Chronic autoimmune thrombocytopenic purpura (AITP) is characterized by platelet-specific autoantibody production that is influenced by enhanced antiplatelet T-helper cell reactivity. Costimulatory signals are absolutely required for T-cell activation and play key roles in the decision between tolerance and immunity. In this study we cultured T cells isolated from patients with chronic AITP to investigate the effects of the B7-blocking agent cytologic T-lymphocyte–associated antigen 4-immunoglobulin (CTLA4-Ig), and cyclosporin A (CsA), alone or in combination, on induction of platelet-specific T-cell anergy. The data showed that in most cases CTLA4-Ig and/or CsA could induce tolerance toward platelet antigens based on anergy. It could be overcome by stimulation with unrelated antigens, demonstrating its platelet specificity. The anergy is associated with lack of interleukin 2 (IL-2) and withheld by exogenous IL-2, emphasizing the pivotal role of IL-2 suppression in the induction of platelet-specific anergy. We also prospectively evaluated the efficacy of CsA therapy in patients with refractory AITP and observed that the response to CsA treatment in vivo was associated with the inhibiting sensitivity of platelet-reactive T cells to CsA in vitro. This suggests that the sensitivity of T cells to CsA in vitro could serve as a reliable parameter in predicting the efficacy of CsA for patients with refractory AITP. CTLA4-Ig may become a promising new therapeutic agent for the treatment of chronic AITP, and the combination of CTLA4-Ig and CsA would be a more powerful strategy for the management of refractory AITP.
Introduction
Chronic autoimmune thrombocytopenic purpura (AITP) is an autoimmune disorder characterized by antiplatelet autoantibody-mediated platelet destruction. Autoantibody production in AITP is associated with both T-cell activation and T-B cognate interaction.1-4 Patients with AITP have enhanced T-cell reactivity against platelets that is measurable at both the bulk polyclonal T-cell level5,6 and at the clonal level.7,8 Optimal T-cell activation requires 2 signals. The first signal is based on T-cell receptor (TCR) recognition of peptide/major histocompatibility complex (MHC) complexes. The second signal is provided by interaction of CD28, CD2, and leukocyte factor antigen-1 (LFA-1) on T cells with their costimulatory ligands B7 (CD80 and CD86), LFA-3, and intercellular adhesion molecule-1 (ICAM-1), respectively, on antigen-presenting cells (APCs). Because the CD28 costimulatory pathway does not depend on intracellular calcium, the B7-CD28/CTLA-4 (cytologic T-lymphocyte–associated antigen 4) interaction is considered to provide the main signal that confers cyclosporin A (CsA)–resistant interleukin 2 (IL-2) production, whereas costimulation through other accessory molecules such as CD2 is calcium dependent and thus could be blocked by CsA.9-11
B7 costimulation is involved not only in the induction stage of autoimmune response but also in the effector activation of autoreactive T cells for propagating autoimmune disease.12-16 Semple et al6 has observed an enhanced expression of CD80 on platelets from patients with AITP, implicating a role of B7/CD28 costimulation in the pathogenesis of AITP.6 T-cell response(s) could be inhibited by administration of the cytotoxic T-lymphocyte–associated antigen 4-immunoglobulin (CTLA4-Ig), a fusion protein comprising the extracellular domain of CTLA-4 and the Fc portion of human IgG1 region that binds to both CD80 and CD86. Multiple studies have demonstrated that blocking B7/CD28 interaction results in prevention and amelioration of autoimmune diseases in a number of animal models such as lupus, encephalomyelitis, thyroiditis, myasthenia gravis, arthritis, and nonobese diabetes, as well as in patients with psoriasis in phase 1 and phase 2 studies.12-21(Bristol-Myers has completed phase 2 clinical trials of the experimental drug CTLA4-Ig in rheumatoid arthritis and has completed phase 1 safety trials in psoriasis and multiple sclerosis.) In particular, Zhang et al9 and Van Gool et al22 reported that CTLA4-Ig, when synergized with CsA, is highly effective in inducing anergy to alloantigens. Therefore, one purpose of our study was to investigate the effects of the B7-blocking agent CTLA4-Ig, and/or CsA on induction of anergy of platelet-reactive T cells in chronic AITP.
Several studies have reported the beneficial effects of CsA in refractory patients.23-25 Although such therapy is successful in some refractory cases, its effect remains inconsistent. It would be helpful to identify laboratory markers that are useful in predicting the efficacy of CsA therapy. Hence, another purpose of our study was to prospectively evaluate the effects of CsA treatment on patients with refractory AITP and identify whether the efficacy of CsA in vivo is correlated with the sensitivity of T cells to CsA inhibition in vitro.
Patients, materials, and methods
Patients and control volunteers
Thirty-six newly diagnosed patients (26 women, 10 men; mean age, 36 years; range, 12-51 years) in the active phase of chronic AITP (platelet counts ≤ 100 × 109/L) were studied. The diagnosis of chronic AITP was based on the previously described criteria.6 These criteria are thrombocytopenia for more than 6 months, increased bone marrow megakaryocytes, absence of splenomegaly, and no secondary immune or nonimmune abnormality that could account for the thrombocytopenia. All patients were evaluated prior to the commencement of specific therapy. Nine patients have not received any specific treatments because they have no clinically significant bleeding symptoms with a platelet count more than 30 to 50 × 109/L, in accordance with the practice guideline.26 The remaining 27 patients with bleeding symptoms and/or platelet count less than 30 × 109/L were treated with prednisone, splenectomy, and/or various other treatment modalities. Eleven patients refractory to prednisone and splenectomy were randomly selected and treated with CsA. Among them, 3 were also refractory to vincristine, 3 to danazol, 2 to cyclophosphamide, and 2 to intravenous immunoglobulin. Platelet counts before CsA treatment were 15.5 to 27 × 109/L. CsA was given orally at an initial dose of 5 mg/kg per day, taken twice daily for 1 week, then adjusted to 3 to 12 mg/kg per day to maintain a serum CsA level between 200 and 400 ng/mL. If complete or partial response was achieved within 12 weeks, CsA was continued but no longer than 6 months. If there had been no response by 12 weeks, CsA was stopped.
