The regulation of cell death in activated naive T cells is not well understood. We examined the expression of A1, an antiapoptotic member of the Bcl-2 family, following activation of naive mouse splenocytes. A1 gene expression was strongly but transiently induced during the first day of activation, with a peak at 2 to 6 hours, whereas Bcl-2 mRNA was simultaneously transiently down-regulated. Transgenic (Tg) overexpression of A1-a in T cells via the lck distal promoter resulted in decreased apoptosis following activation either with concanavalin A or with antibodies to CD3 and CD28 and led to a doubling of T-cell yield by 5 days. Tg A1-a also partially protected thymocytes from several proapoptotic stimuli but did not protect T-cell blasts from cell death induced by reactivation via the T-cell receptor. Tg Bcl-2 and Tg A1-a showed a similar ability to reduce apoptosis in both resting and activated T cells. However, in activated splenocyte cultures, the increase in 5-day T-cell yield observed with Tg Bcl-2 was only half that produced by Tg A1-a. This difference could be attributed at least in part to the fact that A1, unlike Bcl-2, did not inhibit S-phase entry of activated cells. The A1 protein may represent an adaptation of the Bcl-2 gene family to the need for survival regulation in the context of a proliferative stimulus.
Introduction
Activation of T cells via the T-cell receptor (TCR) can lead either to proliferation and differentiation or alternatively to apoptotic cell death. This critical decision is regulated by contextual factors such as the presence of costimulatory signals and cytokines as well as prior activation of the cell.1-11 Studies with T-cell lines indicate that a component of this regulation is the ability of the activated cell to impede apoptotic signaling mediated by death receptors such as Fas.12,13 In cultures of primary T cells, a Fas-dependent pathway of “activation-induced cell death” has been demonstrated in cells subject to repeated stimulation.4,14,15 Similarly, the expansion of T cells activated in vivo by high levels of antigen (in the absence of adjuvant) is limited by the Fas-dependent or tumor necrosis factor α (TNFα)–dependent removal of these cells.16-18 However, the initial activation of naive T cells in natural antigen responses may involve a different set of survival-regulatory mechanisms. Polyclonal activation of cultured naive T cells leads to Fas-independent apoptosis.6 Furthermore, the homeostatic contraction of the response of adoptively transferred TCR-transgenic T cells is Fas independent when the antigen is provided locally with adjuvant rather than systemically.18Similarly, the response of naive donor T cells to alloantigen in vivo leads to Fas-independent apoptosis,19 as does the activation of Vβ8+ T cells in vivo by staphylococcal enterotoxin B.20
The induction of apoptosis following activation of naive T cells appears to be counterbalanced by antiapoptotic mechanisms that are active for at least one to several days after exposure to antigen. In vivo studies of both Fas-independent death after alloantigen stimulation and Fas-dependent death using superantigen indicate that activated cells enter apoptosis only after cell division has occurred.21,22 Similarly, another study of apoptosis induced in naive cells in vivo by superantigen found that during the first 1 to 2 days T cells did not enter apoptosis and were Fas resistant.23 Moreover, at day 1 of the response these activated death-resistant cells experienced a transient elevation of Bax, a proapoptotic member of the Bcl-2 family of survival-regulatory proteins. This finding suggests that activated cells at this early stage have a mechanism of resisting Bax-induced death. More recently, Bim, a member of the BH3-only proapoptotic branch of the Bcl-2 family, has been shown to be up-regulated by TCR ligation in thymocytes and to play an essential role in the apoptosis that ensues.24 Bim has also been shown to be required for the deletion of Vβ8+ T cells following activation in vivo with a superantigen.20 Studies of mice that are deficient in either Bax or a third proapoptotic family member, Bak, have shown that either Bax or Bak is essential for spontaneous cell death in unstimulated T cells,25 for which Bim is also necessary,26 and thus it is conceivable that all of these Bcl-2–related proteins may contribute to a cell death pathway activated following exposure of naive cells to antigen. Consistent with this idea, when the expression of Bcl-2, an antiapoptotic protein that can counteract the activity of these proapoptotic molecules, was eliminated by gene targeting, naive T cells showed increased apoptosis that peaked at 24 hours after activation by anti-CD3.27Bcl-2 can protect lymphocytes from a variety of apoptotic stimuli, although not from the activation of death receptors such as Fas.28,29 However, Bcl-2, though constitutively present in quiescent naive T cells, does not show increased expression during the first few days following polyclonal T-cell activation in vitro,6,30,31 although it can be induced by the addition of interleukin 2 (IL-2).30,32 At least 4 additional antiapoptotic members of the Bcl-2 family (Bcl-xL, A1, Mcl-1, Bcl-w) have the capacity to interfere with Bax-induced cell death.33-35 Of these, Bcl-xL protein expression has been shown to be induced during the first day following activation of naive T cells with anti-CD3. Costimulation via CD28 greatly increases expression and also provides extensive protection from apoptosis.6,7 36-42
The A1 gene is also expressed in the T-cell lineage43,44 and is up-regulated by activation.45,46 A1 is induced as an immediate-early gene by activating stimuli in macrophages.43 In inflammatory macrophages elicited by Toxoplasma gondii, the induction of A1 in vivo parallels that of proapoptotic Bax.47 We therefore asked whether A1 plays a role in the survival of freshly activated T cells. We found that A1 is strongly and transiently up-regulated very early after activation of naive cells. Furthermore, transgenic A1, unlike Bcl-2, is able to protect T cells without interfering with cell cycle progression, and thus may represent a unique adaptation of an antiapoptotic strategy to the onset of proliferative activation.
