexpression of Bcl-2 totally inhibits apoptosis in terminally differentiated MEL cells. These results suggest a distinction in the functional capabilities of Bcl-XL and Bcl-2 to prevent postmaturation apoptosis. There are other reports showing that Bcl-2 and Bcl-XL can differentially regulate apoptosis.27,28 This difference has several possible interpretations. Whereas Bcl-2 and Bcl-XL are likely to have similar tertiary structure, they may function differently by specific interactions with proteins that mediate postmaturation apoptosis. It is not known which is the proapoptotic factor that triggers apoptosis in MEL cells. We have previously reported that the proapoptotic Bax and Bad proteins are present at a high level before and after induction of differentiation in MEL cells, leaving the cells in a proapoptotic condition.14 Although both Bcl-2 and Bcl-XLfunction as death repressors, Bad and Bax could differentially affect their protective ability. It has been reported that Bad interacts strongly with Bcl-XL but weakly with Bcl-2 and can overcome the antiapoptotic effect of Bcl-XL but not Bcl-2.29 Another possibility is that agents that promote apoptosis could biochemically modify Bcl-2 or Bcl-XL. Posttranslational modification of Bcl-2 or Bcl-XL through phosphorylation has been shown to modulate their antiapoptotic activity.30 31 Finally, as Bcl-XL but not Bcl-2 is present in MEL cells, overexpression of Bcl-2 may lead to high levels of resistance to apoptosis due to a combination of Bcl-2 and Bcl-XL expression.
Our results indicate that the suppression of apoptosis by Bcl-2 is not sufficient to induce spontaneous erythroid differentiation in MEL cells and that DMSO induction is required to overcome the block of differentiation in this model. These results are in agreement with those reported by Chida et al,32 showing that expression of the cell survival genes, Bcl-2 and Bcl-XL, is not sufficient for erythroid colony formation from normal erythroid colony-forming units (CFU-Es) in the absence of EPO. Moreover, targeted expression of Bcl-2 in the erythroid lineage in transgenic mice did not induce erythroid differentiation in the absence of EPO.33Therefore, a survival signal is probably necessary but not sufficient for induction of terminal erythroid differentiation. However, further studies using transgenic mice expressing bcl-XL gene are needed to determine whether targeted expression of Bcl-XLin the erythroid lineage would allow by itself an EPO-independent erythroid differentiation.
Several studies have indicated that survival programs are required during the initial phase of cell differentiation to escape apoptosis and complete the differentiation process.34 Although apoptosis-resistant pathways during differentiation of leukemic cells are poorly understood, there is evidence that members of the Bcl-2 family are involved in the regulation of these processes. For example, Mcl-1 and A1, members of the Bcl-2 family, are required to prevent apoptosis during differentiation of leukemic U937 or NB4 cells.35,36 However, it is presently unclear whether these early survival programs are prerequisite for, occur simultaneously with, or are dependent on, cell differentiation. During the chemically induced MEL erythroid differentiation, cells must also possess a mechanism to avoid cell death until terminal differentiation occurs, allowing the development of mature cells to exhibit their functional phenotype. We have previously shown an up-regulation of Bcl-XL in MEL cells induced by DMSO or hexamethylene bisacetamide (HMBA).14 We show here that inhibition of Bcl-XL expression by antisense transcripts or antisense oligonucleotide results in an acceleration of apoptosis in differentiating MEL cells associated with an inhibition of hemoglobin synthesis. This indicates that Bcl-XL is essential to delay apoptosis during MEL erythroid differentiation. Our results are in agreement with several studies, using different approaches, showing that Bcl-XL is required in erythroid differentiation because it protects normal erythroid cells from apoptosis during the late stages of erythroid maturation.4,10 11 On the other hand, we observe that an antisense oligonucleotide to Bcl-XL does not produce apoptosis in MEL cells overexpressing Bcl-2. This indicates that Bcl-2 can overcome premature apoptosis induced by Bcl-XLinhibition in differentiating MEL cells. However, overexpression of Bcl-2 does not prevent Bcl-XL antisense oligonucleotide from inhibiting hemoglobin synthesis in DMSO-treated MEL cells. These results suggest, for the first time, that Bcl-XLhas a role in hemoglobin synthesis independent of its antiapoptotic function. Thus, Bcl-XL could have a dual function during terminal erythroid differentiation, one by delaying apoptosis and allowing cells to complete the differentiation program, the other by interacting directly on hemoglobin synthesis.