Fifteen healthy volunteers (5 men, 10 women) between 18 and 35 years (mean, 24 years) were examined as control subjects. Their platelet counts ranged from 150 to 320 × 109/L.
Signed and informed consent approved by the medical ethical committee of Qilu Hospital of Shangdong University was granted by all study participants.
We used the following response criteria27: complete response (CR), therapy resulted in a platelet count above 100 × 109/L; partial response (PR), therapy resulted in a platelet count above 50 × 109/L; no response (NR), platelet count remained below 50 × 109/L after therapy.
Cell isolation
Peripheral blood mononuclear cells (PBMCs) were obtained from heparinized blood from 36 patients with chronic AITP and 16 healthy control subjects by Ficoll-hypaque (Pharmacia Biotech, Beijing, China) gradient centrifugation. T cells were prepared by double-rosetting of PBMCs with 2-aminoethylosothiouronium bromide-treated sheep red blood cells (SRBCs). After counting, T cells were resuspended in RPMI 1640 medium (Gibco, China) containing 10% pooled inert human group AB serum, 2 mM l-glutamine (Gibco, China), 100 μg/mL penicillin/streptomycin, and 5 × 105 M 2-mercaptoethanol. Fresh autologous PBMCs were irradiated (4000 rad) and used as APCs as described by Kuwana et al2 and Ware and Howard.7
Preparation of platelets
Acid-treated platelets were prepared as described by Semple and Freedman,5 ie, EDTA (ethylenediaminetetraacetic acid)–treated blood from healthy individuals was centrifuged at 150g for 15 minutes at 20°C, and the platelet-rich plasma (PRP) was recovered. The PRP was then centrifuged at 550gfor 6 minutes at 20°C, and the platelets were washed twice in normal saline. Platelet HLA class I immunogenicity was abolished by acid dissociation (pH, 3.0) at 4°C for 15 minutes to reduce possible class I alloreactivity in the PBMC cultures.
In vitro antiplatelet reactivity
For the primary culture, proliferative responses by T cells were performed by the modified methods of Semple and Freedman5and Zhang et al.9 Briefly, T cells (1 ×106 cells/mL) and enriched APCs (0.2 × 106 cells/mL) were cultured with acid-treated normal platelets (100 × 106 cells/mL) in the presence or the absence of CTLA4-Ig (10 μg/mL; gift from Dr Yiqiang Zhao, Ohio University, Athens) and/or CsA (400 ng/mL; Sandoz Pharmaceuticals, Switzerland) in 96-well plates (200-μL volumes) or 24-well culture plates (1-mL volumes). After 6 days of incubation, 0.037 MBq (1 μCi) [3H]thymidine (Beijing Atomic Energy Research Institute, Beijing, China)/well was added to the 96-well plates, the plates were incubated for an additional 24 hours at 37°C, and the cells were harvested and counted for incorporated radioactivity. For the secondary culture, the cells from the 24-well plates were washed, resuspended in culture medium, and rested for 2 days. Subsequently, the cells were resuspended in culture medium and restimulated with original or unrelated antigens (tuberculin, 20 U/mL; gift from Dr Yiqiang Zhao, Ohio University) in the presence of fresh autologous APCs without blocking agents. T-cell activation was assayed by proliferation and by IL-2 production. For proliferation studies, cells were incubated in 96-well plates (2 × 105precultured cells with 0.4 × 105 autologous APCs in a 200-μL final volume). The cells were incubated at 37°C with 5% CO2 for different time periods. During the last 8 hours of incubation, the cells were pulsed with 0.037 MBq (1 μCi) [3H]thymidine/well. For IL-2 production, 1 × 106 cells in 1 mL culture medium were incubated in the presence of 2 μg/mL anti-Tac monoclonal antibody (McAb; Jingmei Biotech, Shenzhen, China) in 24-well plates to block IL-2 consumption. Culture supernatants were collected after antigen rechallenging for 3 days, and the production of IL-2 was tested by stimulation of proliferation of the IL-2–dependent cell line CTLL as described by Semple and Freedman.5
Statistical analysis
The Student t test of paired samples was used to compare the differences of proliferation and IL-2 production for differentially treated samples from patients with chronic AITP. The significance between patient and healthy control groups was determined by the Student t test for analysis of completely randomized 2-group design. The CsA sensitivity in vitro and CsA response in vivo in patients with refractory AITP was analyzed using exact probabilities in 2 × 2 tables, as appropriate. P ≤ .05 was considered significant.
Results
Inhibition of platelet-induced T-cell proliferation by CTLA4-Ig and/or CsA in primary culture
As shown in Figure 1, T cells derived from healthy individuals did not show vigorous proliferation after being incubated with acid-treated platelets and APCs for 7 days. T cells from patients with chronic AITP showed very high reactivity (P ≤ .001). T-cell proliferation was inhibited by CTLA4-Ig (10 μg/mL) or CsA (400 ng/mL) (P ≤ .01 and .02, respectively), and the inhibiting effect was synergistically enhanced by the combination of CTLA4-Ig and CsA (P ≤ .001).
Effects of CTLA4-Ig and/or CsA on platelet Ag–induced T-cell proliferation in primary culture.