Materials and methods
Mice
To generate the A1-a transgene construct, primers were designed to create a polymerase chain reaction (PCR) product consisting of the A1-a open reading frame43 flanked on the 3′ side by aBamHI site and on the 5′ side by the 5′ untranslated region of the p60-SRC protein of Rous sarcoma virus, 5′-CCACTGTGGCCAGGCGGTAGCTGGGACGTGCAGCCGACCACC-3′, which supports efficient translation.48 This product was inserted into pCR II (Invitrogen, Carlsbad, CA). The sequence was checked and the insert excised with BamHI and placed in theBamHI site of the pW120 vector,49 which contains the mouse lck distal promoter region (nucleotides −4032 to +41) upstream of the BamHI site and a human growth hormone gene downstream. This segment of the lck promoter has been demonstrated to direct expression predominantly in T cells.49 The resulting construct was linearized and injected into FVB/N oocyte pronuclei. Oocyte injection and transfer were performed by the Albert Einstein College of Medicine Transgenic and Gene Targeting Core Facility. Chimeric progeny were mated to strain C57BL/6. Experiments were performed with mice obtained after 7 to 10 generations of backcross to C57BL/6. Bcl-2 transgenic mice in a C57BL/6 background28 were a kind gift from Dr Howard Petrie (Memorial Sloan-Kettering Cancer Center, New York, NY). These mice express transgenic Bcl-2 in both T- and B-cell lineages. All mice were maintained in a specific pathogen-free environment.
Cell culture
Thymi and spleens were removed aseptically and teased on a nylon mesh (70 μm, Becton Dickinson, Bedford MA). Thymocytes (1.25 × 106/mL) or splenocytes (2.5 × 106/mL) were cultured in 96-well plates in 0.2 mL/well Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS), 0.5 mM sodium pyruvate, penicillin plus streptomycin, 5 × 10−5 M 2-mercaptoethanol, and vitamins and nonessential amino acids as previously described.50 For induction of apoptosis, thymocytes were either γ-irradiated with a γ cell-40 irradiator (Atomic Energy of Canada, Ottawa, QC, Canada) using a 137Ce source at a dose rate of 80 cGy/min, or treated with αFas (1 μg/mL; clone Jo-2, BD Pharmingen, San Diego, CA) plus cycloheximide (10 μg/mL; Sigma, St Louis, MO), dexamethasone (20 nM; Sigma), or ionomycin (0.5 μg/mL; Sigma). Splenocytes were activated either with 4 μg/mL concanavalin A (Con A; Sigma) or with αCD3 plus αCD28 (each 10 μg/mL; BD Pharmingen). For experiments comparing the effects of A1-a and Bcl-2 on cell cycle, cells were also activated with plate-bound αCD3. Plates coated with αCD3 (clone 2C11) were obtained from Becton Dickinson. For restimulation experiments, splenocytes were activated with 5 μg/mL Con A for 3 days and then supplemented for 2 days with 100 U/mL recombinant human IL-2, a gift from Chiron (Emeryville, CA).51 T lymphoblasts were enriched by centrifugation over Histopaque (1.090 g/mL; Sigma) and cultured in either control or αCD3-coated plates. Purified splenic T cells were obtained by antibody-mediated depletion of non–T cells on a density gradient (SpinSep, Stem Cell Technologies, Vancouver, BC, Canada) according to the manufacturer's instructions. Using this method, 48% of starting splenic T cells were obtained as 99% pure T cells (purity determined by αCD3 staining).
RNA analysis
Cells were pelleted and total RNA extracted with Trizol (Life Technologies, Rockville, MD) according to the manufacturer's instructions, with the addition of an ethanol precipitation step after isopropanol precipitation. Northern blot analysis of A1 and Bcl-2 expression and normalization to 28S rRNA levels using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA) were as previously described.47 Reverse transcription–PCR (RT-PCR) analysis of A1 isoforms was performed according to the method of Hatekeyama et al.52 RNase protection analysis was performed on 1 μg total cellular RNA using the Riboquant system (BD Pharmingen) as previously described.53
Cell cycle, viability, and apoptosis assays
Total viable cells per well were measured by trypan blue exclusion. To determine thymocyte and splenocyte apoptosis and cell cycle status, cells were pelleted, washed in cold phosphate-buffered saline (PBS), and resuspended in a hypotonic propidium iodide (PI) solution (0.1% sodium citrate, 0.1% Triton X-100, 50 μg/mL PI) at 4°C for 4 hours in the dark prior to analysis by flow cytometry. Cell debris, doublets, and aggregates were gated out using a doublet discrimination module (Becton Dickinson). Apoptosis was measured as the percentage of cells with hypodiploid nuclei. The percentage of cycling cells (cells not in G1) was assessed with ModFit (Verity Software, Topsham, ME). To determine viable T-cell accumulation and T-cell apoptosis, splenocyte cultures were pelleted and washed with cold PBS, and 5 × 105 cells were incubated for 30 minutes on ice in 100 μL PBS containing 2% FCS and either 0.5 μg αCD3 (clone 2C11) or 0.1 μg αThy1.2 (clone 53-2.1; BD Pharmingen) conjugated with allophycocyanin. The cells were then washed with PBS plus 2% FCS and suspended in 100 μL 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.4), 140 mM NaCl, 5 mM CaCl2, (buffer A) containing 2 μg/mL PI and fluorescein-labeled annexin V (Roche Applied Science, Indianapolis, IN) at the manufacturer's recommended dilution. After 30 minutes an equal volume of buffer A was added and the samples were analyzed within 30 minutes on a FACSCalibur flow cytometer (Becton Dickinson). A viable lymphocyte gate was generated by forward and right angle scatter and by excluding PI-stained necrotic cells. The fraction of T cells within this gate was determined by assessing CD3+ cells or (for cultures activated with αCD3) Thy1.2+ cells. This fraction was then applied to the total viable splenocyte number determined by trypan blue exclusion to yield the absolute viable T-cell count. To measure the percentage of apoptotic T cells, total CD3+ or Thy1.2+ splenocytes were gated to exclude PI-stained necrotic cells and the annexin V–bright fraction determined.