Incubation of MEL cells with an inducer such as DMSO leads to the stimulation of 2 major programs: one program responsible for hemoglobin synthesis and the other for commitment to terminal maturation.13,25 Several studies have indicated that the coordination of these programs is not obligatory. In this study we found that, although Bcl-XL antisense transcripts affect MEL cells in their ability to synthesize hemoglobin with DMSO, they have no effect on the proliferative aspect of the erythroid differentiation. In MEL cells, both programs of differentiation, erythroid phenotypic differentiation and growth arrest, can be separately regulated. Our data show that, whereas Bcl-XLinterferes with erythroid differentiation, it is not involved in terminal cell division. Terminal differentiation is characterized by growth arrest and accumulation of cells in G0/G1.13 The role of the Cip/Kip family in the cell cycle exit of terminally differentiating cells has been recently described during erythroid differentiation of MEL cells.37 Further studies will be necessary to clarify why the absence of Bcl-XL does not affect the cell cycle withdrawal in differentiating leukemic cells.
Here, we found that Bcl-XL antisense inhibited the production of heme but not the synthesis of α- and β-globin after DMSO induction of MEL cells. Moreover, treatment of DMSO-induced MEL cells expressing Bcl-XL antisense with exogenous hemin overcame the block in hemoglobin synthesis, suggesting that they have a defect in the induction of heme biosynthesis. These results show that Bcl-XL has an essential role in heme synthesis during terminal erythroid differentiation of MEL cells. Heme biosynthesis is carried out by a series of enzymes which are activated during MEL differentiation.19 Increases in the activities of heme pathway enzymes occur sequentially in the order they appear in the heme biosynthetic pathway, and mRNAs for these enzymes accumulate before α- and β-globin mRNAs when MEL cells are induced by DMSO.26 As previously reported, DMSO treatment in MEL cells restores the activity of GATA-1 that is essential for expression of erythroid-specific genes such as globin and heme biosynthetic enzyme genes.38 Because α- and β-globin mRNAs are induced in MEL cells expressing Bcl-XL antisense when treated by DMSO, it is unlikely that the defect in heme synthesis was due to an absence of heme pathway enzymes. Therefore, our results suggest that Bcl-XL is required for a late step of heme synthesis.
Our observation that Bcl-XL accumulated in mitochondria during DMSO-induced MEL differentiation is compatible with a role in heme biosynthesis. Indeed, mitochondria participate in many essential functions during erythroid differentiation, including heme biosynthesis. The first and the 3 last steps of heme synthesis occur in the mitochondria.39 Little is known about how biosynthetic intermediates are shuttled across mitochondrial membranes. A novel mitochondrial transporter, ABC-mitochondrial erythroid (ABC-me), was recently described.40 This protein is induced during erythroid maturation in cell lines and primary hematopoietic cells. ABC-me is believed to mediate critical mitochondrial transport functions related to heme biosynthesis. Since both Bcl-XLand ABC-me are induced during erythroid MEL differentiation and are located in mitochondrial membranes, it is possible that they interact and cooperate, across mitochondrial membranes, in the transport of substrates that are required for heme biosynthesis. ABC-me is a member of the ATP-binding cassette which depends on ATP for its function. It was recently described that when Bcl-XL resides in the outer mitochondrial membrane (OMM), it appears to maintain mitochondrial ATP/ADP exchange across the inner membrane by facilitating OMM permeability to adenine nucleotides.41 42Then it is possible that Bcl-XL may allow MEL cells to maintain sufficient mitochondrial ATP/ADP exchange to provide ATP for ABC-me during terminal erythroid differentiation.
In summary, these studies provide evidence that Bcl-XL has a fundamental role in terminal erythroid differentiation. It appears to be required in heme biosynthesis independently of its antiapoptotic function. The effect of Bcl-XL in heme synthesis seems to be related to its mitochondrial localization. It remains to be determined how Bcl-XL is involved in heme synthesis and whether its dual function, survival and heme formation, found in MEL cells is also present in normal erythroid maturation.
Prepublished online as Blood First Edition Paper, November 21, 2002; DOI 10.1182/blood-2002-02-0478.
Supported by grants from the Association pour la Recherche sur le Cancer (ARC 9551) and from the Association NRB-Vaincre le Cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jacqueline Robert-Lézénès, INSERM U268, Hôpital Paul-Brousse, 14 avenue Paul Vaillant Couturier, 94807 Villejuif cedex, France; e-mail:jrl@infobiogen.fr.