T cells (1 × 106/mL) from 36 patients with chronic AITP (▪) or 15 healthy control subjects (■) were cultured with acid-treated platelets and APCs for 7 days in the presence or the absence of CTLA4-Ig (10 μg/mL) and/or CsA (400 ng/mL). Proliferation was measured by [3H]thymidine incorporation and shown as mean ± SD cpm.
Effects of CTLA4-Ig and/or CsA on platelet Ag–induced T-cell proliferation in primary culture.
T cells (1 × 106/mL) from 36 patients with chronic AITP (▪) or 15 healthy control subjects (■) were cultured with acid-treated platelets and APCs for 7 days in the presence or the absence of CTLA4-Ig (10 μg/mL) and/or CsA (400 ng/mL). Proliferation was measured by [3H]thymidine incorporation and shown as mean ± SD cpm.
Tolerance induction on rechallenge of platelet antigens (Ags)
After the primary stimulation for 7 days, T cells were washed and rested for 2 days and then restimulated with platelets in the presence of autologous APCs. To determine the optimal time point for T-cell proliferative responses during the secondary stimulation, we analyzed 3 randomly selected patients and found that T-cell responses to platelet Ags showed a similar time course and peaked at day 4 (Figure2A). Measurement of T-cell proliferation following secondary stimulation of all patients (n = 36) showed that, for most patients with chronic AITP, CTLA4-Ig (P ≤ .01) or CsA (P ≤ .05) alone drove T cells into a state of tolerance when restimulated with platelets (Figure 2B). Moreover, the combined use of CTLA4-Ig and CsA resulted in platelet-reactive T-cell tolerance in all patients (P ≤ .001).
Induction of T-cell tolerance to platelet Ags for patients with chronic AITP.
T cells (1 × 106/mL) from 36 patients were incubated with acid-treated platelets and APCs in the presence of CTLA4-Ig (10 μg/mL) and/or CsA (400 ng/mL). After 7 days of incubation the cells were washed and rested for 2 days (primary culture), and then rechallenged with acid-treated platelets in the presence of APCs but without CTLA4-Ig or CsA (secondary culture) (A) for different culture periods in the first 3 patients to determine the kinetics of T-cell proliferation during secondary stimulation, and (B) for 4 days in all 36 patients to define the effects of CTLA4-Ig and/or CsA on the induction of T-cell tolerance. Proliferation was measured by incorporation of [3H]thymidine and shown as mean ± SD cpm.
Induction of T-cell tolerance to platelet Ags for patients with chronic AITP.
T cells (1 × 106/mL) from 36 patients were incubated with acid-treated platelets and APCs in the presence of CTLA4-Ig (10 μg/mL) and/or CsA (400 ng/mL). After 7 days of incubation the cells were washed and rested for 2 days (primary culture), and then rechallenged with acid-treated platelets in the presence of APCs but without CTLA4-Ig or CsA (secondary culture) (A) for different culture periods in the first 3 patients to determine the kinetics of T-cell proliferation during secondary stimulation, and (B) for 4 days in all 36 patients to define the effects of CTLA4-Ig and/or CsA on the induction of T-cell tolerance. Proliferation was measured by incorporation of [3H]thymidine and shown as mean ± SD cpm.
Tolerance to platelet Ags was based on anergy rather than clonal deletion
To determine whether the tolerance to platelet Ags could be the result of anergy or clonal deletion, we rendered the T cells from 16 patients tolerant to platelet Ags with CTLA4-Ig and/or CsA as described in “Tolerance induction on rechallenge of platelet antigens (Ags).” The cells were washed and rested for 2 days, and then rechallenged with platelet Ags and autologous APCs in the presence or the absence of IL-2 (10 U/mL) during the secondary culture. The presence of IL-2 during restimulation with original Ags and autologous APCs reversed the tolerance of T cells induced by CTLA4-Ig (P ≤ .001) and CsA (P ≤ .001) alone, or in combination with CsA (P ≤ .001; Figure3). Therefore, the tolerant state in this in vitro model was due to anergy induction rather than to deletion.
IL-2 reversed the T-cell tolerance to platelet Ags induced by CTLA4-Ig and/or CsA.
Following the same procedure for primary culture as that described in Figure 2, the T cells from 16 patients rendered tolerant to platelet Ags by CTLA4-Ig and/or CsA were rechallenged with platelet Ags and APCs in the presence (▪) or the absence (■) of IL-2 (10 U/mL) in the secondary culture. Proliferation was determined by [3H]thymidine incorporation on day 4 and shown as mean ± SD cpm.
IL-2 reversed the T-cell tolerance to platelet Ags induced by CTLA4-Ig and/or CsA.
Following the same procedure for primary culture as that described in Figure 2, the T cells from 16 patients rendered tolerant to platelet Ags by CTLA4-Ig and/or CsA were rechallenged with platelet Ags and APCs in the presence (▪) or the absence (■) of IL-2 (10 U/mL) in the secondary culture. Proliferation was determined by [3H]thymidine incorporation on day 4 and shown as mean ± SD cpm.
T-cell anergy induced by CTLA4-Ig and CsA, alone or in combination, was platelet specific
To confirm the specificity of the T-cell anergy to platelet Ags, T cells from 12 patients who had a history of Mycobacterium tuberculosis bacille Calmette-Guérin (BCG) vaccination(s) and were positive for tuberculin purified protein derivative (PPD), were pretreated with CTLA4-Ig and/or CsA as described in “Tolerance to platelet Ags was based on anergy rather than clonal deletion.” T cells treated with platelet Ags in the presence of CTLA4-Ig and CsA, alone or combined in the primary culture, displayed T-cell proliferation when restimulated with third-party stimulators (P ≤ .001 for all 3 groups; Figure4). No effect was seen when challenged with unrelated antigens (tuberculin 200 U/mL). This finding indicates that only platelet-specific T cells were anergic.