Results
Induction of A1 mRNA during T-cell activation
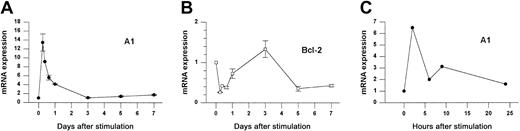
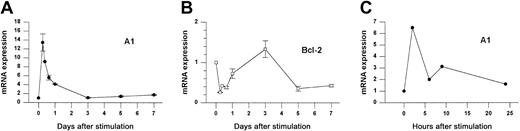
We investigated the expression of Bcl-2 family members following Con A activation of fresh splenocytes from naive animals. As shown in Figure 1A, A1 mRNA was increased 14-fold within 6 hours after stimulation and then rapidly returned to baseline levels by day 3. RT-PCR analysis (not shown) indicated that all A1 isoforms were induced, although the fold induction was greatest for the A1-a isoform. In contrast, Bcl-2 mRNA levels were repressed during the first day of activation but had begun to rebound by 24 hours (Figure1B). A similar induction was observed when purified splenic T cells were activated with αCD3 plus αCD28 (Figure 1C).
A1 and Bcl-2 gene expression in activated splenocytes.
(A-B) Splenocytes from wild-type mice were treated with Con A for the indicated times and gene expression was determined by Northern blot analysis normalized to 28S rRNA. Data from 3 similar experiments are pooled. Within each experiment values are relative to the value for unstimulated cells (set to 1). Each point represents the mean ± SE for 3 to 6 mice, except for 9 hours (n = 1) and 16 hours (n = 2). The total number of measurements is 30 for A1 and 25 for Bcl-2. (C) Purified splenic T cells were activated with αCD3 plus αCD28 for the indicated times and analyzed by the RNase protection assay (RPA). A1 expression is normalized to L32.
A1 and Bcl-2 gene expression in activated splenocytes.
(A-B) Splenocytes from wild-type mice were treated with Con A for the indicated times and gene expression was determined by Northern blot analysis normalized to 28S rRNA. Data from 3 similar experiments are pooled. Within each experiment values are relative to the value for unstimulated cells (set to 1). Each point represents the mean ± SE for 3 to 6 mice, except for 9 hours (n = 1) and 16 hours (n = 2). The total number of measurements is 30 for A1 and 25 for Bcl-2. (C) Purified splenic T cells were activated with αCD3 plus αCD28 for the indicated times and analyzed by the RNase protection assay (RPA). A1 expression is normalized to L32.
Overexpression of A1-a in T cells
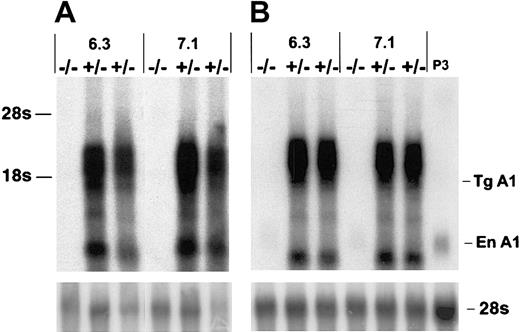
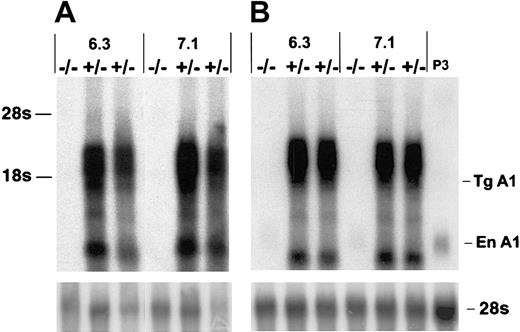
To investigate the potential role of A1 in T-cell survival we constructed A1-a transgenic (Tg) strains in which the distal lck promoter was used to direct expression to the T-cell lineage. Four independently generated A1-a Tg lines were analyzed for transgene mRNA expression in thymus and spleen. Three lines had similar high levels of expression; 2 of these lines, 6.3 and 7.1, were chosen for further investigation. As shown in Figure 2, the transgene produces multiple RNA species, several of which are clustered between 1.6 and 3.0 kb. The 1.6-kb band at the lower end of this cluster agrees with the predicted size of the fully spliced transgenic transcript, whereas 3.0 kb is the predicted size of the primary transcript. Similar clusters have been observed in previous studies using this vector and are likely due to alternative or incomplete splicing of the human growth hormone component of the transcript.49 54
Overexpression of A1-a in Tg mice.
Thymocyte (A) or splenocyte (B) RNA (4 μg/lane) from wild-type (−/−) or A1-a Tg (+/−) littermates was analyzed by hybridization of Northern blots with an A1-a cDNA probe. Two independent A1-a Tg lines, 6.3 and 7.1, are shown. P3 indicates RNA (4 μg) from the macrophagelike cell line P388D1, which constitutively expresses levels of A1 mRNA comparable to activated macrophages or T cells43; Tg A1, location of the predicted fully spliced transgenic transcript; En A1, endogenous A1 mRNA.
Overexpression of A1-a in Tg mice.
Thymocyte (A) or splenocyte (B) RNA (4 μg/lane) from wild-type (−/−) or A1-a Tg (+/−) littermates was analyzed by hybridization of Northern blots with an A1-a cDNA probe. Two independent A1-a Tg lines, 6.3 and 7.1, are shown. P3 indicates RNA (4 μg) from the macrophagelike cell line P388D1, which constitutively expresses levels of A1 mRNA comparable to activated macrophages or T cells43; Tg A1, location of the predicted fully spliced transgenic transcript; En A1, endogenous A1 mRNA.