Specificity of T-cell anergy to platelet Ags induced by CTLA4-Ig and/or CsA.
Following the same procedure for first culture as that described in Figure 2, the T cells rendered tolerant to platelet Ags were rechallenged with the original (■) or unrelated Ags (tuberculin, 20 U/mL; ▪) in the secondary culture in 12 patients. After 4 days, proliferative response was measured by [3H]thymidine incorporation and shown as mean ± SD cpm.
Specificity of T-cell anergy to platelet Ags induced by CTLA4-Ig and/or CsA.
Following the same procedure for first culture as that described in Figure 2, the T cells rendered tolerant to platelet Ags were rechallenged with the original (■) or unrelated Ags (tuberculin, 20 U/mL; ▪) in the secondary culture in 12 patients. After 4 days, proliferative response was measured by [3H]thymidine incorporation and shown as mean ± SD cpm.
Role of IL-2 in platelet-specific anergy induced by CTLA4-Ig, CsA, or their combination
To disclose whether the CTLA4-Ig and/or CsA-induced T-cell anergy to platelet Ags was correlated with the inhibition of IL-2 production, we have also measured the IL-2 level after platelet rechallenge in 20 patients. As shown in Figure 5, either CTLA4-Ig or CsA could block IL-2 production on rechallenge for most patients (P ≤ .01 in each of the 2 Student ttests of paired samples). When CTLA4-Ig and CsA were combined, this combination resulted in almost a complete block of IL-2 production in all patients (P ≤ .001). This finding suggests that lack of IL-2 might be responsible for the anergy induction.
Effects of CTLA4-Ig and/or CsA on IL-2 production on platelet Ag rechallenge.
Following the same procedure for first culture as that described in Figure 2, the T cells (1 × 106/mL) from 20 patients were rechallenged with the original Ags. Anti-Tac McAb (2 μg/mL) was added to the medium to block IL-2 consumption during the secondary culture. The supernatants were collected after 3 days, and IL-2 was measured by a bioassay on cytotoxic T-lymphocyte line (CTLL).
Effects of CTLA4-Ig and/or CsA on IL-2 production on platelet Ag rechallenge.
Following the same procedure for first culture as that described in Figure 2, the T cells (1 × 106/mL) from 20 patients were rechallenged with the original Ags. Anti-Tac McAb (2 μg/mL) was added to the medium to block IL-2 consumption during the secondary culture. The supernatants were collected after 3 days, and IL-2 was measured by a bioassay on cytotoxic T-lymphocyte line (CTLL).
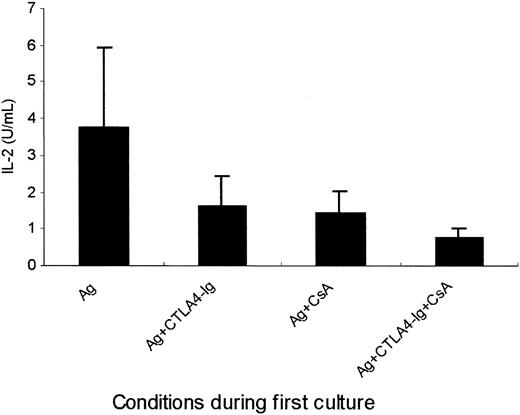
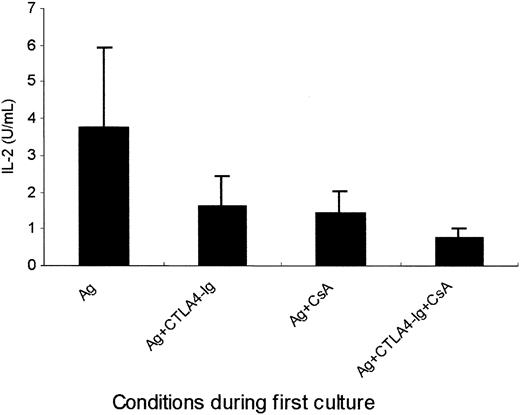
Because IL-2 has been shown to prevent the anergic state of T cells anergized in the absence of costimulatory signals, recombinant IL-2 was, therefore, added to the first culture of PBMCs in 8 patients. We found that platelet-specific anergy induction could also be prevented by adding IL-2 during the primary culture in the presence of CTLA4-Ig (P ≤ .001), CsA (P ≤ .02) or CTLA4-Ig and CsA together (P ≤ .001; Figure6), demonstrating the pivotal role of defective IL-2 production in the induction of platelet-specific T-cell anergy.
Prevention of T-cell anergy by exogenous IL-2 in the primary culture.
T cells (1 × 106/mL) from 8 patients were cultured following the same procedure as that described in Figure 2 except that the primary culture was performed in the presence (▪) or the absence (■) of exogenous IL-2 (10 U/mL). Proliferation on rechallenge with platelets was measured and shown as [3H]thymidine incorporation (mean ± SD cpm).
Prevention of T-cell anergy by exogenous IL-2 in the primary culture.
T cells (1 × 106/mL) from 8 patients were cultured following the same procedure as that described in Figure 2 except that the primary culture was performed in the presence (▪) or the absence (■) of exogenous IL-2 (10 U/mL). Proliferation on rechallenge with platelets was measured and shown as [3H]thymidine incorporation (mean ± SD cpm).