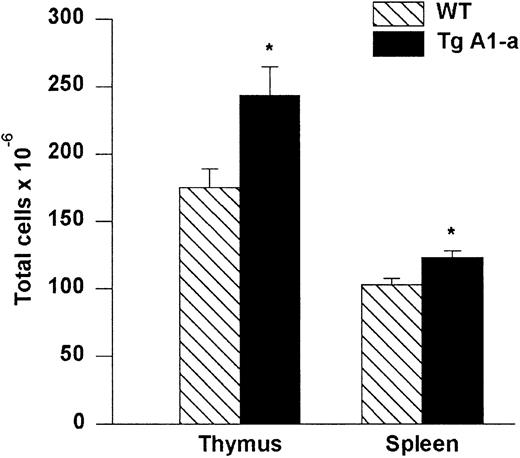
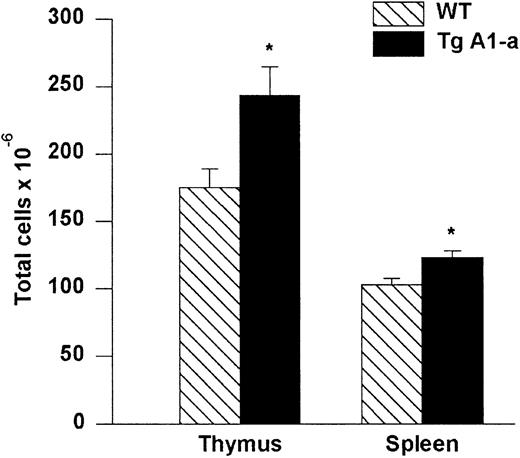
Increased cellularity of lymphoid organs in A1-a Tg mice
Thymi and spleens collected from A1-a Tg mice had greater cellularity than control littermates (Figure3). We did not observe a difference in the relative proportions of CD4 or CD8 single-positive or double-positive subsets in either thymus or spleen (data not shown).
Absolute cell numbers of A1-a Tg thymus and spleen.
Dissected thymi and spleens from wild-type (WT) and Tg littermates were meshed and cells in suspension were counted. The values represent mean ± SE of 9 thymi and 17 spleens. *P < .01 byt test.
Absolute cell numbers of A1-a Tg thymus and spleen.
Dissected thymi and spleens from wild-type (WT) and Tg littermates were meshed and cells in suspension were counted. The values represent mean ± SE of 9 thymi and 17 spleens. *P < .01 byt test.
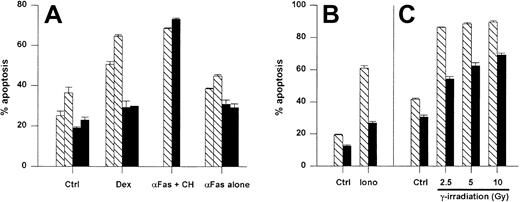
Resistance to spontaneous and induced cell death in A1 Tg mouse thymocytes
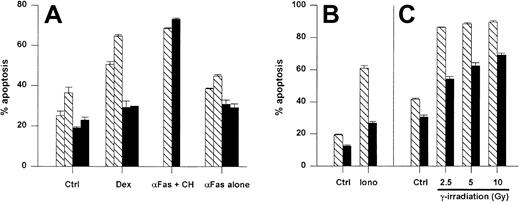
Initially, we examined the antiapoptotic activity of the A1-a transgene in thymocytes because this cell type has been used frequently to analyze the effects of Bcl-2 and Bcl-xL transgenes. Thymocytes overexpressing either Bcl-2 or Bcl-xL show reduced spontaneous apoptosis in culture as well as resistance to cell death induced by agents such as ionizing radiation, glucocorticoids, and ionomycin-induced cell death.28,55,56 As shown in Figure4, the A1-a transgene inhibited spontaneous thymocyte apoptosis. By day 7, all the wild-type thymocytes were dead, whereas 30% of the A1-a Tg cells remained viable (data not shown). An increase in the proportion of double-positive CD4+CD8+ thymocytes was noted by day 4 in the Tg cultures (data not shown). A1-a Tg thymocytes were partially protected from cell death induced by either dexamethasone, ionomycin, or γ irradiation (Figure 4A-C). In contrast, no protection was observed in cultures treated with αFas plus cycloheximide. In the absence of cycloheximide, a modest induction of apoptosis by αFas was reproducibly observed over 9 experiments, and a consistent small inhibition of this death was observed in A1-a Tg cells (Figure 4A). However, the degree of inhibition was no greater than the inhibition of spontaneous apoptosis, so that no conclusion could be drawn regarding a protective effect of the transgene. Overall, this pattern of antiapoptotic activity in thymocytes is similar to previous studies of Bcl-2 and Bcl-xL Tg cells.28 55
A1-a protects thymocytes from spontaneous and induced apoptosis.
Panels A-C represent separate experiments. Thymocytes from wild-type (WT) and A1-a Tg littermates were cultured for 1 day (A,C) or 16 hours (B) either in control medium (Ctrl) or in the presence of dexamethasone (Dex), αFas with or without cycloheximide (CH), or ionomycin (iono), or were irradiated prior to culture. Apoptosis was determined as percent hypodiploid nuclei by flow cytometry. Each bar represents the mean ± SEM for one mouse. The data are representative of 3 to 9 experiments. ▧ indicates WT; ▪, A1-a Tg.
A1-a protects thymocytes from spontaneous and induced apoptosis.