The response to CsA therapy in vivo was associated with T-cell sensitivity to CsA inhibition in vitro: clinical implications in the treatment of refractory AITP with CsA
In experiments using PBMCs from 11 patients with refractory AITP, we found that T-cell–antiplatelet reactivity decreased to normal ranges (normal ranges were established as the mean ± 2 SD of results from healthy control subjects)5 6 in 6 patients in the presence of CsA during primary culture. However, in the other 5 patients CsA was not sufficient to suppress T-cell proliferation completely. The lack of complete inhibition in vitro by CsA alone was consistent with the pattern in clinical treatment of refractory AITP with CsA, which could achieve response in only a certain percentage of patients. This finding led us to explore the association between the sensitivity in vitro and the response to CsA in vivo in the treatment of refractory AITP. Of the 11 patients with refractory AITP treated with CsA (Table 1), 5 of 6 patients showed platelet reactivity that was completely inhibited by CsA in vitro (CsA sensitive) and were also responsive to CsA treatment (3 CR and 2 PR cases; peak platelet count occurred between 35 and 98 days [mean, 60.2] after CsA beginning). However, all of the 5 cases whose platelet reactivity could not be completely inhibited by CsA in vitro (CsA insensitive) showed no response to CsA therapy. The results suggested that the CsA efficacy in vivo was significantly different between CsA-sensitive and CsA-insensitive groups (P ≤ .02), and CsA sensitivity in vitro could serve as a reliable parameter in predicting the efficacy of CsA therapy in refractory AITP. In 2 patients CR lasted for more than 2 years off treatment. Another CR patient relapsed 1 year after the end of treatment and attained PR after readministration of CsA. The platelet counts of the 2 cases of PR dropped to 30 × 109/L or less after stopping CsA. Readministration synergistically with low-dose prednisone (20 mg per day) could maintain platelets at a safe level. The side effects during CsA treatment included nausea in 4 patients, gingival hyperplasia in 3, and slight creatinine level elevation in 1 patient. These complaints usually resolved after dose reduction or discontinuation for a few days.
Discussion
In accordance with previous reports showing a strong in vitro antiplatelet T-cell response in chronic AITP,5-8 we found that T cells freshly isolated from the patients proliferate against platelets in the presence of autologous APCs. However, Garcia-Suarez et al27,28 demonstrated in chronic AITP that highly purified T cells lacking in APC showed defective activation and proliferation following anti-CD3 monoclonal antibody or phytohemagglutinin (PHA) stimulation. Therefore, it is likely that the interaction of APCs with T cells provides signals that could overcome the defective response to mitogenic stimuli through the TCR/CD3 complex. On the basis of previous work on the murine model of type I diabetes that showed a similar autoimmune pathogenesis at the level of T cells,29 we would like to propose that in chronic AITP the CD28 costimulatory pathway is functional. A specific defect in signal 1 transduction through the TCR/CD3 complex that could be compensated for at least partially by signal 2 mediated through CD28 molecules, may underlie the hyporesponsiveness of the purified T cells.
Autoimmunity may occur only in the presence of proper coactivation.30 Most tissue does not express costimulatory molecules and should not be able to costimulate T cells.31If costimulatory signals were aberrantly provided to platelet-reactive T cells, AITP may occur. The data presented provide the first evidence that in most patients with chronic AITP, blocking the B7-CD28 interaction results in platelet-specific T-cell tolerance, thus implicating the CD28 blockade as a promising approach to the treatment of chronic AITP.
We also observed that in a few cases, B7-blocking agent alone failed to induce tolerance, suggesting the existence of other pathways to prevent anergy. This finding could be explained from several aspects. First, although B7-CD28 is believed to be the most important costimulatory pathway of T-cell activation, T-cell surface receptor molecules other than CD28, including CD2 and LFA-1, could mediate costimulation. The LFA-3/CD2 pathway and LFA-1/ICAM-1 pathway have been found to stimulate antigen-independent cell adhesion and proliferation.32Second, CD28-mediated signals are also essential for the survival of CD4+CD25+ suppressive T cells (Ts).31 Blocking CD28 costimulation may inhibit the activation of this subset of immunoregulatory T cells, as in the case of certain autoimmune diabetic models.33 Third, T-cell tolerance is achieved by coupling the fate of the cell to the integration of signals received through the TCR, costimulatory molecules as well as cytokine receptors, especially the IL-1 receptor and tumor necrosis factor (TNF) receptor family members. It has been shown that IL-1 or TNF could synergize with TCR stimulation by mediating activation of the IL-2 promoter through sphingomyelin/nuclear factor-κB (NF-κB) in certain organ-specific autoimmune diseases. This renders CD28 signaling functionally redundant.30,34,35 The interfamily redundancy of CD28, CD2, and LFA-1 costimulatory pathways offers an opportunity to regulate distinct T-cell response profiles in various microenvironments.32 By combining the use of CTLA4-Ig and CsA during the primary culture, we observed that the platelet-induced T-cell responses were inhibited consistently in the secondary culture. Moreover, we also performed second restimulation with platelets (tertiary culture) in 4 patients with chronic AITP and found that the T cells made unresponsive during the primary culture with CTLA4-Ig and CsA displayed no platelet reactivity (data not shown), indicating the persistence of platelet-specific tolerance.
Autoreactive T cells to the glycoprotein GPIIb/IIIa have been reported to escape thymic deletion and exist in the periphery of healthy individuals but remain tolerized.36,37 Therefore, peripheral mechanisms that control these potentially pathogenic T cells are necessary for preventing autoimmunity. Peripheral tolerance is believed to include several not mutually exclusive mechanisms: deletion, anergy, ignorance, and suppression.16 The existence of distinct pathways of T-cell tolerance suggests that different types of antigens induce tolerance by distinct mechanisms.38 Our experiments demonstrated that the tolerance could be broken down and that the T cells resume proliferation on rechallenge with IL-2 in addition to original Ags and autologous APCs, suggesting that the T-cell tolerance to platelet Ags was ascribed more likely to anergy than to deletion. The data also show that the T-cell tolerance induced by CTLA4-Ig, CsA, or their combination is platelet Ag specific. When restimulated with third-party stimulators (unrelated Ags), the T cells displayed the ability to proliferate, indicating that the tolerance is not likely due to the effects of T-cell suppression.