Panels A-C represent separate experiments. Thymocytes from wild-type (WT) and A1-a Tg littermates were cultured for 1 day (A,C) or 16 hours (B) either in control medium (Ctrl) or in the presence of dexamethasone (Dex), αFas with or without cycloheximide (CH), or ionomycin (iono), or were irradiated prior to culture. Apoptosis was determined as percent hypodiploid nuclei by flow cytometry. Each bar represents the mean ± SEM for one mouse. The data are representative of 3 to 9 experiments. ▧ indicates WT; ▪, A1-a Tg.
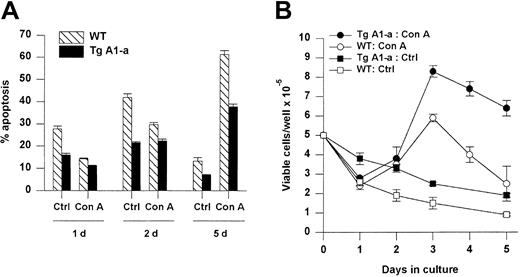
A1-a protects activated splenocytes from apoptosis
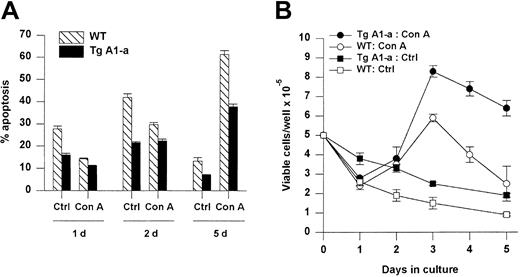
We next assessed the effect of Tg A1-a on the apoptosis of splenocytes either cultured in control medium or activated with the T cell–specific mitogen Con A (Figure 5). Tg A1-a reduced the frequency of spontaneous cell death in control cultures by about half. CD4+ and CD8+ subsets were protected to similar extents (data not shown). Wild-type cells treated with Con A showed significantly reduced apoptosis during the first day, corresponding to the period of endogenous A1 gene expression observed in Figure 1. The expression of the A1-a transgene produced a modest but significant degree of additional protection at day 1. Continued culture of wild-type cells in Con A (during which endogenous A1 gene expression returns to baseline [Figure 1]) led to sharply increased apoptosis. Tg A1-a remained protective throughout the culture, and by day 5 the Tg A1-a–induced inhibition of apoptosis had increased to 41%. This protection of activated cells is reflected in the accumulation of viable cells following activation (Figure 5B). A significant difference between control and A1 Tg viable cell number became apparent by day 3 after activation and this difference increased at later time points.
Effect of Tg A1-a on splenocyte apoptosis.
Splenocytes were cultured for the indicated times in either control medium (Ctrl) or with Con A. (A) Apoptosis was determined as percent hypodiploid nuclei. (B) Viable cell accumulation was measured by trypan blue exclusion. Values represent the mean ± SE for 3 mice. The data are representative of 7 similar experiments.
Effect of Tg A1-a on splenocyte apoptosis.
Splenocytes were cultured for the indicated times in either control medium (Ctrl) or with Con A. (A) Apoptosis was determined as percent hypodiploid nuclei. (B) Viable cell accumulation was measured by trypan blue exclusion. Values represent the mean ± SE for 3 mice. The data are representative of 7 similar experiments.
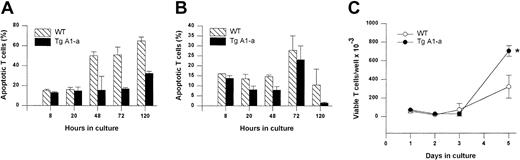
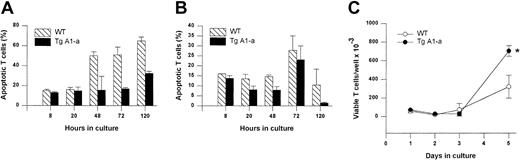
We next sought to confirm these results using a second apoptosis assay method (annexin binding) that permitted specific analysis of T cells. In this experiment, the splenocytes were activated with αCD3 plus αCD28 to verify that the effect of the A1-a transgene was not limited to mitogen-stimulated cells. Once again we observed that Tg A1-a inhibited apoptosis throughout the postactivation period, and that this inhibition was especially prominent at day 5, at which point a pronounced increase in T-cell yield became evident (Figure6).
Effect of Tg A1-a on apoptosis and accumulation of T cells activated via the TCR.
Splenocytes were cultured for the indicated times in (A) control medium or (B-C) with αCD3 plus αCD28. (A-B) T-cell apoptosis was determined by staining with αThy1.2 and annexin. (C) Viable T-cell number was assessed by trypan blue exclusion. *P < .05 by Student t test.
Effect of Tg A1-a on apoptosis and accumulation of T cells activated via the TCR.
Splenocytes were cultured for the indicated times in (A) control medium or (B-C) with αCD3 plus αCD28. (A-B) T-cell apoptosis was determined by staining with αThy1.2 and annexin. (C) Viable T-cell number was assessed by trypan blue exclusion. *P < .05 by Student t test.