A wealth of previous data has pointed to the importance of IL-2 in preventing induction of T-cell anergy.32 33 Our data also confirmed that the platelet-specific T-cell anergy induced by CTLA4-Ig and/or CsA is correlated with inhibition of IL-2 production and could be prevented if exogenous IL-2 is added, indicating that defective IL-2 production is partially responsible for the platelet-specific anergy induction.
We also prospectively evaluated the efficacy of CsA therapy in a small series of selected patients with refractory AITP. The clinical management of refractory AITP, with a natural evolution to persistent, severe, and uncontrolled thrombocytopenia, remains a difficult dilemma. Although there are optimistic reports for new modalities used to treat patients with refractory AITP, there are no rigorous clinical trial data on the relative benefits and risks on which to make evidence-based recommendations for the choice of interventions.30 In our experiments of CsA treatment, despite the small number of patients evaluated, we saw a consistent response in 5 of 11 patients. Further study showed that the response to CsA treatment in vivo is associated with the sensitivity of platelet-reactive T cells to CsA inhibition in vitro. Therefore, in patients with severe refractory AITP showing CsA-sensitive antiplatelet T-cell reactivity, CsA may offer a useful alternative to other immunosuppressive drugs with the side effects comparing favorably to those of other drugs. It would be reasonable to recommend the usefulness of CsA as first-line immunosuppressive therapy in this particular subpopulation of patients to avoid myelotoxicity and mutagenic effects.
The ultimate goal for the treatment of chronic AITP, and possibly of other autoimmune diseases, might be to achieve autoantigen-specific immunologic tolerance. In this sense CTLA4-Ig may become a promising new therapeutic agent for the treatment of chronic AITP. CTLA4-Ig has specific interaction, minimal toxicity, and low risk of blunting resistance to infection and carcinogenesis. The blockade of 2 costimulatory signals with CTLA4-Ig and CsA combination would make the tolerance achieved more easily, providing a powerful strategy for the management of refractory AITP.
We would like to thank Prof Xueyong Wang for invaluable contributions, Dr Jiajun Liu for referring patients, and Dr Xiaoli Liu for technical assistance. We also thank Dr Yiqiang Zhao (Ohio University, Athens) for providing reagents and for helpful suggestions and scientific discussions. We gratefully acknowledge the invaluable help of Dr Michael P. Fautsch (Department of Ophthalmology, Mayo Clinic, Rochester, MN) for critically reading this manuscript.
Prepublished online as Blood First Edition Paper, November 21, 2002; DOI 10.1182/blood-2002-06-1666.
Supported in part by grant no. 96374218 of the Excellent Young Scientist Research Fund from the Science and Technology Committee of Shandong Province, People's Republic of China.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jun Peng, Department of Hematology, Cancer Center, Qilu Hospital of Shandong University, Jinan, Shandong, 250012, P R China; e-mail: junpeng88@sina.com.cn.

![Fig. 1. Effects of CTLA4-Ig and/or CsA on platelet Ag–induced T-cell proliferation in primary culture. / T cells (1 × 106/mL) from 36 patients with chronic AITP (▪) or 15 healthy control subjects (■) were cultured with acid-treated platelets and APCs for 7 days in the presence or the absence of CTLA4-Ig (10 μg/mL) and/or CsA (400 ng/mL). Proliferation was measured by [3H]thymidine incorporation and shown as mean ± SD cpm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-06-1666/3/m_h80734058001.jpeg?Expires=1769908082&Signature=VBenLamrTIALPqEzIONZrcyW5l62QtvctVQppt7UOB8tMfYTrZHJkCp-GS191oa2wJR2nAE9pLSLObKfBuFmGNlP1G745coNhLVqmRU5gwozxAF6mvG~4NwPc94EhU64mwjTK5IKbEsjqJstclDCPlIlcQrKRlV7BH8RHbewJlxSV~YJqzezRrcCHX71uH7UedbWGPKpfp2zHlDPQRpWXn2spxmg3-0jS7I~QUA402m4-7NeUzaBKW4PNPfLKSNzZcFa41~VXMWONBB0E1NFNkS-yVBKTVzRxYj9WlN5cWu3MaDgpJL9bs6Zc2PD3Ts4SfG3XH1ySCmobJMNFYRCQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Induction of T-cell tolerance to platelet Ags for patients with chronic AITP. / T cells (1 × 106/mL) from 36 patients were incubated with acid-treated platelets and APCs in the presence of CTLA4-Ig (10 μg/mL) and/or CsA (400 ng/mL). After 7 days of incubation the cells were washed and rested for 2 days (primary