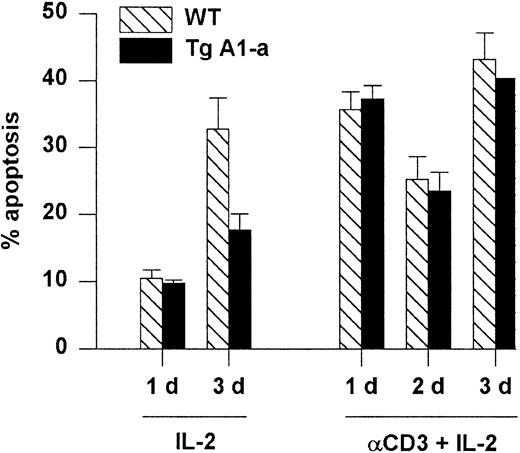
A1-a does not protect T cells from death induced by restimulation of the TCR
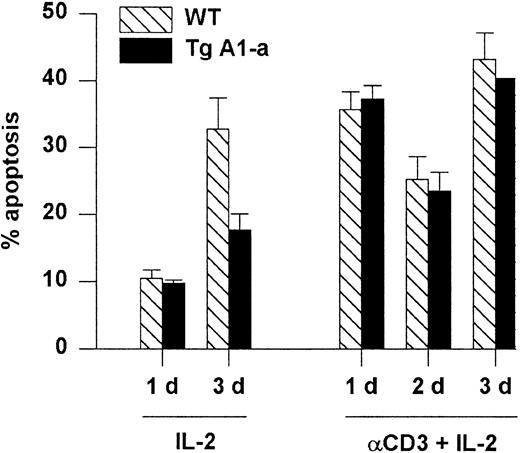
Apoptosis associated with the initial activation of naive T cells is thought to occur via a Fas-independent mechanism.6 In contrast, restimulation of previously activated cells leads to Fas-dependent, activation-induced cell death (AICD) that is sensitized by previous exposure to IL-2.57 We asked whether the A1-a transgene would protect splenocytes from AICD. Activated splenocytes obtained by stimulation with Con A followed by culture in IL-2 were reactivated with plate-bound αCD3 plus IL-2. Reactivated cells entered cell cycle (data not shown) and showed elevated apoptosis compared with activated cells cultured with IL-2 alone (Figure7). However, this cell death was not prevented by the transgene. In contrast, the cells cultured with IL-2 alone showed elevated apoptosis by day 3 (presumably due to IL-2 depletion) that was effectively inhibited by the transgene. The inability of A1 to prevent AICD resembles the previously demonstrated ineffectiveness of transgenically overexpressed Bcl-2 in this regard.29
Tg A1-a does not protect against reactivation-induced AICD.
Following primary activation with Con A and IL-2, splenocytes were cultured in the presence of IL-2 either on control or αCD3-coated plates. Apoptosis was measured as percent hypodiploid nuclei. Values represent the mean ± SE for 3 mice. At 1 day after reactivation the mean percentage of T cells in the cultures (measured as the percentage of PI-excluding cells expressing either CD4 or CD8) was 86% (range, 83%-89%). The data are representative of 3 similar experiments.
Tg A1-a does not protect against reactivation-induced AICD.
Following primary activation with Con A and IL-2, splenocytes were cultured in the presence of IL-2 either on control or αCD3-coated plates. Apoptosis was measured as percent hypodiploid nuclei. Values represent the mean ± SE for 3 mice. At 1 day after reactivation the mean percentage of T cells in the cultures (measured as the percentage of PI-excluding cells expressing either CD4 or CD8) was 86% (range, 83%-89%). The data are representative of 3 similar experiments.
Overexpression of A1, unlike Bcl-2, does not inhibit the proliferation of activated T cells
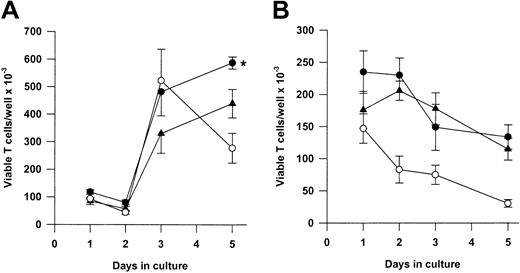
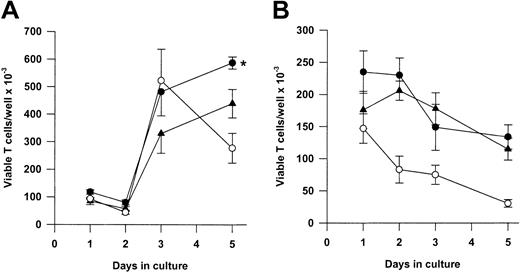
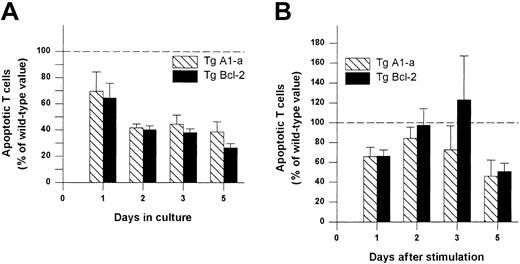
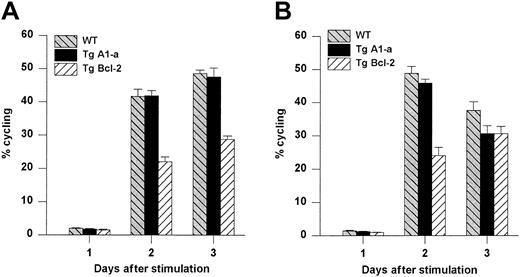
We next asked whether Tg A1-a and Tg Bcl-2 were comparable in their ability to support the survival and growth of activated T cells. As shown in Figure 8A, viable T-cell accumulation at day 5 after Con A treatment was increased 93% by the A1-a transgene, but only 49% by the Bcl-2 transgene. Similar differences between Tg A1-a and Tg Bcl-2 splenocyte accumulation were observed in 2 additional experiments in which unfractionated cells were assessed (data not shown). These results might reflect differential effects of the 2 transgenes on either T-cell survival or proliferation. As shown in Figure 9, the overall effectiveness of Tg Bcl-2 as an antiapoptotic agent in either resting or activated T cells was similar to that of Tg A1-a. Similarly, there was no difference between the 2 transgenes with respect to viable T-cell accumulation in unstimulated cultures (Figure 8B). Therefore, it is unlikely that the differential effect of the transgenes on T-cell accumulation is due to either a difference in transgene expression or a general difference in the apoptotic mechanisms affected. One possibility is that a specific apoptotic mechanism generated after activation is inhibited more efficiently by Tg A1-a than by Tg Bcl-2. The variability of the data in Figure 9B do not permit a definitive answer to this question. A second possibility is that the transgenes differentially affect T-cell proliferation. It has been shown that transgenic overexpression of Bcl-2 in T cells can delay cell cycle progression following activation.27,58 59 Figure10 confirms this finding. In contrast, cell cycle progression was not affected by Tg A1-a after activation with either Con A or plate-bound αCD3.
Comparative effectiveness of Tg A1-a and Tg Bcl-2 in supporting viable T-cell accumulation.
Splenocytes were cultured either with (A) Con A or (B) control medium. Viable T cells were measured as described in “Materials and methods.” The data represent the mean ± SE for 4 mice. In graphs, ● indicates Tg A1-a, ▴ indicates Tg Bcl-2, and ○ indicates WT. The asterisk indicates significant difference between Tg Bcl-2 and Tg A1-a (P < .05 by Student ttest).
Comparative effectiveness of Tg A1-a and Tg Bcl-2 in supporting viable T-cell accumulation.
Splenocytes were cultured either with (A) Con A or (B) control medium. Viable T cells were measured as described in “Materials and methods.” The data represent the mean ± SE for 4 mice. In graphs, ● indicates Tg A1-a, ▴ indicates Tg Bcl-2, and ○ indicates WT. The asterisk indicates significant difference between Tg Bcl-2 and Tg A1-a (P < .05 by Student ttest).
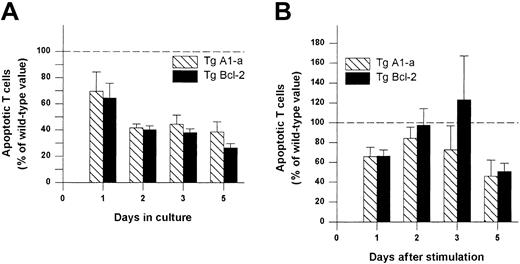
Comparative effectiveness of Tg A1-a and Tg Bcl-2 in inhibition of T cell apoptosis.
Splenocytes were cultured in (A) control medium or (B) with Con A. The cultures were the same as those used for Figure 8. The percentage of apoptotic (annexin-binding) CD3+ T cells was determined and the value for wild-type mice at each time point was set to 100 (dashed line). The data represent the mean ± SE for 4 mice.
Comparative effectiveness of Tg A1-a and Tg Bcl-2 in inhibition of T cell apoptosis.
Splenocytes were cultured in (A) control medium or (B) with Con A. The cultures were the same as those used for Figure 8. The percentage of apoptotic (annexin-binding) CD3+ T cells was determined and the value for wild-type mice at each time point was set to 100 (dashed line). The data represent the mean ± SE for 4 mice.
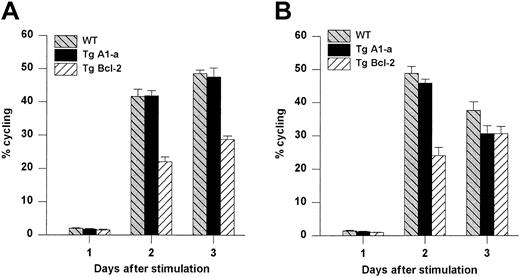
Comparative effects of Tg A1-a and Tg Bcl-2 on cell cycle progression.
Splenocytes were activated with either (A) Con A or (B) plate-bound αCD3. The cultures were the same as those used for Figure 8. The percentage of cycling cells (cells not in G1) was determined by flow cytometry. The data represent the mean ± SE for 4 mice. For Con A stimulation, the data are representative of 7 similar experiments for Tg A1-a and 4 similar experiments for Tg Bcl-2. For αCD3 stimulation, the data are representative of 3 similar experiments for Tg A1-a and 2 similar experiments for Tg Bcl-2.
Comparative effects of Tg A1-a and Tg Bcl-2 on cell cycle progression.
Splenocytes were activated with either (A) Con A or (B) plate-bound αCD3. The cultures were the same as those used for Figure 8. The percentage of cycling cells (cells not in G1) was determined by flow cytometry. The data represent the mean ± SE for 4 mice. For Con A stimulation, the data are representative of 7 similar experiments for Tg A1-a and 4 similar experiments for Tg Bcl-2. For αCD3 stimulation, the data are representative of 3 similar experiments for Tg A1-a and 2 similar experiments for Tg Bcl-2.
Discussion
Evidence from several model systems of T-cell activation supports the notion that the activated cell moves through an early period of death resistance followed by a period of increased susceptibility to apoptosis. This window of resistance might reflect either a delay in the onset of proapoptotic signals or the temporary provision of an antiapoptotic mechanism. The latter model is supported by recent studies showing early up-regulation of proapoptotic factors (Fas, FasL, Bad, Bax, Bim) in activated cells,23,24 as well as by several studies demonstrating induction of Bcl-xL expression within 1 day of activation.6,7,40 Our results lend further support to this view by showing that an early event after activation is a strong, transient increase in A1 gene expression. A caveat is that this expression has not been verified at the protein level, and recent studies have found discordance between mRNA and protein expression for Bcl-231,60-62 as well as for several proapoptotic family members.63,64 These results may be related to the relative stability of these proteins61,63,65; in contrast, A1 has been reported to have a short half-life65 consistent with its pattern of acute regulation. Our findings suggest that A1, perhaps in concert with Bcl-xL and other unknown factors, may act to generate the death-resistant window, and also that the subsequent down-regulation of A1 may contribute to a stage of increased postactivation apoptosis.