culture), and then rechallenged with acid-treated platelets in the presence of APCs but without CTLA4-Ig or CsA (secondary culture) (A) for different culture periods in the first 3 patients to determine the kinetics of T-cell proliferation during secondary stimulation, and (B) for 4 days in all 36 patients to define the effects of CTLA4-Ig and/or CsA on the induction of T-cell tolerance. Proliferation was measured by incorporation of [3H]thymidine and shown as mean ± SD cpm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-06-1666/3/m_h80734058002.jpeg?Expires=1769908082&Signature=H5Jbju46K9JzBnEU3uB8gB~xHsrkvzcuTpkbIcwVp4mDozETYLf3QFfm45dj8FH2aT8ESN0DCOprta4IZChRl8viWyXkvVr9J6RNE9walmZvnHr01JO~WPOxHnDP2DbS1pceMsf~~SVaFXHDb9HyKHfWCaHSliaDPcDwmtwbwxXVYEGqB7dHJTITPBSeTLlkSWZt6NFAGqGY5NTKp5EgWXtc6FpybUmYNrjgL-2mCB3oQO8rJ4EEUyobUgtumWRkLiA6QpcHx~1Dx3q~Rigu2hPzv-VJkkBnFbexMMF4AyTCEkez7LWXEIngeYeVd4PSUxKPum3zwqpNLkiDbUVLcQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. IL-2 reversed the T-cell tolerance to platelet Ags induced by CTLA4-Ig and/or CsA. / Following the same procedure for primary culture as that described in Figure 2, the T cells from 16 patients rendered tolerant to platelet Ags by CTLA4-Ig and/or CsA were rechallenged with platelet Ags and APCs in the presence (▪) or the absence (■) of IL-2 (10 U/mL) in the secondary culture. Proliferation was determined by [3H]thymidine incorporation on day 4 and shown as mean ± SD cpm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-06-1666/3/m_h80734058003.jpeg?Expires=1769908082&Signature=skbb7Pj~XkJ5IaFhtslmJTQpfRRKpCo-qo~4OrGiLmjpgwZFcP3Kb9kDrstvjennqW1Maf6saWQSUythEu-x34Y6gzBNn0~nfL3L-jh5XonZeZWd-XVyVGPYFPKLC5x8e3amzYjHKXEkxvgm0P~bPhx8fHYYZEGuJNoktelVQsyR7o-D2lUbMennohin-nJ7PDTuXz539CN-WZAgj~lgN-BVN5bpgBdLkJTf~wV-4blrlLHptM4VoLUaHiIoqgVbb5ZGdQ2rIbD~4OpEvWTTlZvXK30LDuJ2qnRDRAJJEqv2oJ76bDzWeHsUw2CTHgyNgr2vnMM9DYAxCvCSbLQ7Og__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Specificity of T-cell anergy to platelet Ags induced by CTLA4-Ig and/or CsA. / Following the same procedure for first culture as that described in Figure 2, the T cells rendered tolerant to platelet Ags were rechallenged with the original (■) or unrelated Ags (tuberculin, 20 U/mL; ▪) in the secondary culture in 12 patients. After 4 days, proliferative response was measured by [3H]thymidine incorporation and shown as mean ± SD cpm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-06-1666/3/m_h80734058004.jpeg?Expires=1769908082&Signature=Gdu5ATyQWCRlYuxFRSdEKZqGhuB9~4y5zkscVV3JgKpjd0FHZj74y2cHWoneeIVBcOIUvE5Ml5M9gI7Pha7SYcVLudZpwWmtUJhM6jD1kICe7zVzooOvlnavv3La0Sf1E9rAF2qMYlu~eFp7bhK008bGUeoX-4JXAU7nXrCfMq~cTo~vDUm-qGIvMzi7kwJ-A1MAQmFWKD0pqGSHB60PcMPPTi3cXfzg6ls9jksHLvW4IG8qzg9pXE-S6hylhq2LVmwMDIF7Zn8QfO3VZNSDY14218KWJHNUGnTzFOcWpicQMwtpIZ5ZeYybE117hwRRuXnk~1J2VtTUomJqzRwI2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Prevention of T-cell anergy by exogenous IL-2 in the primary culture. / T cells (1 × 106/mL) from 8 patients were cultured following the same procedure as that described in Figure 2 except that the primary culture was performed in the presence (▪) or the absence (■) of exogenous IL-2 (10 U/mL). Proliferation on rechallenge with platelets was measured and shown as [3H]thymidine incorporation (mean ± SD cpm).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-06-1666/3/m_h80734058006.jpeg?Expires=1769908082&Signature=GF5L~m7yP6uqouhIoGJVbkOTCqbpTzud047pWKVZzseWaV2Ca01KAvatw4k4o-VD3LXWJ3pZxH0JtU4MausBINzO1xo23rW3bdQgLNEgY3aVJ4D2Xr7sLHT3Jxj9NCDK8NYtCZwkWr63ZdI6Qh~MpNiMYYWNuqfTssQd4dBTUIyyGXqe0U4SpyuqmrjGuf4bdV6qzCTZ9fvlBroKFqW92V5jqVenNhih9Q7lSzu-2NsBz0yhBGo-iGgXlvPMMVNGVbQX3AF~5zmEl~Y1UYL-SNA5Pt~rCYE~odmmAqLu28Cb-EoJ4at3lMWEfIbtnRCTdSHUHgJ2kYctz2E9ydEZkg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. Effects of CTLA4-Ig and/or CsA on platelet Ag–induced T-cell proliferation in primary culture. / T cells (1 × 106/mL) from 36 patients with chronic AITP (▪) or 15 healthy control subjects (■) were cultured with acid-treated platelets and APCs for 7 days in the presence or the absence of CTLA4-Ig (10 μg/mL) and/or CsA (400 ng/mL). Proliferation was measured by [3H]thymidine incorporation and shown as mean ± SD