To investigate these ideas further, we generated strains of transgenic mice in which the A1-a isoform of A1 is overexpressed in T cells. The A1-a transgene was strongly expressed in both thymocytes and splenocytes and provided protection from apoptosis to quiescent cells from both tissues, similar to previous studies of the effects of transgenic Bcl-2 and Bcl-xL 2.7 55 Tg A1-a and Tg Bcl-2 were quantitatively comparable with respect to protection of resting splenic T cells from spontaneous apoptosis in culture. After Con A activation, however, the yield of viable T cells was significantly greater with Tg A1-a than with Tg Bcl-2. We were able to relate this finding to the fact that Tg A1-a had no effect on the cell cycle status of T cells activated with either Con A or αCD3, whereas Tg Bcl-2 reduced the proliferative index by about half 48 hours after either stimulus.
These results suggest a model in which activated T cells progress through successive stages that are characterized by distinct antiapoptotic mechanisms. In particular, in view of the evidence that during the first day after activation T cells move through cell cycle transition points that are sensitive to Bcl-2,27,58 it is logical to hypothesize that at this stage Bcl-2 is repressed and A1 activated. A caveat to this idea is that alterations of the T-cell Bcl-2 mRNA level do not always correspond to changes in protein expression,40 perhaps due to the relatively long half-life of Bcl-2 protein compared with A1.65 However, a study of Bcl-2 protein expression in human peripheral blood lymphocytes found evidence of reduced expression during the first day after activation.31 In addition, it is possible that Bcl-2 repression may include both a transcriptional and a posttranslational component, because a recent report indicates that the cell cycle inhibitory effects of Bcl-2 protein can be specifically blocked by phosphorylation mediated by cyclin-dependent kinases.66 In any case, the postulated repression of Bcl-2 cannot be complete because removal of endogenous Bcl-2 was shown to accelerate entry into S phase in αCD3-activated splenocytes.27 In the latter study, an inhibitory effect of endogenous Bcl-2 on IL-2 gene transcription was evident as early as 1 hour after activation, suggesting that even if our model is correct, the “A1 for Bcl-2” switch may not occur rapidly enough to prevent a degree of inhibition.
Our model suggests that after the first day of activation, A1 may be replaced by other survival regulators, such as Bcl-2 or Bcl-xL. This transition might be mediated by IL-2, which can both induce Bcl-2 and down-regulate A1 gene expression.30,32 The antiproliferative effects of Bcl-2 and Bcl-xL59 might be minimized once the sensitive stage of the initial cell cycle has been traversed. At least in the case of Bcl-2 this seems likely, because the sensitive control point appears to be IL-2 transcription mediated by nuclear factor of activated T cells (NFAT),27 and this transcription subsequently becomes NFAT independent.67 Bcl-xL, however, is induced during the first day of activation,6,7 40 and the mechanism of its cell cycle inhibitory effects have been less well characterized. It would be helpful to compare the kinetics of induction of the A1 and Bcl-xL proteins. This analysis has been prevented to date by the insensitivity of A1 protein assays.
The notion that after the first day other Bcl-2 family members replace A1 might seem to be in conflict with our suggestion that the decline in endogenous A1 expression contributes to the acceleration of postactivation apoptosis. However, Bcl-2 protein expression is highly heterogeneous for several days after splenocyte activation,30 and thus may be protective of only a selected subset of activated cells. It will be of interest to determine whether activation-induced A1 protein expression is comparatively more homogeneous. Alternatively, A1 may function to counteract certain proapoptotic signals generated during activation and against which Bcl-2 is less effective. In this case, the down-regulation of endogenous A1 may be essential to permit apoptosis necessary for the focusing or resolution of the immune response, or for the elimination of self-reactive cells. Comparative studies of the apoptotic pathways affected by Tg A1-a and Tg Bcl-2 may address this issue.
Relatively few studies have indicated functional distinctions among antiapoptotic Bcl-2 family members, particularly with regard to functions other than cell survival. We previously reported that the spontaneous granulocytic differentiation of 32D cells was inhibited by Bcl-2 but not by A1.68 The biochemical basis for a functional disparity between these 2 molecules is unknown. The N-terminal BH4 region of Bcl-2 has been shown to specifically mediate the antiproliferative rather than the antiapoptotic effects of the protein.69 Interestingly, mouse A1 lacks a BH4 region and shows no sequence similarity to other family members in this region.
In vivo studies indicate that the postactivation apoptosis of previously naive T cells occurs as a highly regulated physiologic process dependent on cell division and IL-2 signaling.21,22 A recent study indicated that CD4+ splenocytes activated in vitro also undergo apoptosis via a mechanism that is “metered” by cell division.70The immunologic properties, if any, that distinguish apoptotic and surviving cells at this juncture are unknown. Consequently, it is as yet unclear whether this phenomenon represents a general homeostatic dampening of the activated population or a more specific tailoring of the immune response. A study of the retention of adoptively transferred TCR-Tg T cells after immunization showed enhanced retention of Tg Bcl-2 donor cells, indicating that postactivation apoptosis in vivo is susceptible to modulation by Bcl-2 family members.18 A comparative analysis of the effects of Bcl-2 family member transgenes on T-cell kinetics in vivo should help to evaluate the hypotheses generated by the current study.
We thank Lirong Wan for maintenance of the Tg A1-a mouse colony and assistance with RNA analysis. We thank Nicole Kawachi for technical assistance and Edmond Kloscewski of the Albert Einstein College of Medicine FACS Facility for guidance in flow cytometric analysis.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-04-1229.
Supported by funds from National Institutes of Health grant AI-43401 (M.B.P.).
J.G. and A.O. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Amos Orlofsky, Albert Einstein College of Medicine, Department of Pathology, F717N, 1300 Morris Park Ave, Bronx, NY 10461; e-mail: orlofsky@aecom.yu.edu.