cpm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-06-1666/3/m_h80734058001.jpeg?Expires=1770405500&Signature=usRe0Nvh9v6C-0IodkWg7zj71ejROCTGwG8qXoSkq1EbdM5GNin7c7xCb-juxey6FVUGK4mm3CTOyrlMfAkjpVMF4AB4ZYhIf9RvxNoN3sBP-xs6oSK7wE7GxmufBCNOpQM6dxFhY-vkM8Qq7DBL7TQNY~XMJpFd1rSk-iqSCO4Hgz7umxYx3ktgQruZ3V39BGjGsT8YOGT3zg3FkzcptCYTiNNqlAfMsc63bJiHfo-nhfuJ~-aA8TGe55bDeDT3~dC-kP7~HuqCvjeOzrqP0S5aSMKCI8gN0lHGrMfyxbCIl2C6lCoQTqSJr4~891CT6-OgSs6sesK-HoU-uZgWzg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Induction of T-cell tolerance to platelet Ags for patients with chronic AITP. / T cells (1 × 106/mL) from 36 patients were incubated with acid-treated platelets and APCs in the presence of CTLA4-Ig (10 μg/mL) and/or CsA (400 ng/mL). After 7 days of incubation the cells were washed and rested for 2 days (primary culture), and then rechallenged with acid-treated platelets in the presence of APCs but without CTLA4-Ig or CsA (secondary culture) (A) for different culture periods in the first 3 patients to determine the kinetics of T-cell proliferation during secondary stimulation, and (B) for 4 days in all 36 patients to define the effects of CTLA4-Ig and/or CsA on the induction of T-cell tolerance. Proliferation was measured by incorporation of [3H]thymidine and shown as mean ± SD cpm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-06-1666/3/m_h80734058002.jpeg?Expires=1770405500&Signature=XcvDUGQ9S2gQi4LeugkM72gR4IG9n5aYyr6cimtzudsrIzFkcDEch53J542RnUEobvOcmqSj1-XeovzLjO8qmfnhOwo5j3thUupIsiL1w7qbuUYVE2CB-MRlGzl59X0MXSA6HbjwoJwv1eJ4y8N1wmS1QzLIoR2Z~HUvgu7QJAmn6D7MvcUQvjDLh-iwp82Mp9YXxWSV7GgY-7jL6v6UptGVtf4TXWbDLD-ybRBbHlbpTTErbGE-PNrLdmFrg9fW9y3LOKoKSji-YwK0aV8yykSGAUDjpSA6kVoCnS71btFyRbV2UxbUIoDUjH9Y~Q4-JvAgqRQdGygRQioZWB5Fag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. IL-2 reversed the T-cell tolerance to platelet Ags induced by CTLA4-Ig and/or CsA. / Following the same procedure for primary culture as that described in Figure 2, the T cells from 16 patients rendered tolerant to platelet Ags by CTLA4-Ig and/or CsA were rechallenged with platelet Ags and APCs in the presence (▪) or the absence (■) of IL-2 (10 U/mL) in the secondary culture. Proliferation was determined by [3H]thymidine incorporation on day 4 and shown as mean ± SD cpm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-06-1666/3/m_h80734058003.jpeg?Expires=1770405500&Signature=Zmi~A6zM3~r-Q7spkZRzehI~rtvFN~3qFqd12y49kcMOIDhV44bRVoCNk1RKzl7Powq4CS7j1K0T-~m-x0exy2gPUR1~dmfnbLaX5dJFtLZCzTWngGgiCIqPog4SOhlJewh-bjLancqMvVxRuzacRM9-jWJSJ91Qtj32BBqCP6UwLqySwZwC1Dq7Zrr-vTlq1fheOarGZczUNzgyLeik5ghuLDT4bowNa4cyaF-PhDDm~-mFQcBIeMeKu5xQy5iea5G7VR1K5b~0TqRfwe-ebZekJl3hatD2jgMQUn8cf~CwERwx0SYTXknibifs6afaxV076mdxOgxgSKFYruztjQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Specificity of T-cell anergy to platelet Ags induced by CTLA4-Ig and/or CsA. / Following the same procedure for first culture as that described in Figure 2, the T cells rendered tolerant to platelet Ags were rechallenged with the original (■) or unrelated Ags (tuberculin, 20 U/mL; ▪) in the secondary culture in 12 patients. After 4 days, proliferative response was measured by [3H]thymidine incorporation and shown as mean ± SD cpm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-06-1666/3/m_h80734058004.jpeg?Expires=1770405500&Signature=Bj6jOmNboPKvBsjJ74bz7PWx-RaDmWaZpgoH1exGmo1rh~hycvLSsAvpCHhSUZNPHRDmBbEiwH-HRtLhciTCLbpmckAY3mwwDCpXg3xSbXTB01tvCiDdO9hgevLAWcbE~ap9GAdTk-TKLqUTnfC76ZUTg5GXJ9juoOvJTnuJRP~D8JoxuSbSXLZ5B5yuWJXuJs6UmP2FKDemLd1VtLHZjmOTA0Kz7goQs900oESYsTY9yE7IFL4zf4RGj~meGIq8SAmaJEiAJUHqJk8LlIM~-YjwPz5GEZqCHyVIrWrWTSvFzLAXy2PBUMMfLmfHzuRzr71aqC98jki1uCZ6ZKg3iA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Prevention of T-cell anergy by exogenous IL-2 in the primary culture. / T cells (1 × 106/mL) from 8 patients were cultured following the same procedure as that described in Figure 2 except that the primary culture was performed in the presence (▪) or the absence (■) of exogenous IL-2 (10 U/mL). Proliferation on rechallenge with platelets was measured and shown as [3H]thymidine incorporation (mean ± SD cpm).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-06-1666/3/m_h80734058006.jpeg?Expires=1770405500&Signature=N0g8xCudGJ8nrKwVS1uFLBGzDFrv8fTU2RZdBDDQPZbHJ7SjLIsjRDcDLZoTviAStujZ-DwPFzhnU15B9FysBFmP-tbF~ToTVIwCgq0W9p~4Uwtaxqx6tqH9nbtOnhYRdng4dOaF83q4mQUkOBz7NWd5neJZuG~S8NW9F5lRtqc3SAtIoUvBn1g3TtfQKE-h-nZia4jv03i43lMFmoLHDwyMGN3ZClDU1wiDbRj~CRPSDI3RgME3luuYskdy-EFSlDWd7uJzIjkiWBOd~Hluc77v0PQgMzDNZWjsftTmHmMCkSVG2JeVNpRy3-CKyvCgkhIin4SB3WW1fcoq9RnVsw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)